Mpho Mashigo1*  , Kennedy J Ngwira 2
, Kennedy J Ngwira 2 , Mpho Choene 3
, Mpho Choene 3  and Ida Risenga1
and Ida Risenga1
1Department of Animal, Plant and Environmental Sciences, University of the Witwatersrand, Johannesburg, South Africa.
2Molecular Sciences Institute, Department of Chemistry, University of the Witwatersrand, Johannesburg, South Africa.
3Department of Biochemistry, Faculty of Science, University of Johannesburg, Johannesburg, South Africa.
Corresponding Author E-mail: mashigo.mpho@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2810
Abstract
Carpobrotus edulis is an edible medicinal plant from South Africa and is used for the treatment of different ailments and disorders, including diabetes mellitus. Diabetes is a persistent metabolic condition distinguished by high levels of glucose concentrations in the bloodstream. Due to climate change-related conditions, plants may be subjected to extreme temperature events such as cold fronts and heat waves. Hence the aim of the study was to expose Carpobrotus edulis leaves to temperature conditions and then assesses their antidiabetic activity against alpha-amylase in different solvents. The objective was to keep plants in growth chambers set at either 15/10oC and 45/35oC (day and night), respectively, and harvested at 48-hour intervals (48, 96, and 144). These were compared to plant samples in control conditions (25/15oC). Under control (25/15°C) conditions, the aqueous extract displayed effective inhibition of alpha-amylase (IC50 = 195mg/ml). In contrast to the control extracts, the hexane solvent consistently exhibited the highest inhibitory activity against alpha-amylase under both low (15/10°C) and high (45/35°C) temperature conditions. This trend was observed across all three harvest durations. After 48 hours of high-temperature conditions, the IC50 value was 131mg/ml. While after 96 and 144 hours of low-temperature conditions, the IC50 values were 214mg/ml and 131mg/ml, respectively. The results suggest that Carpobrotus edulis, exposed to low and high temperature conditions, has potential antidiabetic properties against alpha-amylase. This is an interesting aspect of how environmental conditions can impact medicinal properties. The outcome may have significant implications for the use of the plant by indigenous people, who depend on it for the treatment of various ailments, including diabetes. It will also have implications for the antidiabetic research of the plant as well as climate change research.
Keywords
Antidiabetic activity; Alpha-amylase; Carpobrotus edulis; Climate change; Temperature conditions
Download this article as:| Copy the following to cite this article: Mashigo M, Ngwira K. J, Choene M, Risenga I. Alpha-amylase Inhibition of Carpobrotus edulis (L.) Bolus Exposed to Low and High-Temperature Conditions. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Mashigo M, Ngwira K. J, Choene M, Risenga I. Alpha-amylase Inhibition of Carpobrotus edulis (L.) Bolus Exposed to Low and High-Temperature Conditions. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3HblQvj |
Introduction
Indigenous medicinal plants have been valued for thousands of years due to their nutritional and pharmacological properties, which continue to be essential in various health care systems worldwide1. These plants have had a substantial impact on the traditional medicine of indigenous tribes and have also been integrated into modern Western medicine through drug discovery processes 2. One such plant with a long history of traditional medicinal use is Carpobrotus edulis (L.) Bolus, commonly known as sour fig 3 (Figure 1). The species is native to South Africa and is commonly invasive in the coastal environments of many parts of the world 3. Sour fig belongs to the Aizoaceae family and the Carpobrotus genus. This genus has thirteen species, seven of which occur in southern Africa 4. The Carpobrotus edulis species is a flat-growing, perennial, succulent herb that forms dense mats 5,6.
 |
Figure 1: Image showing Carpobrotus edulis leaves and flower. |
Carpobrotus edulis is used by traditional healers, herbalists, and local people of South Africa for the treatment of various ailments such as mouth infections, sore throats, stomach aches, diabetes mellitus, tuberculosis, and human immunodeficiency virus (HIV)-related infections 7. The leaves juice has been shown through studies to have antiseptic properties for soothing burns, sores, allergies, cuts, and other skin conditions 8. In other parts of Africa, the mixture of honey, olive oil in water, and leaf extract is used as a treatment for vaginal thrush and tuberculosis 7.
Diabetes mellitus is an enduring metabolic condition distinguished by elevated concentrations of glucose in the blood (hyperglycemia), caused by impaired insulin secretion from the pancreas 9. Its prevalence has been steadily increasing worldwide 10, especially in low- and middle-income countries like South Africa 11. This escalating burden of diabetes poses substantial challenges to public health, healthcare systems, and the overall well-being of the population in the country 12. Diabetes can be classified into different types, with type 2 being the most prevalent in South Africa 13. Accounting for approximately 85–90% of all cases, type 2 diabetes is primarily linked to lifestyle elements such as lack of physical activity, unhealthy eating habits, and excess body weight 14. Although less common in developing countries, type 1 diabetes also affects a significant number of individuals 15. It causes long-term complications associated with small blood vessels, i.e., microvascular complications 15. Researchers focus on various mechanisms to develop effective anti-diabetic drugs 16. Among these approaches, utilizing medicinal plants in drug development for diabetes treatment is preferred over synthetic drugs because of the numerous harmful side-effects linked with the latter 16.
