Basma A. Ibrahim 1 , Abdelmonem Awad Hegazy 2,3,*
, Abdelmonem Awad Hegazy 2,3,* , Mai Ahmed Gobran 4
, Mai Ahmed Gobran 4 , Mohamed Abdallah Zaitoun 5
, Mohamed Abdallah Zaitoun 5 , Fayig Elmigdadi 2
, Fayig Elmigdadi 2 , Gehane A. El-Gindy 6
, Gehane A. El-Gindy 6 , Salwan Abdelmonem Hegazy 7
, Salwan Abdelmonem Hegazy 7 , Elsayed M. Alashkar 8
, Elsayed M. Alashkar 8 and Walaa E. Omar 1
and Walaa E. Omar 1
1Medical Biochemistry and Molecular Biology Department, Faculty of Medicine, Zagazig University, Zagazig City 44519, Egypt.
2Basic Medical and Dental Sciences Department, Faculty of Dentistry, Zarqa University, Zarqa City 13110, Jordan.
3Human Anatomy and Embryology Department, Faculty of Medicine, Zagazig University, Zagazig City 44519,, Egypt.
4Pathology Department, Faculty of Medicine, Zagazig University, Zagazig City 44519, Egypt.
5General Surgery Department, Faculty of Medicine, Zagazig University, Zagazig City 44519, Egypt.
6Clinical Pharmacology Department, Faculty of Medicine, Mutah University, Alkarak 61710, Jordan.
7Dermatology Department, Faculty of Medicine, Zagazig University, Zagazig, Zagazig City 44519, Egypt.
8Physics Department, Faculty of Science, Al-Azhar University, Nasr City 11765, Egypt.
Corresponding Author E-mail: ahegazy@zu.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/2806
Abstract
Objective: Breast cancer (BC) is the most common cause of cancer-related death among women worldwide. Let-7d and microRNA-195 (miR-195) are members of microRNAs that are known tumor suppressors and are involved in the regulation of apoptosis, invasion, and other cellular functions. However, the roles of these microRNAs in BC progression remain controversial. This study aimed to explore the correlation between the expression of let-7d and miR-195 and apoptosis-related genes (ARGs) “B-cell lymphoma 2 (BCL2) and caspase-3 (CASP3)” as potential biomarkers of breast carcinogenesis. Methods: It was a retrospective case-control study in which expression of let-7d, miR-195, CASP3, and BCL2 was assessed using quantitative real-time PCR (qRT-PCR); and immunohistochemical (IHC) staining was used to determine expression of BCL2 and CASP3 in BC tissue versus normal breast tissue (NT) samples. Results: The expression of let-7d and miR-195 was significantly reduced within BC tissues compared to NT (P: < 0.0001); and there was a statically positive correlation between them (r=0.314, P: 0.005). They have also been correlated to biomarkers’ expression of genes related to apoptosis. There was a statistically significant positive association between CASP3, and both let-7d, and miR-195 relative gene expression (r=0.713, P: <0.0001 and r=0.236, P: 0.03, respectively). In contrast, there was a statistically significant negative association between the relative gene expression of BCL2, with let-7d, and miR-195 (r=-0.221, P: 0.04 and r=-0.311, P: 0.005, respectively). Conclusion: Let-7d and miR-195 have been suggested to be involved in BC through modulation of the ARGs including BCL2 and CASP3. The qRT-PCR and IHC studies demonstrated that decreased expression of let-7d and miR-195 prohibits apoptosis via downregulating CASP3 and increasing BCL2 expressions promoting BC progression. These results also hypothesize that let-7d and miR-195 along with apoptotic biomarkers (BCL2 and CASP3) can be used in the future to introduce novel, non-invasive molecular biomarkers for BC into clinical practice.
Keywords
Apoptotic Biomarkers; Breast Cancer; Immunohistochemistry; Micrornas; Novel Molecular Markers
Download this article as:| Copy the following to cite this article: Ibrahim B. A, Hegazy A. A, Gobran M. A, Zaitoun M. A, Elmigdadi F, El-Gindy G. A, Hegazy S. A, Alashkar E. M, Omar W. E. Expression of microRNAs ‘let-7d and miR-195’ and Apoptotic Genes ‘BCL2 and Caspase-3’ as Potential Biomarkers of Female Breast Carcinogenesis. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Ibrahim B. A, Hegazy A. A, Gobran M. A, Zaitoun M. A, Elmigdadi F, El-Gindy G. A, Hegazy S. A, Alashkar E. M, Omar W. E. Expression of microRNAs ‘let-7d and miR-195’ and Apoptotic Genes ‘BCL2 and Caspase-3’ as Potential Biomarkers of Female Breast Carcinogenesis. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/48wsisE |
Introduction
Breast cancer (BC) is the most common malignant tumor among women worldwide. The incidence of BC specifically ranks first in both developed and developing countries; and the death rate among women ranks second in developed countries and 15th in developing countries. This variation between countries may be due to changes in exposure to risk factors including lifestyle, behaviors, environmental and genomic alterations 1. Risk factors for BC in general in low- and middle-income countries include smoking, alcohol use, and low intake of vegetable and fruit. In high-income countries, other factors, including overweight and obesity as well as higher smoking and alcohol use, are also important causes of cancer 2. Furthermore, hormone replacement therapy, particularly estrogen and progesterone combinations, has been suggested to increase the risk of invasive BC via the steroid receptors to which the hormone can bind 3.
MicroRNAs are small endogenous non-coding RNAs that have gene regulatory functions at the post-transcriptional level. MicroRNAs play important roles in tumorigenesis and progression through oncogenic or tumor suppressor properties, and their abnormal expression is closely associated with the incidence of some tumors 4.
