Manuscript accepted on :21-11-2023
Published online on: 29-12-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ramdas Bhat and Dr. Durgeshranjan Kar
Second Review by: Dr. Randa Salah Gomaa
Final Approval by: Dr. Patorn Piromchai
Zafar Isomiddinovich Sanoev1,2 , Dilnoza Safaralievna Ismailova3
, Dilnoza Safaralievna Ismailova3 , Sukhrob Davlatyor ogli Rakhimboev1
, Sukhrob Davlatyor ogli Rakhimboev1 , Tolmas Tolibovich Khamroev1
, Tolmas Tolibovich Khamroev1 , Burkhon Zhuraevich Elmuradov3
, Burkhon Zhuraevich Elmuradov3 , Ibrokhimjon Tuychievich Abdinazar1
, Ibrokhimjon Tuychievich Abdinazar1 and Sokhib Zamon ogli Rashidov1
and Sokhib Zamon ogli Rashidov1
1Department of pharmacology and toxicology of the Institute of Chemistry of Plant Substances named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, 100170, Mirzo-Ulugbek str., 77, Tashkent, Uzbekistan.
2Department microbiology and pharmacology of Tashkent State Dental Institute, 100047, Yashnobod dist., Taraqqiyot str., 103, Tashkent, Uzbekistan,
3Department of Organic synthesis of the Institute of Chemistry of Plant Substances named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan, 100170, Mirzo-Ulugbek str., 77, Tashkent, Uzbekistan.
Corresponding Author E-mail: zafarsano19@mail.ru
DOI : https://dx.doi.org/10.13005/bpj/2820
Abstract
Epilepsy is a chronic non-communicable disease of the brain, which is estimated that five million people are diagnosed with epilepsy every year worldwide. In this regard the study studied the anticonvulsant properties of annulated 4-(6-phenyl-7H-[1,2,4] triazolo [3,4-b] [1,3,4] thiadiazin-3-yl)-aniline. Anticonvulsant activity was evaluated in various experimental models of convulsions in laboratory rodents. Oral dosages of 3, 10, 30, and 60 mg/kg of the study substances were given one hour prior to the experiment. Pentylenetetrazole (PTZ) convulsions were modeled using PTZ at a dose of 7 mg/kg subcutaneously, isoniazid convulsions were modeled using an oral dose of 300 mg/kg, and bicuculline convulsions were modeled using a subcutaneous dose of 2,7 mg/kg. Also, comparatively studied the antiepileptic activity of triazole derivatives with the well-known antiepileptic drug carbamazepine. In the conducted studies the studied compound showed carbamazepine-like activity at doses of 3 and 10 mg/kg in the model of seizures caused by strychnine, while at a dose of 30 mg/kg it showed high activity and in models induced using bicuculin and isoniazid, the studied drug showed activity similar to carbamazepine. On the contrary, the model of seizures induced by PTZ showed high activity at doses of 3, 10 and 30 mg/kg. The results obtained showed that triazole derivative had a pronounced antiepileptic activity, and further study can be proposed as a potential antiepileptic drug.
Keywords
Benzodiazepines; Carbamazepine; Epilepsy; Pentylenetetrazole; Seizures; Sodium Channels
Download this article as:| Copy the following to cite this article: Sanoev Z. I, Ismailova D. S, Rakhimboev S. D. O, Khamroev T, T, Elmuradov B. Z, Abdinazar I. T, Rashidov S. Z. O. Synthesis and Research Anticonvulsant Activity of Annulated Triazolo-Thiadiazine Derivative in Laboratory Animals. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Sanoev Z. I, Ismailova D. S, Rakhimboev S. D. O, Khamroev T, T, Elmuradov B. Z, Abdinazar I. T, Rashidov S. Z. O. Synthesis and Research Anticonvulsant Activity of Annulated Triazolo-Thiadiazine Derivative in Laboratory Animals. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3tJ1Gpr |
Introduction
A chronic, non-communicable brain disorder, epilepsy affects approximately 50 million people globally 1. It is believed that 4 to 10 persons out of every 1000 are estimated to have active epilepsy, meaning they require medication or have periodic seizures. An estimated five million people receive an epilepsy diagnosis each year worldwide. 49 new cases per 100,000 people are reported each year in high-income nations. The rate can be more than twice as high (139 cases per 100,000 people) in low- and middle-income nations.