Damage to body cells caused by harmful molecules (oxidative stress) and inflammation caused by substances that form when sugars react with proteins in the body (advanced glycation end products (AGEs)), are mechanisms that exacerbate the problems of diabetes mellitus 16. The antiglycation activity of C. edulis extracts was investigated, and significant inhibition of fluorescent advanced glycation end products was observed in the ethanol-water extract of C. edulis 17. This antiglycation property and the inhibition of AGEs are crucial in preventing and alleviating diabetes-related complications 17. In the development of diabetes mellitus treatment drugs, one important mechanism involves the inhibition of α-glucosidase and α-amylase activity. Inhibitors targeting these carbohydrate-metabolising enzymes have been effective in controlling postprandial hyperglycemia (high blood sugar levels after eating a meal), which is a significant strategy in managing diabetes mellitus 18,19. Postprandial hyperglycemia is closely associated with abnormalities in glucose homeostasis, and its regulation plays a pivotal role in the early stages of diabetes pathophysiology, especially in type 2 diabetes mellitus 16. Carpobrotus edulis leaf extracts have shown potential anti-diabetic activity through the inhibition of α-glucosidase, as demonstrated by its aqueous, methanol (50%), and acetone (70%) extracts 9. This property suggests that C. edulis could be a valuable natural resource for managing blood glucose levels after carbohydrate catabolism and potentially contribute to diabetes management.
Due to climate change, prolonged extreme temperature events, such as cold fronts and heat waves, may have unknown effects on the plant, especially its antidiabetic activity. Consistent with global trends, South Africa has observed a reduction in cold temperature extremes and a rise in warm temperature extremes 20. It has been stated that the secondary metabolite composition of some medicinal plants could be affected by climate change 21,22. These secondary metabolites, known for their various biological activities, include compounds with potential antidiabetic properties. Changes in temperature and other environmental factors can influence the synthesis of these compounds in plants. While various authors have shown how plants respond to different abiotic stresses, there remains a scarcity of information on how indigenous medicinal plants such as Carpobrotus edulis are affected by extreme temperature conditions. This study is guided by the ethnomedicinal uses of Carpobrotus edulis and the temperature extremes experienced in South Africa for an extended period. It aims to investigate the antidiabetic activity of its leaves under low and high temperature conditions, using aqueous, methanolic, and hexane extracts. The outcomes of these temperature conditions are compared to ambient conditions. The study seeks to shed light on how climate-induced temperature variations may impact the medicinal properties of Carpobrotus edulis. The hypothesis is that low and high temperature conditions have an effect on the antidiabetic activity of Carpobrotus edulis.
Materials and Methods
The chemicals and solvents used for this study were of analytical grade. Methanol, hexane, dimethyl sulfoxide (DMSO) (purity > 99.5%), phosphate buffer, alpha-amylase, potato starch and 3,5-dinitrosalicylic acid (DNSA) (purity > 99%) were all procured from Sigma-Aldrich, Johannesburg, South Africa.
Collection of plant material
The aerial parts (fresh cuttings) of Carpobrotus edulis plants were collected at the City of Tshwane (Pretoria North: 25.6776° S, 28.1755° E), Gauteng, South Africa, in January 2021 (summer season), planted in five-litre plastic pots in commercial potting soil, and allowed to grow for three months in the green house at the University of the Witwatersrand, Johannesburg, South Africa. The prepared taxonomic identification of the voucher specimen (IMR 0009522) was done and stored at the University of the Witwatersrand, Braamfontein East Campus. Dr Ida Risenga authenticated the plant material.
Control plants (five pots with three cuttings each) were watered daily with 100ml of water and kept under 25/15ºC (day and night) in the green house. Two sets of fifteen experimental pot plants (three cuttings in each pot) were transferred into separate plant growth chambers (Conviron CMP6010) and kept at either 15/10oC or 45/35oC (day and night), respectively, watering them once daily for a period of up to six days. The plants were kept under a photoperiod of 14-hour light and 10-hour darkness. The control and temperature treatment samples were harvested episodically at 48-hour intervals (48, 96, and 144 hours), dried using a freeze dryer (VaCo 2, Zirbus technology GmbH Hilfe Gottes 1, 37539 Bad Grund) and pulverised.