Let-7 (lethal-7) was initially identified as a heterochronic gene in C. elegans (the second microRNA gene identified after lin-14), where its expression determines adult cell fate in the worm 5. The let-7 family of microRNAs, which consists of multiple paralog genes located in different chromosomes, has been identified as one of the largest and most conserved families of microRNAs across different species, ranging from worms to humans. In humans, there are 10 mature let-7 microRNAs, including let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, let-7i, miR-98, and miR-202, which are derived from 13 precursor genes 6. In cancer cells, the let-7 family of microRNAs generally acts as tumor suppressor inhibiting tumor growth and metastasis. Let-7 microRNAs are also involved in the suppressing cancer stem cell characteristics, including sphere/colony formation, tumor growth, differentiation, and regenerative potential 7. Let-7d, which belongs to the let-7 microRNA family, has been shown to directly target oncogenes including c-Myc, H-RAS, k-RAS, HMGA2 and PBX3, inhibiting their expression and thus suppressing cancer proliferation and metastases 6,8. The let-7d contributes to the pathogenesis of some cancers. For example, let-7d has been reported to exert both anti-oncogenic and oncogenic effects in osteosarcoma 8. On the other hand, microRNA-195 (miR-195) is also a member of the microRNA-15 family. It is a gene is located in the 17p13.1 region and is distributed in clusters with miR-497. Dysregulation of miR-195 expression in tumor tissues has been shown to be closely associated with different tumorigenesis. Furthermore, expression of miR-195 in the serum of BC patients is much higher than that in healthy individuals 9.
The number of cells in the human body is controlled not only by the rate of cell division, but also by the rate of cell death. If cells of any structure are no longer needed, they undergo a programmed cell death called apoptosis. Any error in the apoptotic program can lead to diseases such as cancer and azoospermia 10,11. In cancer, for example, excessive and uncontrollable cell proliferation is not associated with adequate apoptosis. Given the great value of cell cycle control through apoptosis, recent therapeutic research is focusing on the potential of this cell death program in controlling cancers. Genes that act as critical mediators of apoptosis include B-cell lymphoma 2 (BCL2) and caspase 3 (CASP3) 11. Moreover, investigation of the regulators of genes involved in cell fate determination such as the BCL2 gene is of great interest 12.
CASP3, encoded by the CASP3 gene, is a member of the cysteine-aspartic acid pro-tease (or caspase) family, which is sequentially activated and plays a key role in the execution phase of apoptosis 11. It is the primary caspase effector that implements apoptosis and plays an important role in cancer development and progression. Polymorphisms of the CASP3 gene can affect the production and/or activity of the CASP3 protein, contributing to genetic susceptibility to lung cancer 13. Among the prognostic parameters studied, the CASP3 and BCL2 genes, as well as their proteins, have attracted much attention as promising predictive markers for therapy response among BC patients. The process of apoptosis, which plays an important role in tumor growth and aggressiveness is regulated by many genes, including activation of primary cellular oncogenes and loss of function of tumor suppressor genes such as BCL2 14.
It has been proposed that in BC cells, miR-195 targets BCL2 and downregulates its expression by binding to its 3′ untranslated region (3′UTR) 15. At the same time, the let-7 family of microRNAs, namely let-7a, let-7d, and let-7e, regulates the expression of CASP3 during apoptosis 16. Down-expressed let-7d, in particular, has a significant impact on the formation of tumor cells that are resistant to irradiation and chemotherapy and responsible for cancer metastasis 17. Therefore, we decided to evaluate the expression of let-7d and miR-195 as potential biomarkers for detection and prognostic evaluation of BC. Moreover, we aimed to study BCL2 and CASP3 as potential targets for these microRNAs.
Methods
Patients and clinical data
This was a retrospective case-control study, conducted in the Departments of Medical Biochemistry & Molecular Biology, Pathology, Dermatology and General Surgery, University Hospitals, during the period between November 2022 and April 2023. Patients were classified into two groups. Group A (control group) included 80 healthy females who underwent reduction mammoplasty; and group B included 80 female patients with newly discovered BC who were diagnosed by mammogram and underwent surgery for invasive breast duct carcinoma; 49 operated with modified radical mastectomy and 31 operated with conservative breast surgery (CBS) (Fig. 1). Samples were obtained from BC cases and controls after operations performed in the Department of General Surgery, and tissue samples were taken by either mastectomy or excisional biopsy. All patients provided informed consent following Institutional Review Board (IRB) approval (Reference number is ZU-IRB #10133). Written informed consent was obtained from subjects who joined the study to allow the use of clinical samples and data without clarifying their identity. Consent was provided with a custom form consistent with the Declaration of Helsinki. All BC patients were newly diagnosed cases who had not received chemotherapy, radiotherapy, or immunotherapy before sampling. Samples from women with renal, heart, liver, metabolic, or any systemic disease were excluded. None of the patients had distant metastasis and had received adjuvant/adjuvant therapies and radiotherapy before mastectomy, and were not diagnosed with serious complications (e.g., cachexia) or other malignancies. BC cases were diagnosed by hematoxylin and eosin (H&E) and using IHC staining for BCL2 and CASP3. Estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) status as well as pathological data of the samples including tumor grade, size and lymph node (LN) status were obtained.
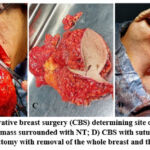 |
Figure 1: Breast surgery: A) Conservative breast surgery (CBS) determining site of surgery; B) CBS during removal of mass; C) CBS after removal of the mass surrounded with NT; |
Quantitative real-time PCR (qRT-PCR)
Breast tissue samples were homogenized, followed by extraction of total cellular RNA from the tissue homogenate according to the instructions for the TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc, USA). The total extracted RNA was reverse transcribed to complementary DNA (cDNA) with miRCURY LNA™ Universal cDNA synthesis kit (Qiagen, USA) based on the manufacturers’ recommendations. Expression of let-7d and miR-195 was quantified using the SYBR Green qRT-PCR master mix (Enzynomics, Korea) following the manufacturer’s instructions. qRT-PCR was performed as follows: 10 µl TOPreal™ qRT-PCR 2x PreMIX (SYBR Green with low ROX), 1 µl forward and reverse primers, 2 µl cDNA, and 6 µl RNase-free water in a final volume of 20 µl. U6 snRNA gene expression was utilized as an internal control. qRT-PCR included 40 cycles at 95°C for 12 min, followed by 95°C for 20 s, 60°C for one min, and 72°C for one min. The thermal program for the replication was done under the same conditions. The let-7d and miR-195 relative gene expressions were normalized to U6 snRNA as a housekeeping gene and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the internal reference for CASP3 and BCL2 genes. The forward and reverse primers used for each gene are listed in Table 1. The calculation of the levels was performed using the CT system threshold process (2-ΔΔCT method) 18.