Sample preparation and extraction
The powdered leaves of Carpobrotus edulis from the control and the two sets of temperature treatments were separately extracted with aqueous, methanol, and hexane (1 gram to 20 millilitres solvent ratio). As is generally known, different solvents will dissolve different compounds depending on the polarity index 23. Water (Aqueous) is a polar solvent with an index of 10.2, methanol is a polar solvent with an index of 5.1and hexane is a non-polar solvent with an index of 0.123. The methanol and hexane extracts were evaporated on a fume hood, while the water extracts were evaporated with a freeze dyer. Thereafter, 1% DMSO was used to resuspend the powdered extracts.
In-vitro inhibition of alpha-amylase activity
Inhibition of α-amylase activity by the aqueous, methanol, and hexane extracts of Carpobrotus edulis followed the techniques outlined by Kamtekar et al. 24 with slight modifications (on the concentrations of plant extract). Plant extracts of varying concentrations (0,5 – 2 mg/ml, 500μL) were mixed with 500μL α-amylase solution, with a concentration of 2 units/ml. The enzyme solution was prepared by dissolving 0.001 g of α-amylase in 100 ml of 0.02 M sodium phosphate buffer at pH 6.9, containing 6.7 mM sodium chloride. The mixture was incubated at 32°C for 10 minutes. Subsequently, 500μL of 1% w/v corn starch solution in distilled water was added to the reaction mixture and incubated for 10 minutes at 32°C. After incubation, 1ml of DNSA reagent was introduced to each reaction mixture tube, followed by incubation in a hot water bath at 85°C for 5 minutes. The reaction mixtures were removed from the water bath and cooled to room temperature, and each tube was diluted to a final volume of 5ml with distilled water. The absorbance of the resulting orange-red colour was measured at 540nm using a spectrophotometer. An enzyme unit is defined as the quantity of enzyme required to release 1mg of maltose from 1% starch within 5 minutes under specified room-temperature conditions. To calculate the IC50 (inhibitory concentration) value, which represents the sample concentration (mg/ml) needed to reduce the absorbance of alpha-amylase, a logarithmic regression curve was generated by plotting the percentage of alpha-amylase inhibition against sample concentration.
The percentage of α-amylase inhibition was assessed by using the following formula:
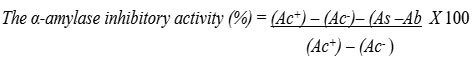
Where Ac+ is the absorbance of 100% enzyme activity (only solvent with enzyme).
Ac– is the absorbance of 0% enzyme activity (only solvent without enzyme).
As is the absorbance of the test sample with enzyme.
Ab is the absorbance of a test sample without an enzyme.
Data analysis
The obtained study results were analysed using Microsoft Excel GraphPad Prism 10.1.0 software. A one-way analysis of variance (ANOVA) was used to determine a significant difference between the control and the temperature treatments’ inhibition percentage within the same concentration. Additionally, the ANOVA was used to determine a significant difference between the IC50 values of the temperature treatments and the control. These were determined at a significance level of p ≤ 0.05. Where a significant difference was observed, a two-sample T-test was used to identify the difference. An asterisk (*) represents the number of zeros after the decimal point. All analyses were done in triplicate, and the data were presented as the mean and standard error.
Results and discussion
Alpha-amylase is an eminent enzyme that is involved in the breakdown of carbohydrates into simple sugars in the digestive system 25,26. Therefore, inhibiting the alpha-amylase enzyme can suppress the digestion of carbohydrates and eventually reduce blood sugar levels 27. The alpha-amylase enzyme inhibition percentage for Carpobrotus edulis aqueous extract at control (25/15oC) conditions was compared to extracts at low (15/10oC) and high (45/35oC) temperature conditions at different durations (48-hour, 96-hour, and 144-hour harvest) (Figures 2a, 3a, and 4a, respectively). The results showed that the inhibition percentage of Carpobrotus edulis aqueous extracts against the alpha-amylase enzyme is increasing for all the treatments in a concentration-dependent manner (Figures 2a, 3a, and 4a, respectively). For both the 48-hour and 96-hour harvests, the low-temperature treatment had the highest inhibition percentage compared to the control, followed by the high-temperature treatment (Figures 2a and 3a). However, for the 144-hour harvest, the control treatment had the highest inhibition percentage compared to both the low and high temperature treatments (Figure 4a).