Table 1: Primers used for qRT-PCR
|
|
Forward Primer (5′-3′) |
Reverse Primer (5′-3′) |
|
let-7d |
GCGAACTGTTTGCAGAGG |
CAGTGCGTGTCGTGGAGT |
|
miR-195 |
CGTAGCAGCACAGAAAT |
GTGCAGGGTCCGAGGT |
|
U6 snRNA |
CTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
|
CASP3 |
TGTCATCTCGCTCTGGTACG |
AAATGACCCCTTCATCACCA |
|
BCL2 |
CCTGTGGATGACTGAGTACC |
GAGACAGCCAGGAGAAATCA |
|
GAPDH |
CGCTCTCTGCTCCTCCTGTTC |
ATCCGTTGACTCCGACCTTCAC |
Histopathology
All tissue samples were received in the operating room and then transferred to the pathology department. Samples were fixed in 10% neutral formalin for 24 hours. Nodal and tumor samples were assessed by routine H&E staining. Experienced pathologists confirmed the assessment obtained from the patient’s records including all histopathological features, such as tumor size, histopathological type, grade, nodal status, and peritumoral lymph-vascular invasion. All BC patients were of infiltrating duct carcinoma -not otherwise specified (IDC-NOS) according to the 2012 WHO classification 19. Histological grades were assigned according to Elston and Ellis grades 20. Then, BC staging was performed according to the American Joint Committee on Cancer (AJCC) 8th edition 14.
IHC staining
Representative sections of paraffin-embedded tissues from BC cases and control samples used for immunohistochemistry. For BCL2 examination, an anti-BCL2 mouse monoclonal (clone 124; Dako) was used. For CASP3, an anti-CASP3 monoclonal anti-body (ab2171; Abcam, USA) was used 21. For antigen retrieval, sections were treated with 98C Target Retrieval Solution pH 9 (Dako) for 40 min. After blocking non-specific activity, the sections were incubated for 30 min at room temperature with anti-BCL2 antibody diluted to 1:100, while anti-CASP3 diluted to 1:50 Bound antibodies were detected utilizing the mouse EnVision+, HRP kit (Dako). Appropriate negative and positive controls were included in each batch of immunostains 22.
IHC analysis
CASP3-stained sections were evaluated using an optical microscope at × 400 magnification, based on manual counting of positive cells in each tissue. Cases of disagreement were jointly reviewed for a consensus score. The percentage of positive cells and the intensity of CASP3 staining were scored. The intensity was graded as negative (score 0), weak (score 1), moderate (score 2), or strong (score 3), and the percentage of positive cells was graded as <5% (score 0), 5–25% (score 1), 26–50% (score 2), 51–75% (score 3), and >75% (score 4). The final score of CASP3 expression was determined by multiplying the intensity score and percentage score, with a range of 0–12. According to the scoring results, all patients were divided into two groups: Low (score 1–5) and high (score 6–12) expression 22. The BCL2 staining was assessed according to the estimated proportion of cytoplasmic or membranous staining of positive tumor cells. Scoring criteria were as follows (in the form of a proportion of nuclear and/or cytoplasmic staining = score): ab-sent = 0+, no staining; weakly positive (1+), staining in fewer than 10 percent of the tumor; moderate (2+), staining in 10% to 75% of the tumor; and strongly positive (3+), staining in more than 75% of the tumor. BCL2 scores of 0+ and 1+ were negative, and scores of 2+ and 3+ were positive 14. The percentage of tumor cells with unequivocal nuclear staining for ER, and PR, was recorded semi-quantitatively (0, no staining; 1, <10%; 2, 11-25%; 3, 26-50%; 4, 51-75%; 5, >75%). Membranous staining was scored for HER2 according to the Hercep Test (Dako) as follows: 0, no staining or faint incomplete staining in <10% cells; 1, faint incomplete staining in >10% cells; 2, weak to moderate complete staining in >10% cells; 3, strong complete staining in >10% cells. The cut off value threshold was 10% for ER, PR & score of 3+ for HER2 23.
Statistical methods
The Statistical Package of Social Sciences (SPSS) (version 26) was used to generate the results. The normality of the data was tested using the Kolmogorov-Smirnov test. Qualitative data were described as numbers and percentages. Relation between qualitative data was done using the Chi-square test or Fisher’s exact test as appropriate. Numerical variables were presented as mean and standard deviation (SD) or median and (range). To compare the two groups, the Mann-Whitney test was used, and the Kruskal-Wallis test was used to compare more than two groups. Spearman correlation was used to correlate continuous data. A P: ≤0.05 was considered significant.
Results
Clinical characteristics of the BC group
The mean age of the studied patients was 43.4 years and ranged between 35 to 65 years. Eleven IDC-NOS patients (13.75%) had grade I tumors, sixty-two (77.5%) had grade II tumors, and seven (8.75%) had grade III tumors. Sixteen patients (20%) had stage I disease, forty-two patients (52.5%) had stage II disease, eighteen patients (22.5%) had stage III disease, and four patients (5%) had stage IV disease. Sixty-eight patients (85%) had positive ER, while sixty-one patients (76.25%) had positive PR. At the same time, only eight patients (10%) had positive HER2 (Table 2).
Table 2: Clinicopathological characteristics of the BC group
|
Variable |
Results |
No. (Total: 80) |
% |
|
Grade
|
I II III |
11 62 7 |
13.75 77.5 8.75 |
|
Stage
|
I II III IV |
16 42 18 4 |
20 52.5 22.5 5 |
|
ER |
Negative Positive |
12 68 |
15 85 |
|
PR |
Negative Positive |
19 61 |
23.75 76.25 |
|
HER2 |
Negative Positive |
72 8 |
90 10 |
|
LN metastasis |
Negative Positive |
31 49 |
38.75 61.25 |
|
Tumor size
|
<2 cm 2-5 cm >5 cm |
24 46 10 |
30 57.5 12.5 |
Expression of let-7d, miR-195, CASP3, and BCL2
Let-7d was statistically lower in BC tissues compared to NT (0.26 ±0.21 versus 1.01 ±0.12, P: <0.0001). In addition, miR-195 was also statically suppressed in BC tissues compared to NT (0.41 ±0.18 versus 1.05 ±0.08, P: <0.0001) (Table 3; Figs. 2,3). Furthermore, CASP3 was statistically lower in BC tissues compared to NT (0.35± 0.27 versus 1.04±0.07, P: <0.0001). On the other hand, BCL2 was statically upregulated in BC tissues compared to NT (2.40 ±0.75 versus 0.93 ±0.18, P: <0.0001) (Table 3; Figs. 4,5). Regarding let-7d gene expression, there were significant correlations with tumor size, stage, and LN metastasis (P: 0.024, 0.001 and 0.018, respectively); however, no statistically significant difference was found with age, grade, PR, ER, and HER2 (P: 0.405, 0.147, 0.406, 0.255, and 0.138, respectively). At the same, there was a statistically significant relationship between miR-195 expression and the following parameters: tumor grade, stage, size, and LN metastasis (P: 0.020, <0.001, <0.001, and 0.037, respectively); however, no statistically significant difference was found between miR-195 and age, PR, ER and HER2 (P: 0.380, 0.790, 0.210, and 0.100, respectively) (Table 4). On the other hand, there was a statically positive correlation between let-7d and miR-195 relative gene expressions (r=0.314, P: 0.005) (Table 5; Fig. 6). There was also a statically positive correlation between CASP3, and the relative gene expression of let-7d and miR-195 (r=0.713, P: <0.0001 and r=0.236, P: 0.03, respectively). Conversely there was a statically negative correlation between BCL2, let-7d, and miR-195 relative gene expression (r=-0.221, P: 0.04 and r=-0.311, P: 0.005, respectively) (Table 5).
Table 3: Relative genes expression of let-7d, miR-195, CASP3, and BCL2.
|
Variable |
NT |
BC tissue |
P |
|
Let-7d |
1.0161± 0.12 |
0.2601± 0.21 |
<0.0001* |
|
miR-195 |
1.0549± 0.08 |
0.4117± 0.18 |
<0.0001* |
|
CASP3 |
1.0401± 0.07 |
0.3598± 0.27 |
<0.0001* |
|
BCL2 |
0.9375± 0.18 |
2.4003± 0.75 |
<0.0001* |
*= Significant
Table 4: Comparison of let-7d and miR-195 relative gene expression in in BC cases
|
Clinical pathological criteria |
No. (Total: 80) |
Let-7d |
P |
miR-195 |
P |
|
Age |
|||||
|
≤50 |
60 |
0.27±0.21 |
0.405 |
0.43±0.16 |
0.380 |
|
>50 |
20 |
0.23±0.18 |
0.36±0.22 |
||
|
Histological grade |
|||||
|
I |
11 |
0.27±0.20 |
0.147 |
0.37±0.23 |
0.020* |
|
II |
62 |
0.27±0.21 |
0.44±0.15 |
||
|
III |
7 |
0.12±0.13 |
0.20±0.22 |
||
|
PR |
|||||
|
Negative |
19 |
0.27±0.19 |
0.406 |
0.38±0.21 |
0.790 |
|
Positive |
61 |
0.26±0.21 |
0.42±0.17 |
||
|
ER |
|||||
|
Negative |
12 |
0.29±0.19 |
0.255 |
0.31±0.23 |
0.210 |
|
Positive |
68 |
0.25±0.21 |
0.42±0.16 |
||
|
HER2 |
|||||
|
Negative |
72 |
0.25±0.20 |
0.138 |
0.39±0.18 |
0.100 |
|
Positive |
8 |
0.33±0.22 |
0.52±0.10 |
||
|
LN metastasis |
|||||
|
Negative |
31 |
0.33±0.23 |
0.018* |
0.47±0.13 |
0.037* |
|
Positive |
49 |
0.22±0.18 |
0.38±0.20 |
||
|
Stage |
|||||
|
I&II |
58 |
0.29±0.21 |
0.024* |
0.47±0.16 |
<0.001* |
|
III&IV |
22 |
0.16±0.15 |
0.25±0.21 |
||
|
Tumor Size |
|||||
|
<2 cm |
24 |
0.29±0.2 |
<0.001* |
0.45±0.12 |
0.001* |
|
2-5 cm |
46 |
0.29±0.21 |
0.45±0.15 |
||
|
>5 cm |
10 |
0.051±0.032 |
0.145±0.22 |
||
*= Significant
Table 5: Correlation between qRT-PCR data of let-7d, miR-195, CASP3, and BCL2 genes
|
Genes |
r |
P-value |
|
Let-7d & miR-195 |
0.314 |
0.005* |
|
Let-7d & CASP3 |
0.713 |
<0.0001* |
|
Let-7d & BCL2 |
-0.221 |
0.04* |
|
miR-195 & CASP3 |
0.236 |
0.03* |
|
miR-195 & BCL2 |
-0.311 |
0.005* |
*= Significant, r=Spearman’s Correlation
 |
Figure 2: Expression of let-7d was downregulated in BC tissues compared
|
 |
Figure 3: Expression of miR-195 was downregulated in BC tissues compared
|
 |
Figure 4: Expression of CASP3 was downregulated in BC tissues compared
|
 |
Figure 5: Expression of BCL2 was upregulated in BC tissues compared to NT (n=160). ** P <0.0001.
|
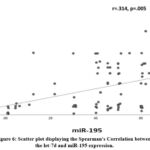 |
Figure 6: Scatter plot displaying the Spearman’s Correlation between the let-7d and miR-195 expression.
|
IHC analysis of BCL2 and CASP3
BCL2 expression was significantly higher in BC samples (85%) compared to the NT samples (8.75%), (P: <0.00001), while CASP3 positive expression was significantly higher in control specimens (71.25%) than in BC (25%), (P: <0.00001) (Table 5; Fig. 7A-D). Regarding BCL2 IHC expression, we observed a statistically significant relationship between it and tumor grade, ER, PR, HER2 (P: <0.00001), LN metastasis, stage, tumor size (P =0.018983, 0.000981 and <0.00001 respectively). BCL2 IHC score was significantly higher in low grades I&II, and low stages of BC. There was no significant association between BCL2 expression and age (P: 1.000) (Table 6; Fig. 7A-D). Regarding CASP3 IHC expression, a significant association was observed between it and grade, HER2, LN metastasis, tumor stage and size (P: 0.00037, 0.00002, 0.011821, 0.000171 and <0.00001, respectively). Regarding the CASP3 score, it was significantly high in high grades II, III, and stages with positive LN metastasis. There was no significant association between CASP3 and the age, ER and PR expression (P: 0.233, 1.000, and 0.28 respectively) (Table 7; Fig. 7E-H).