Based on its IC50 value of 195 mg/ml, the antidiabetic effect of C. edulis aqueous extract at control (25/15oC) conditions had a higher inhibitory activity via alpha-amylase than the low (15/10oC) and high (45/35oC) temperature conditions extracts at 48-hour (297 and 441 mg/ml, respectively), 96-hour (524 and 429 mg/ml, respectively), and 144-hour harvest (830 and 343 mg/ml, respectively) (Figures 2b, 3b, and 4b, respectively). Due to the available synthetic inhibitors that have been shown to have negative side effects, plant-based alternative therapeutic agents have been advocated for because they have been reported to have good safety profiles, have fewer side effects, do not cause hypoglycemia, and do not stimulate insulin secretion but effectively manage postprandial hyperglycemia 18, 28, 29.
It has been established in this present study that the plant has potential antidiabetic effects based on the IC50 value of the aqueous extract at control/ambient/normal conditions (25/15oC). In another study, the potential antidiabetic activity of C. edulis aqueous leaf extracts via the inhibition of alpha-glucosidase (an enzyme found in the intestine that further degrades the simple monosaccharides to glucose, which, upon absorption, enters the blood stream) showed that aqueous extracts had an overall strong inhibitory activity against the enzyme with an IC50 value of 5μg/ml 9. This present study not only substantiates the use of the C. edulis plant leaves for the treatment of diabetes but also the use of water as a medium of preparation in traditional medicine. Based on the half-maximal inhibitory concentration (IC50) of the temperature treatments, it was revealed that the plant could be significantly losing some of its antidiabetic activity when it is exposed to either low or high temperature conditions. In South Africa, if a cold front could persist for six days, as predicted in previous studies 20 and reported in the past couple of weeks in Gauteng and other parts of the country 30, the plant might have reduced inhibitory activity against alpha-amylase using water as an extraction solvent. The same applies for a heat wave that could persist for six days; the plant might have reduced inhibitory activity against alpha-amylase.
Various studies have reported that the antidiabetic activity of certain medicinal plantscould be attributed to the presence of their phenolic compounds 31, 32. Recently, Sabiu et al.26 reported on the presence of cyanidin, a phenolic compound identified in C. edulis as one of the inhibitors of alpha-amylase and alpha-glucosidase. In Table 1 of this study, the phytochemical profile of C. edulis showed a low presence of phenolic compounds after 48 and 96 hours of low temperature conditions but was absent after 144 hours of low temperature conditions. After 48, 96, and 144 hours of high-temperature conditions, the presence of phenolic compounds was absent. The low presence and absence of phenolic compounds in the low-temperature and high-temperature conditions, respectively, might cause a potential synergistic or antagonistic interaction of the other compounds that are highly present, altering their individual or collective impact on alpha-amylase inhibition 33. These could explain the difference in the IC50 value of these extracts compared to the control treatment. As part of adaptive responses, these changes could be due to the conformational changes in alpha-amylase caused by the enzymes present due to temperature variations 34, 35, 36, 37. This process can modify its substate specificity or affinity.
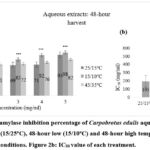 |
Figure 2a: Alpha-amylase inhibition percentage of Carpobrotus edulis aqueous extracts under control (15/25oC), 48-hour low (15/10oC) and 48-hour high temperature conditions. Figure 2b: IC50 value of each treatment. |
 |
Figure 3a: Alpha-amylase inhibition percentage of Carpobrotus edulis aqueous extracts at control (15/25oC), 96-hour low (15/10oC) and 96-hour high temperature conditions. Figure 3b: IC50 value of each treatment. |
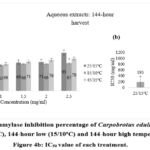 |
Figure 4a: Alpha-amylase inhibition percentage of Carpobrotus edulis aqueous extracts at control (15/25oC), 144-hour low (15/10oC) and 144-hour high temperature conditions. Figure 4b: IC50 value of each treatment. |
The alpha-amylase enzyme inhibition percentage for Carpobrotus edulis methanolic extract at control (25/15oC) conditions was compared to extracts at low (15/10oC) and high (45/35oC) temperature conditions (48-hour, 96-hour, and 144-hour harvest) (Figures 5a, 6a, and 7a, respectively). The results showed that the inhibition percentage of Carpobrotus edulis methanolic extracts against the alpha-amylase enzyme is increasing for all the treatments in a concentration-dependent manner (Figures 5a, 6a, and 7a, respectively). The control extract had the highest inhibition percentage compared to both the 48-hour and 96-hour temperature treatments (Figures 5a and 6a). However, for the 144-hour harvest, the highest inhibition percentage was observed at the high temperature treatment (Figure 7a) compared to the control and the low temperature treatment (Figures 5a and 6a, respectively).
Based on its IC50 value of 1680 mg/ml, the antidiabetic effect of C. edulis methanolic extract under control (25/15oC) conditions had a lower inhibitory activity against alpha-amylase than the low (15/10oC) and high (45/35oC) temperature conditions extracts at 48-hour (265 and 1122 mg/ml, respectively), 96-hour (235 and 262 mg/ml, respectively), and 144-hour harvest (197 and 487 mg/ml, respectively) (Figures 5b, 6b, and 7b, respectively). Based on these IC50 values, the 144-hour low-temperature treatment had a statistically significant increase in inhibitory activity against alpha-amylase. This could mean that the plant’s antidiabetic effect improves after being exposed to low-temperature conditions for six days. Exposure to temperatures can lead to the activation or enhancement of certain secondary metabolites, such as polyphenols and flavonoids, which are known for their potential health benefits 38, 39. The phytochemical profile of C. edulis exposed to low-temperature conditions for 144 hours (methanolic extract) in Table 1, showed a high presence of compounds, including phenolics and terpenoids. Exposure to low temperatures led to the enhancement of terpenoids and steroids. Some of these compounds have been shown to possess antidiabetic properties, including alpha-amylase inhibition, by interfering with enzyme-substrate interactions 40. The observed increase in inhibitory activity after exposure to temperature conditions aligns with this phenomenon reported in other plant extracts. The observed changes in alpha-amylase inhibition might be influenced by the duration of exposure to low-temperature conditions; prolonged exposure enhanced the activity over time. The extract might also have other enzymes that influence the inhibition 36.
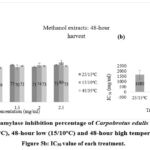 |
Figure 5a: Alpha-amylase inhibition percentage of Carpobrotus edulis methanol extracts at control (15/25oC), 48-hour low (15/10oC) and 48-hour high temperature conditions. Figure 5b: IC50 value of each treatment. |
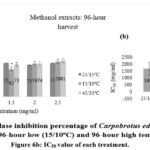 |
Figure 6a: Alpha-amylase inhibition percentage of Carpobrotus edulis methanol extracts at control (15/25oC), 96-hour low (15/10oC) and 96-hour high temperature conditions. Figure 6b: IC50 value of each treatment. |
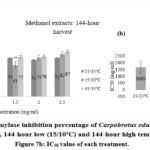 |
Figure 7a: Alpha-amylase inhibition percentage of Carpobrotus edulis methanol extracts at control (15/25oC), 144-hour low (15/10oC) and 144-hour high temperature conditions. Figure 7b: IC50 value of each treatment. |
The alpha-amylase enzyme inhibition percentage for Carpobrotus edulis hexane extract at control (25/15oC) conditions was compared to extracts at low (15/10oC) and high (45/35oC) temperature conditions (48-hour, 96-hour, and 144-hour harvest) (Figures 8a, 9a, and 10a, respectively). The results showed that the inhibition percentage of Carpobrotus edulis hexane extracts against the alpha-amylase enzyme is increasing for all the treatments in a concentration-dependent manner (Figures 8a, 9a, and 10a, respectively).
Based on its IC50 value of 274 mg/ml, the antidiabetic effect of C. edulis hexane extract at control (25/15oC) conditions had a lower inhibitory activity against alpha-amylase than the low (15/10oC) and high (45/35oC) temperature conditions extracts at 48-hour (226 and 131 mg/ml, respectively), 96-hour (214 and 220 mg/ml, respectively), and 144-hour harvest (131 and 164 mg/ml, respectively) (Figures 8b, 9b, and 10b, respectively). Based on these IC50 values, the 48-hour high-temperature treatment and the 144-hour low-temperature treatment had a significantly higher inhibitory activity against alpha-amylase. The impact of temperature on the composition and bioactivity of plant extracts has been well documented in scientific literature 39, 40. Al-Huqail, et al. 41 investigated the impact of climate change, temperature, and water stress on secondary metabolites in sweet basil (Ocimum basilicum). The researchers found that increasing temperatures (35, 45, and 55°C) significantly increased the levels of certain phenolic compounds, including flavonoids. Another study by Ljubej et al. 42 examined the effects of cold stress on secondary metabolites in kale (Brassica oleracea), and the results indicated that cold stress (8°C) increased the levels of phytochemicals such as phenolic acids, flavonoids, carotenoids, and glucosinolates (3%, 5%, 15%, and 21%, respectively), which are bioactive compounds with potential health benefits, including antidiabetic effects. The above pattern was observed in this study, as presented in Table 1 (hexane extracts). Both high-temperature and low-temperature conditions for 48 and 96 hours, respectively, triggered the biosynthesis of flavonoids, saponins, cardiac glycosides, and volatile oils. These compounds have been reported to have antidiabetic effects 43, 44. The observed changes in alpha-amylase inhibition might be influenced by the duration of exposure to low-temperature conditions, with prolonged exposure leading to an enhancement in activity over time. This phenomenon could be associated with the activation of specific enzymes under these conditions, either facilitating the breakdown of inhibitory compounds or inducing conformational changes in the alpha-amylase enzyme as adaptive responses 35, 36, 37. However, in the context of high-temperature conditions, unique inhibitory outcomes may be attributed to specific combinations of temperature and duration, as indicated by previous studies 36, 45.