Table 6: CASP3 and BCL2 IHC markers expression in BC patients versus controls
|
CASP3, BCL2 expression |
BC cases n=80 |
Controls n=80 |
X² |
P |
|
CASP3 positive |
20(25%) |
57(71.25%) |
27.2198 |
<0.00001* |
|
CASP3 negative |
60(75%) |
23(28.75%) |
||
|
BCL2 positive |
68(85%) |
7(8.75%) |
93.3898 |
<0.00001* |
|
BCL2 negative |
12(15%) |
73(91.25%) |
*= Significant
Table 7: Relation between CASP3, BCL2, and clinical and pathological features.
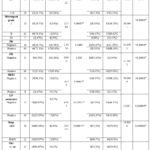 |
Table 7: Relation between CASP3, BCL2, and clinical and pathological features
|
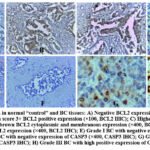 |
Figure 7: IHC expression in normal “control” and BC tissues: A) Negative BCL2 expression in control (×400, BCL2 IHC); B) Grade II BC with score 3+ BCL2 positive expression (×100, BCL2 IHC); C) Higher magnification of Grade II BC with score 3+ positive brown BCL2 cytoplasmic and membranous expression (×400, BCL2 IHC);
|
Correlations of microRNAs qRT-PCR data and both CASP3 and BCL2 IHC data
There were significant associations between let-7d and both CASP3 and BCL2 (P: 0.045 and 0.036, respectively). At the same time, miR-195 showed statistically significant relationship with CASP3 and BCL2, (P: 0.002 and 0.044, respectively) (Table 8).
Table 8: Association of qRT-PCR data of let-7d and miR-195 with IHC data of CASP3 and BCL2.
|
|
No. |
Let-7d |
P |
miR-195 |
P |
|
CASP3 |
|
||||
|
Negative |
60 |
0.21±0.18 |
0.045* |
0.46±0.14 |
0.002* |
|
Positive |
20 |
0.12±0.12 |
0.27±0.21 |
||
|
BCL2 |
|
||||
|
Negative |
12 |
0.29±0.19 |
0.036* |
0.31±0.23 |
0.044* |
|
Positive |
68 |
0.18±0.17 |
0.43±0.16 |
||
*= Significant
Discussion
BC, like all other malignancies, early diagnosis and treatment play vital roles in a good prognosis 24. Hence, identification of reliable biomarkers to predict tumor progression and drug response is a priority 25. In recent years, many microRNAs from tumor cells have been found to be associated with prognostic factors such as BC staging, vascular invasion, proliferation index, ER, and/or PR status 9,26,27. MicroRNAs have also emerged as key therapeutic agents against cancer. Dysregulation of microRNAs can lead to drug resistance in many cancers, and correcting these microRNAs through the use of microRNAs mimics or antagomirs may offer exciting opportunities for cancer therapy 28.
The let-7d has been recognized as a tumor suppressor microRNA. Its reduced expression has been detected in various types of cancer, such as colon, lung, gastric, breast, and ovarian cancer 29-31. Let-7 regulation has been shown in BC. For example, it can occur via regulation by other microRNAs, such as the interaction with miR-107, which downregulates let-7 during tumor progression 32,33.
The tumor suppressive function of miR-195 has been associated with the downstream effectors of NF-kB, especially IKKa and TABP3, linking inflammation to tumorigenesis 34. Downregulation of MiR-195 has been observed in several types of cancers and may be associated with the copy number loss of a segment of chromosome 17p13.1, as observed in serous ovarian carcinoma 35.Moreover, epigenetic alterations, namely, promoter CpG methylation, have also been associated with miR-195 downregulation in gastric cancer, with consequent deregulation of its direct target CDK6 36. On the other hand, the expression of circulating microRNA was investigated in several types of tumors (breast, colon, prostate, kidney, and melanoma) compared to normal individuals and concluded that miR-195 was only upregulated in BC, suggesting that miR-195 could be a potential biomarker for BC 37.
BCs with a high apoptosis index have a better prognosis than those with low or absent levels of apoptosis 38. Some studies also showed that apoptosis factors were overexpressed in advanced BC. CASP3 was found to play a dominant role in the apoptotic signaling pathway and regulation of cellular apoptosis 39. On the other hand, BCL2 proteins are anti-apoptotic factors. Targeting these proteins through developing new drugs could help regulate apoptosis and thus anti-cancer therapy 40. Increasing evidence has shown that the downregulation of CASP3 is correlated with the development of BC 39.MicroRNA let-7, i.e., let-7a and let-7e, regulate the expression of CASP3 during apoptosis 41. BCL2 is a critical pro-survival protein that suppresses apoptosis in a variety of cell systems. BCL2 regulates cell death primarily by controlling mitochondrial membrane permeability and functions together with caspases and other proteins in a feedback loop system 42. It has also been shown that miR-195 regulates biological processes such as apoptosis, cell cycle, and proliferation by targeting CDK4, CDK6, cyclin D1, cyclin E1, E2F3, E2F5, and WEE1 43-45.
The current study explored role of let-7d and miR-195 in apoptosis in BC patients by investigating their correlation with the relative expression levels of apoptotic regulator genes “CASP3 and BCL2”. To our knowledge, this is the first study to investigate this relationship in Egypt. The study showed that expression of both let-7d and miR-195 was dramatically downregulated in BC tissues, and there was a statically positive correlation between them (r=0.314, P: 0.005). Tumor size, grade, stage, and LN metastasis were significantly associated to their expression. Moreover, CASP3 expression was decreased in BC samples compared to the NT samples by qRT-PCR and the IHC staining intensity, while BCL2 expression was increased within tumoral tissues in comparison with NT (P: <0.0001). CASP3 IHC expression was significantly associated with tumor grade, size, stage, HER2, and LN metastasis. BCL2 IHC expression was also statistically significantly associated with tumor grade, ER, PR, HER2, LN metastasis, stage, and tumor size. This can confirm the roles of BCL2 and CASP3 as prognostic IHC markers in BC. Consistent with our findings, a recent study also confirmed that BCL2 IHC expression is decreased in BC compared to normal tissue and is associated with lower histological grade and thus higher survival46. However, the authors added that there is some racial/ethnic variation in BC incidence and outcomes that can be taken into account.
Downregulation of miR-195 and let-7d was significantly correlated with the expression of ARGs; CASP3 and BCL2. Hence, these biomarkers could contribute as regulators in advanced clinical stages of BC and should be considered as markers for diagnosis, prognostic assessment, and targeted therapy. In agreement with our results, expression of miR-195 has been reported to be low in BC cells (and multi-drug-resistant BC tissues), and upregulation of miR-195 increases the sensitivity of BC cells towards the chemotherapeutic drug “Adriamycin” 47. MiR-195 was also found to be downregulated in the circulation of a BC animal model 16,48. This is inconsistent with the results of other studies showing that serum miR-195 expression was significantly up-regulated in BC 5,37,49,50. Moreover, compared with prostate cancer, kidney cancer, and colon cancer, serum miR-195 is a relatively characteristic microRNA of BC 37. In line with our findings in BC, Chen and colleagues found in their study of diabetic nephropathy in rats that treating animals with a miR-195 mimetic contributes to apoptosis by increasing CASP3 as well as reducing BCL2 levels 45. BCL2 regulates apoptosis by affecting mitochondrial membrane permeability and acting with caspases in a feedback loop system 42,45.
In line with this study’s results, a previous study showed that expression of miR-195 can differentiate BC from other cancer types 37. Indeed, the upregulation of miR-195 and let-7a showed a significant correlation with clinicopathological variables, such as nodal status and estrogen receptor status 49. In contrast to the results of this study, let-7d was upregulated in a variety of gynecological malignancies; significant let-7d upregulation between normal and tumor tissues was observed for invasive breast carcinoma, ovarian serous cystadenocarcinoma, endometrial carcinoma, and uterine carcinosarcoma 51.
In harmony with our results, other authors confirmed that CASP3 protein expression was higher in controls (69%) than in BC cases (48.9%) 52. However, other studies have disagreed with our results, concluding that CASP3 activation is higher in BC than in non-malignant breast tissue, which may contradict the prevailing belief that apoptosis is reduced in malignancy 53,54.
It has been reported that BCL2 staining was weaker in normal breast epithelium compared with adjacent neoplastic cells suggesting that some tumors overexpress the protein 55. However, other authors failed to agree as they revealed that normal and hyperplastic breast tissues were exclusive of BCL2(+), and the foci of carcinoma in situ and those of invasive carcinomas were 83% and 66% positive, respectively, for BCL2 56. This is because normal, hyperplastic, and neoplastic breast epithelial cells expressing BCL2 are immature cells that ought to form part of the stem-cell subpopulation, which is committed to the development. The disparity between this study and other studies is because patients with specific clinical, biological, racial, geographic, and/or environmental factors were included in the analysis.
The predominance of mortality in breast cancer cases is the result of local invasion as well as distant metastasis. Therefore, it is important to identify the factors underlying the processes of invasion and metastasis. This may help in developing new therapeutic strategies and thus improve patient survival57. MiR-195 and let-7 have been shown to play key roles in regulating cell proliferation and apoptosis by targeting cell cycle proteins and the gene encoding the antiapoptotic protein BCL2. They negatively regulate BCL2 expression by binding to its 3′UTR mRNA 15,39. Consistent with our findings, it has been also exhibited that microRNAs “let-7 and miR-195” can affect the expression levels of several key ARGs including the genes encoding BCL2, CASP3, and caspase-8 16,45,58. The miR-195 mimics can negatively regulate BCL2 at the post-transcriptional level, resulting in reduced BCL2 protein levels and activation of the caspase cascade. As a result, the cells become apoptotic. MiR-195 mimics affect an endogenous substance that can regulate BCL2 45.
Limitations
There are some limitations to this study. The main limitation of the current study may be the relatively small number of cases. This may be due to the limited number of cases recorded in the same study period. This study also included only one nationality with a relatively limited sample size. Furthermore, environmental variables, such as drinking habits and educational levels, may have affect the outcomes that were not discussed in this analysis. Therefore, further analyzes with a broader sample size and diverse ethnic populations are needed. Further studies are required to investigate the interaction between genetic variants and gene expression, and blood levels of miR-195, let-7d, CASP3, and BCL2 in BC.
Conclusion
The relative expression of let-7d and miR-195 as well as CASP3 and BCL2 in tissue samples of BC could be used as biomarkers of breast carcinogenesis. Let-7d, miR-195, and CASP3 are significantly downregulated in neoplastic specimens when compared to healthy samples, while BCL2 expression is significantly increased within tumor samples. Therefore, let-7d and miR-195 have been suggested to be involved in BC by modulating the ARGs including BCL2 and CASP3. Moreover, both the BCL2 and CASP3 IHC markers may play a useful prognostic role in BC as they have been shown to correlate significantly with tumor grade, stage, size, and LN metastasis. These results may help to elucidate the mechanism underlying the tumor regulator functions of let-7d and miR-195; and demonstrate that the let-7d/miR-195/CASP3/BCL2 interaction can be used in the future to introduce novel molecular markers for BC into clinical practice, which can improve personalized treatment approach.
Acknowledgement
We acknowledge all patients participating in this work and approved it for publication. We also thank Dr. Ahmed Refat, AG Professor of Community Medicine, Faculty of Medicine, Zagazig University for his assistance in conducting the statistical analysis of the study data. We would like to thank the members of the IRB Unit at Zagazig University, Egypt for their good cooperation. We would like to thank the Deanship of Scientific Research at Zarqa University of Jordan for their interest and support.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
This research received no external funding.
References
- Francies FZ, Hull R, Khanyile R, Dlamini Z. Breast cancer in low-middle income countries: abnormality in splicing and lack of targeted treatment options. Am J Cancer Res. 2020;10:1568-91.
- Pedroso CF, Pereira CC, Cavalcante AMRZ, Guimarães RA. Magnitude of risk factors for chronic noncommunicable diseases in adolescents and young adults in Brazil: A population-based study. PloS one. 2023;18(10), e0292612.
- Perkins MS, Louw-du Toit R, Africander D. Hormone Therapy and Breast Cancer: Emerging Steroid Receptor Mechanisms. J Mol Endocrinol. 2018;61:R133-60.
- Cuk K, Zucknick M, Heil J, Madhavan D, Schott S, Turchinovich A, et al. Circulating microRNAs in plasma as early detection markers for breast cancer. Int J Cancer. 2013;132:1602-12.