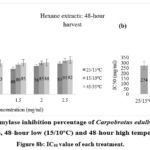 |
Figure 8a: Alpha-amylase inhibition percentage of Carpobrotus edulis hexane extracts at control (15/25oC), 48-hour low (15/10oC) and 48-hour high temperature conditions. Figure 8b: IC50 value of each treatment. |
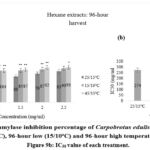 |
Figure 9a: Alpha-amylase inhibition percentage of Carpobrotus edulis hexane extracts at control (15/25oC), 96-hour low (15/10oC) and 96-hour high temperature conditions. Figure 9b: IC50 value of each treatment. |
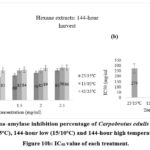 |
Figure 10a: Alpha-amylase inhibition percentage of Carpobrotus edulis hexane extracts at control (25/15oC), 144-hour low (15/10oC) and 144-hour high temperature conditions. Figure 10b: IC50 value of each treatment. |
 |
Table 1: Phytochemical profile of Carpobrotus edulis exposed to low and high temperature conditions compared to normal conditions. |
Conclusion
In this study, the effects of temperature conditions, solvent types, and harvest durations on the antidiabetic activity (against alpha-amylase) of Carpobrotus edulis leaves were investigated. The leaves were subjected to low (15/10°C) and high (45/35°C) temperature conditions using aqueous, methanol, and hexane solvents, with harvests conducted at 48, 96, and 144 hours. A control treatment at 25/15°C was used for comparison. Under control (25/15°C) conditions, the aqueous extract displayed effective inhibition of alpha-amylase, as evidenced by the low IC50 value of 195mg/ml. In contrast to the control extracts, under low (15/10°C) and high (45/35°C) temperature conditions, respectively, the hexane extracts consistently exhibited the highest inhibitory activity against alpha-amylase across all three harvest durations (48, 96, and 144 hours). After 48 hours of high-temperature conditions, the IC50 value was 131mg/ml. After 96 and 144 hours of low-temperature conditions, the IC50 values were 214mg/ml and 131mg/ml, respectively. The research offers valuable insights into how environmental factors, particularly temperature, affect the medicinal properties of Carpobrotus edulis. Therefore, it aligns with the initial hypothesis. This is especially significant in the context of climate change. The emphasis on plant-based therapeutic agents, considering the side effects associated with synthetic drugs, holds great relevance in today’s pharmacological landscape. Examining the impact of temperature stress on the antidiabetic potential of a traditional medicinal plant is a novelty and it contributes substantial value to ethnopharmacological research.
A deeper analysis of the hexane extracts is needed to identify the specific bioactive compounds responsible for the observed effect under the specified temperature conditions. There is limited discussion on the underlying mechanisms of how temperature stress alters the phytochemical composition, leading to the enhanced alpha-amylase inhibition of the extracts. Additionally, the study lacks in vivo validation of the antidiabetic effect of the extracts, which is important for establishing their efficacy and safety. Therefore, a comprehensive understanding of the plant’s antidiabetic properties could be achieved through a multifaceted approach encompassing both in vitro and in vivo studies, along with phytochemical analyses. Future research could explore the impact of other environmental stressors, such as salinity or drought, on the medicinal properties of Carpobrotus edulis. Conducting long-term studies to evaluate the impacts of chronic exposure to varying temperatures could offer a more profound understanding of how medicinal plants adapt to climate change. To bridge the gap between traditional use and clinical application, translational research, including pharmacokinetic and pharmacodynamic studies, is essential.
Acknowledgement
The authors thank the Department of Animal, Plant and Environmental Sciences, University of the Witwatersrand for providing the essential laboratory facilities.
Conflict of Interest
The authors declare that they have no apparent conflicts of interest that might have seemed to influence the findings presented in this paper.
Funding source
No funding source to declare.
References
- Khan M S A, Ahmad I. Herbal medicine: current trends and future prospects. InNew Phytomed. 2019; 3-13.
- Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 2020;19(5): 1199-1209.
- D’Antonio C M, Mahall B E. Root Profiles and Competition Between the Invasive, Exotic Perennial, Carpobrotus edulis, and Two Native Shrub Species in California Coastal Scrub. A J Bot. 1991; 78 (7):885-894.