- Balzeau J, Menezes MR, Cao S, Hagan JP. The LIN28/let-7 Pathway in Cancer. Front Genet. 2017;8:31.
- Ma Y, Shen N, Wicha MS, Luo M. The Roles of the Let-7 Family of MicroRNAs in the Regulation of Cancer Stemness. Cells. 2021;10: 2415.
- Staszel T, Zapała B, Polus A, Sadakierska-Chudy A, Kieć-Wilk B, Stępień E, et al. Role of microRNAs in endothelial cell pathophysiology. Pol Arch Med Wewn. 2011;121(10):361-6.
- Wei Y, Liu G, Wu B, Yuan Y, Pan Y. Let-7d Inhibits Growth and Metastasis in Breast Cancer by Targeting Jab1/Cops5. Cell Physiol Biochem. 2018; 47(5):2126-35.
- Liu Y, Tang D, Zheng S, Su R, Tang Y. Serum microRNA-195 as a potential diagnostic biomarker for breast cancer: a systematic review and meta-analysis. Int J Clin Exp Pathol. 2019;12:3982-91.
- Hegazy R, Hegazy A, Ammar M, Salem E. Immunohistochemical measurement and expression of Mcl-1 in infertile testes. Front Med. 2015;9:361-7.
- Hussar P. Apoptosis Regulators Bcl-2 and Caspase-3. Encyclopedia. 2022;2:1624–36.
- Gong F, Sun L, Wang Z, Shi J, Li W, Wang S, et al. The BCL2 gene is regulated by a special AT-rich sequence binding protein 1-mediated long range chromosomal interaction between the promoter and the distal element located within the 3′-UTR. Nucleic Acids Res. 2011;39:4640-52.
- Jang JS, Kim KM, Choi JE, Cha SI, Kim CH, Lee WK, et al. Identification of polymorphisms in the Caspase-3 gene and their association with lung cancer risk. Mol Carcinog. 2008; 47:383-90.
- Van Nguyen C, Nguyen Q.T, Vu HTN, Phung HT, Pham KH, Le RD. Combined p53 and Bcl2 Immunophenotypes in prognosis of vietnamese invasive breast carcinoma: A single institutional retrospective analysis. Technol Cancer Res Treat. 2020; 19: 1533033820983081.
- Singh R, Saini N. Downregulation of BCL2 by miRNAs augments drug-induced apoptosis–a combined computational and experimental approach. J Cell Sci. 2012;125:1568-78.
- Marques MM, Evangelista AF, Macedo T, Vieira RADC, Scapulatempo-Neto C, Reis RM, et al. Expression of tumor suppressors miR-195 and let-7a as potential biomarkers of invasive breast cancer. Clinics (Sao Paulo). 2018; 73: e184.
- Kolenda T, Przybyła W, Teresiak A, Mackiewicz A, Lamperska KM. The mystery of let-7d – a small RNA with great power. Contemp Oncol (Pozn). 2014;18:293-301.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402-8.
- Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, Van de Vijver MJ. WHO classification of tumours of the breast. 4th ed. Lyon: IARC Press. 2012;143-7.
- Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403-10.
- Honma N, Horii R, Ito Y, Saji S, Younes M, Iwase T, et al. Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy. BMC Cancer. 2015; 15:698.
- Wang Q, Sun L, Yan J, Wang S, Zhang J, Zheng X. Expression of vascular endothelial growth factor and caspase-3 in mucinous breast carcinoma and infiltrating ductal carcinoma-not otherwise specified, and the correlation with disease-free survival. Oncol Lett. 2017;14(4):4890-6.
- Callagy GM, Pharoah PD, Pinder SE, Hsu FD, Nielsen TO, Ragaz J, et al. Bcl-2 is a prognostic marker in breast cancer independently of the Nottingham Prognostic Index. Clin Cancer Res. 2006;12(8):2468-75.
- Rogoz B, Houzé de l’Aulnoit A, Duhamel A, Houzé de l’Aulnoit D. Thirty-Year Trends of Survival and Time-Varying Effects of Prognostic Factors in Patients with Metastatic Breast Cancer-A Single Institution Experience. Clin Breast Cancer. 2018;18(3):246-53.
- Yu W, Liang X, Li X, Zhang Y, Sun Z, Liu Y, et al. MicroRNA-195: a review of its role in cancers. Onco Targets Ther. 2018;11:7109-23.
- Sharifi Z, Talkhabi M, Taleahmad S. Identification of potential microRNA diagnostic panels and uncovering regulatory mechanisms in breast cancer pathogenesis. Sci Rep. 2022;12(1):20135.
- Zhang Z, Zhang H, Yu J, Xu L, Pang X, Xiang Q, et al. miRNAs as therapeutic predictors and prognostic biomarkers of neoadjuvant chemotherapy in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2022;194(3):483-505.
- Szczepanek J, Skorupa M, Tretyn A. MicroRNA as a potential therapeutic molecule in cancer. Cells. 2022;11:1008.
- Wang Y, Le Y, Xue JY, Zheng ZJ, Xue YM. Let-7d miRNA prevents TGF-β1-induced EMT and renal fibrogenesis through regulation of HMGA2 expression. Biochem Biophys Res Commun. 2016 ;479(4):676-82.
- García-Vázquez R, Gallardo Rincón D, Ruiz-García E, Meneses García A, Hernández De La Cruz ON, Astudillo-De La Vega H, et al. Let-7d-3p is associated with apoptosis and response to neoadjuvant chemotherapy in ovarian cancer. Oncol Rep. 2018 ;39(6):3086-94.
- Jiang J, Liu HL, Tao L, Lin XY, Yang YD, Tan SW, et al. Let‑7d inhibits colorectal cancer cell proliferation through the CST1/p65 pathway. Int J Oncol. 2018;53(2):781-90.
- Shahabi A, Naghili B, Ansarin K, Montazeri M, Dadashpour M, Zarghami N. Let-7d and miR-185 impede epithelial-mesenchymal transition by downregulating Rab25 in breast cancer. Asian Pac J Cancer Prev. 2021 ;22(1):305-313.
- Chen PS, Su JL, Cha ST, Tarn WY, Wang MY, Hsu HC, et al. miR-107 promotes tumor progression by targeting the let-7 microRNA in mice and humans. J Clin Invest. 2017;127(3):1116.