- Malan C, Notten A. Carpobrotus edulis (L.) L. Bolus. (Aizoaceae). PlantZAfrica. http://pza.sanbi.org/carpobrotus-edulis. 2006
- Ordway D, Hohmann J, Viveiros M, Viveiros A, Molnar J, Leandro C, Arroz M J, Gracio MA, Amaral L. Carpobrotus edulis Methanol Extract Inhibits the MDR Efflux Pumps, Enhances Killing of Phagocytosed S. Aureus and Promotes Immune Modulation. Phyto Res. 2003;17(5):512-519.
- Global Invasive Species Database. Species profile: Carpobrotus edulis. Downloaded from http://www.iucngisd.org/gisd/species.php?sc=1010 on 28-11-2021.
- Omoruyi BE, Bradley G, Afolayan AJ. Antioxidant And Phytochemical Properties of Carpobrotus edulis (L.) Bolus Leaf Used for the Management of Common Infections in HIV/AIDS Patients in Eastern Cape Province. BMC Comp Alt Med. 2012; 12(1):1-9.
- Van Wyk BE, Van Oudtshoorn B, Gericke N. Medicinal Plants of South Africa. Pretoria, Briza Publications.1997.
- Mulaudzi RB, Aremu AO, Rengasamy KR, Adebayo SA, McGaw LJ, Amoo SO, Van Staden J, Du Plooy CP. Antidiabetic, Anti-inflammatory, Anticholinesterase and Cytotoxicity Determination of Two Carpobrotus Species. S Afr J Bot. 2019;125:142–148.
- International Diabetes Federation (Ed.), IDF Diabetes Atlas (8th ed), International Diabetes Federation. 2017.
- International Diabetes Federation. IDF Diabetes Atlas, 9th edn. Brussels, Belgium: International Diabetes Federation, 2019.
- Atsalos C, Payk M, O’Neill A, Inglis S, Cheung NW, Jackson D. Meeting the Challenges Posed by an Escalating Diabetes Healthcare Burden: A Mixed Methods Study. Contemp Nur 2019; 55(6):469-485.
- Sifunda S, Mbewu AD, Mabaso M, Manyaapelo T, Sewpaul R, Morgan JW, Harriman NW, Williams DR, Reddy SP. Prevalence and Psychosocial Correlates of Diabetes Mellitus in South Africa: Results from the South African National Health and Nutrition Examination Survey (SANHANES-1). Inter J Environ Res Pub Health. 2023;20(10):5798.
- Jangid H, Chaturvedi S, Khinchi MP. An Overview on Diabetes Mellitus. AJPRD. 2017; 5(3):1-11.
- Wunna W, Tsoutsouki J, Chowdhury A, Chowdhury TA. Advances in the Management of Diabetes: New Devices for Type 1 Diabetes. P Med J 2021; 97(1148):384-390.
- Akinyede KA, Ekpo O, Oguntibeju OO. Ethnopharmacology, Therapeutic Properties and Nutritional Potentials of Carpobrotus edulis: A Comprehensive Review. Sci Pharm 2020;88(3):1-39.
- Hafsa J, Hammi KM, Khedher MRB, Smach MA, Charfeddine B, Limem K, Majdoub H. Inhibition of Protein Glycation, Antioxidant and Antiproliferative Activities of Carpobrotus edulis Extracts. Biomed Pharm 2016; 84:1496-1503.
- Laoufi H, Benariba N, Adjdir S, Djaziri R. In-Vitro α-amylase and α-glucosidase Inhibitory Activity of Ononis angustissima Extracts. J App Pharm Sci 2017; 7(2):191-198.
- Rubilar M, Jara C, Poo Y, Acevedo F, Gutierrez C, Sineiro J, Shene C. Extracts of Maqui (Aristotelia chilensis) and Murta (Ugni molinae Turcz.): Sources of Antioxidant Compounds and α-Glucosidase/α-Amylase Inhibitors. J Agr F Chem. 2011;59(5):1630-1637
- Kruger AC, Sekele SS. Trends in Extreme Temperature Indices in South Africa: 1962–2009. Int J Clim. 2013; 33:661-676.
- Figueiredo AC, Barroso JG Pedro LG. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour and Fragrance Journal, 2008; 23: 213-226.
- Cavaliere C. The Effects of Climate Change on Medicinal and Aromatic Plants. Herb Gram. 2009; 81:44-57.
- Abubakar AR, Haque M, Preparation of Medicinal Plants: Basic Extraction and Fractionation Procedures for Experimental Purposes. J. Pharm. Bioal. Sci. 2020; 12(1):1-10.
- Kamtekar S, Keer V, Patil V. Estimation of Phenolic Content, Flavonoid Content, Antioxidant and Alpha Amylase Inhibitory Activity of Marketed Polyherbal Formulation. J App Pharma Sci, 2014; 4(9): 061-065.
- Alqahtani AS, Hidayathulla S, Rehman MT, ElGamal AA, Al-Massarani S, Razmovski-Naumovski V, … AlAjmi MF. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomol. 2019; 10(1):61.
- Sabiu S, Balogun FO, Amoo SO. Phenolics Profiling of Carpobrotus edulis (L.) NE Br. and Insights Into Molecular Dynamics of their Significance in Type 2 Diabetes Therapy and its Retinopathy Complication. Mol. 2021; 26(16):4867.
- Kajaria D, Tripathi J, Tripathi YB, Tiwari S. In-Vitro α-Amylase and Glycosidase Inhibitory Effect of Ethanolic Extract of Antiasthmatic Drug—Shirishadi. J Ad Pharma Tech Res. 2013; 4(4):206.
- Padhi S, Nayak AK, Behera A. Type II Diabetes Mellitus: A Review on Recent Drug Based Therapeutics. Biomed Pharma. 2020;131:110708.
- Rehana D, Mahendiran D, Kumar RS., Rahiman AK. In Vitro Antioxidant and Antidiabetic Activities of Zinc Oxide Nanoparticles Synthesized using Different Plant Extracts. Biopro Biosys Eng. 2017; 40(6):943-957.
- South African Weather Service (SAWS). https://www.weathersa.co.za/home/mediareleases
- Ali Asgar MD. Anti-Diabetic Potential of Phenolic Compounds: A Review. Int J F Prop, 2013; 16(1):91-103.
- Rasouli H, Hosseini-Ghazvini SMB, Adibi H, Khodarahmi R. Differential α-Amylase/α-Glucosidase Inhibitory Activities of Plant-Derived Phenolic Compounds: A Virtual Screening Perspective for the Treatment of Obesity and Diabetes. F Fun. 2017; 8(5):1942-1954.
- Freeman BL, Eggett DL, Parker TL. Synergistic and Antagonistic Interactions of Phenolic Compounds Found in Navel Oranges. J Food Sci. 2010;75(6):C570-6.
- Vierling E. The Roles of Heat Shock Proteins in Plants. Annual Rev Plant Biol. 1991;42(1):579-620.
- Thomashow MF. Plant Cold Acclimation: Freezing Tolerance Genes and Regulatory Mechanisms. Annual Rev Plant Biol. 1999;50(1):571-99.
- Wahid A, Gelani S, Ashraf M, Foolad MR. Heat Tolerance in Plants: An Overview. Environ Exp Bot. 2007 Dec 1;61(3):199-223.
- Miura K, Furumoto T. Cold Signaling and Cold Response in Plants. Int J Mol Sci. 2013;14(3):5312-37.
- Afrisham R, Aberomand M, Ghaffari MA, Siahpoosh A, Jamalan M. Inhibitory Effect of Heracleum persicum and Ziziphus jujuba on Activity of Alpha-Amylase. J Boty. 2015; 2015:1-8.
- Sharma A. Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants Under Abiotic Stress. Mol. 2019; 24(13): 2452.
- Salehi B, Ata A, Anil Kumar N, Sharopov F, Ramírez-Alarcón K, Ruiz-Ortega A, … Sharifi-Rad J. Antidiabetic Potential of Medicinal Plants and their Active Components. Biomol. 2019; 9(10):551.
- Al-Huqail A, El-Dakak RM, Sanad MN, Badr RH., Ibrahim MM, Soliman D, Khan F. Effects of Climate Temperature and Water Stress on Plant Growth and Accumulation of Antioxidant Compounds in Sweet Basil (Ocimum basilicum L.) Leafy Vegetable. Sci. 2020; 2020:1-12.
- Ljubej V, Karalija E, Salopek-Sondi B, and Šamec D. Effects of Short-Term Exposure to Low Temperatures on Proline, Pigments, and Phytochemicals Level in Kale (Brassica oleracea var. acephala). Horticul. 2021; 7(10):341.
- Jhajhria A, Kumar K. Fenugreek with its Medicinal Applications. Int J Pharm Sci Rev Res. 2016: 41(1):194-201.
- Owolabi MS, Ogundajo LA, Satyal P, Abdulkabeer BI, Olubokola DR, Setzer WN. Essential Oil Constituents and Study of Antioxidant, Antidiabetic Activities of Ixora coccinea L. Extract from South West, Nigeria. A J Essen Oils Nat Prod. 2022; 10(1):20-26.
- Iba K. Acclimative Response to Temperature Stress in Higher Plants: Approaches of Gene Engineering for Temperature Tolerance. Annual Rev Plant Biol. 2002;53(1):225-45.