- Ding J, Huang S, Wang Y, Tian Q, Zha R, Shi H, et al. Genome-wide screening reveals that miR-195 targets the TNF-α/NF-κB pathway by down-regulating IκB kinase alpha and TAB3 in hepatocellular carcinoma. Hepatology. 2013 58(2):654-66.
- Flavin RJ, Smyth PC, Laios A, O’Toole SA, Barrett C, Finn SP, et al. Potentially important microRNA cluster on chromosome 17p13.1 in primary peritoneal carcinoma. Mod Pathol. 2009;22(2):197-205.
- Deng H, Guo Y, Song H, Xiao B, Sun W, Liu Z, et al. MicroRNA-195 and microRNA-378 mediate tumor growth suppression by epigenetical regulation in gastric cancer. Gene. 2013;518(2):351-9.
- Heneghan HM, Miller N, Kelly R, Newell J, Kerin MJ. Systemic miRNA-195 differentiates breast cancer from other malignancies and is a potential biomarker for detecting noninvasive and early stage disease. Oncologist. 2010;15(7):673-82.
- Xiang M, Su H, Shu G, Wan D, He F, Loaec M, et al. Amplexicaule A exerts anti-tumor effects by inducing apoptosis in human breast cancer. Oncotarget. 2016;7(14):18521-30.
- Yang X, Zhong DN, Qin H, Wu PR, Wei KL, Chen G, et al. Caspase-3 over-expression is associated with poor overall survival and clinicopathological parameters in breast cancer: a meta-analysis of 3091 cases. Oncotarget. 2017;9(9):8629-8641.
- Qian S, Wei Z, Yang W, Huang J, Yang Y, Wang J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front Oncol. 2022;12:985363.
- Tsang WP, Kwok TT. Let-7a microRNA suppresses therapeutics-induced cancer cell death by targeting caspase-3. Apoptosis. 2008;13(10):1215-22.
- Del Poeta G, Bruno A, Del Principe MI, Venditti A, Maurillo L, Buccisano F, et al. Deregulation of the mitochondrial apoptotic machinery and development of molecular targeted drugs in acute myeloid leukemia. Curr Cancer Drug Targets. 2008;8(3):207-22.
- Lin Y, Wu J, Chen H, Mao Y, Liu Y, Mao Q, et al. Cyclin-dependent kinase 4 is a novel target in micoRNA-195-mediated cell cycle arrest in bladder cancer cells. FEBS Lett. 2012;586(4):442-7.
- Bhattacharya A, Schmitz U, Wolkenhauer O, Schönherr M, Raatz Y, Kunz M. Regulation of cell cycle checkpoint kinase WEE1 by miR-195 in malignant melanoma. Oncogene. 2013;32(26):3175-83.
- Chen YQ, Wang XX, Yao XM, Zhang DL, Yang XF, Tian SF, et al. MicroRNA-195 promotes apoptosis in mouse podocytes via enhanced caspase activity driven by BCL2 insufficiency. Am J Nephrol. 2011;34(6):549-59.
- Al-Alem U, Rauscher GH, Alem QA, Kajdacsy-Balla A, Mahmoud AM. Prognostic Value of SGK1 and Bcl-2 in Invasive Breast Cancer. Cancers (Basel). 2023;15(12):3151. doi: 10.3390/cancers15123151.
- Yang G, Wu D, Zhu J, Jiang O, Shi Q, Tian J, et al. Upregulation of miR-195 increases the sensitivity of breast cancer cells to Adriamycin treatment through inhibition of Raf-1. Oncol Rep. 2013;30(2):877-89.
- Waters PS, McDermott AM, Wall D, Heneghan HM, Miller N, Newell J, et al. Relationship between circulating and tissue microRNAs in a murine model of breast cancer. PLoS One. 2012;7(11):e50459.
- Heneghan HM, Miller N, Lowery AJ, Sweeney KJ, Newell J, Kerin MJ. Circulating microRNAs as novel minimally invasive biomarkers for breast cancer. Ann Surg. 2010;251(3):499-505.
- Jang JY, Kim YS, Kang KN, Kim KH, Park YJ, Kim CW. Multiple microRNAs as biomarkers for early breast cancer diagnosis. Mol Clin Oncol. 2021;14(2):31.
- De Santis C, Götte M. The Role of microRNA Let-7d in Female Malignancies and Diseases of the Female Reproductive Tract. Int J Mol Sci. 2021;22(14):7359.
- Jha K, Shukla M, Kumar M, Shukla VK, Pandey M. Expression of caspase 3 and inhibition of apoptosis lowers survival in breast cancer. World J Surg Med Radiat Oncol. 2017;31: 6.
- Nakopoulou L, Alexandrou P, Stefanaki K, Panayotopoulou E, Lazaris AC, Davaris PS. Immunohistochemical expression of caspase-3 as an adverse indicator of the clinical outcome in human breast cancer. Pathobiol. 2001;69(5):266-73.
- O’Donovan N, Crown J, Stunell H, Hill AD, McDermott E, O’Higgins N, et al. Caspase 3 in breast cancer. Clin Cancer Res. 2003;9(2):738-42.
- Nathan B, Anbazhagan R, Dyer M, Ebbs SR, Jayatilake H, Gusterson BA. Expression of bcl-2-like immunoreactivity in the normal breast and in breast cancer. Breast. 1993;2(3):134-7.
- Yu B, Sun X, Shen HY, Gao F, Fan YM, Sun ZJ. Expression of the apoptosis-related genes BCL-2 and BAD in human breast carcinoma and their associated relationship with chemosensitivity. J Exp Clin Cancer Res. 2010;29(1):107
- Attia AS, Mohamed AH, Hegazy AA, Elwan A, Salah M, Abdelhamid MI, et al. Prognostic value of aquaporin-3, vimentin and E-cadherin expressions in invasive breast carcinoma: an immunohistochemical study. Middle East J Cancer. 2020;11(4):423-37.
- Mansoori B, Mohammadi A, Shirjang S, Baghbani E, Baradaran B. Micro RNA 34a and Let-7a Expression in Human Breast Cancers is Associated with Apoptotic Expression Genes. Asian Pac J Cancer Prev. 2016;17(4):1887-90.