The most popular method of treating epilepsy is medication. There are currently over 30 antiepileptic medications on the market. Antiepileptic medications can lessen the frequency and severity of epileptic seizures as well as successfully prevent them. One medication is used in small doses at first, and then its dosage is raised. On the other hand, it is occasionally necessary to combine multiple antiepileptic medications (AEDs) in order to control seizures 2.
Anticonvulsants combine a large group of chemicals that can suppress or prevent seizures caused by various convulsive agents. They are conditionally divided into universal anticonvulsants and antiepileptics. From a chemical point of view, these groups are represented by derivatives of 3,4-benzodiazepine (diazepam, phenazepam, clobazam), barbituric and thiobarbituric acid (phenobarbital, sodium thiobarbital, hexenal), a derivative of tricyclic iminostilbene (carbamazepine), valproic acid (sodium valproate, calcium valproate) and representatives of other classes (local anesthetics, anesthetics, etc.) 3. Often, anticonvulsants in their spectrum also have tranquilizing (anti-anxiety) properties, for example, 3,4-benzodiazepine derivatives (diazepam, phenazepam, tazepam, lorazepam). Tranquilizing and anticonvulsant properties have also been described for some 7-hydroxycoumarin derivatives 4, although the latter are not common and are usually mild.
The pharmaceutical industry in the world produces dozens of names of antiepileptic drugs belonging to different structural classes and differing in the mechanism of action in different nuances. The most widely used anticonvulsant drug carbamazepine was chosen as the reference drug and prototype 5. For the treatment of epilepsy and pain related to genuine trigeminal neuralgia, carbamazepine is recommended 6. Specifically, mixed seizures, partial seizures with complicated symptoms, and generalized tonic-clonic seizures have all been successfully treated with carbamazepine 6,7. Additionally, bipolar disorder patients with manic episodes and mixed manic-depressive episodes should be treated with carbamazepine 6. Treatment for restless legs syndrome and alcohol withdrawal syndrome are two unapproved uses of carbamazepine 8,9. Its therapeutic index is narrow for carbamazepine7. When taking carbamazepine, some adverse events may occur, especially at the beginning of treatment. These include dyspeptic disorders, headache, dizziness, drowsiness, disturbance of accommodation, etc. The drug depresses psychomotor reactions, and therefore it is not recommended to prescribe it to transport drivers and representatives of similar professions. The tolerance of ethyl alcohol against the background of the action of carbamazepine is reduced. With the appearance of allergic reactions, leukopenia, or thrombocytopenia, the drug is canceled. In connection with the possibility of the last two complications, systematic monitoring of the composition of peripheral blood is necessary.
Triazolo[3,4-b][1,3,4]thiadiazines are an important class of bicyclic heterocyclic compounds. There is sufficient information in the literature on the synthesis 10,11, as well as bactericidal, fungicidal 12, anti-inflammatory, antimicrobial 13-16, and other types of activity 17. Therefore, the synthesis of new heterocyclic compounds containing fragments of this bicycle and the study of their biological properties is very interesting. Compounds with a 1,2,4-triazole ring in their structures have a strong potential to prevent seizures, according to the literature 18,19. The aim of this study is to create an anticonvulsant drug based on 4-(6-phenyl-7H-[1,2,4] triazolo [3,4-b] [1,3,4] thiadiazin-3-yl)-aniline ( compound 2).
Material and Methods
The “European Convention for the Defence of Vertebrates Used for Experiments or Other Scientific Purposes” (Strasbourg, 03/18/1986) governed the conduct of the experiments and its international recommendations. Before the experiment began, the Republic of Uzbekistan’s Ethic Committee gave its approval (protocol № 1/1-1628, 14/02/2023).
The experiments were carried out in the daytime, under natural light, on mature male mice weighing 18-24 g. For all preparations for each test, 6-10 animals were used. Throughout the experimental investigations, every lab animal was housed in a typical vivarium with unrestricted access to water and a full laboratory diet.
The objects of study were 4-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-aniline (compound 2) and the reference drug carbamazepine. Acute toxicity, locomotor activity, research activities, anti-anxiety activity 20 and the spectrum of anticonvulsant action were determined, their activity was studied on models of pentylenetetrazole (PTZ) (Sigma Aldrich), strychnine (Sigma Aldrich), bicuculin (Sigma Aldrich) and isoniazid (Acros) seizures. The doses used for compound 2 were 3;10;30 and 60 mg/kg orally in all of experiments.
The presence of GABAergic properties of the selected compounds was studied on the model of corazole and bicuculin seizures. The mechanism of the convulsive action of corazole is due to the inhibitory effect on the GABA-site, which leads to a weakening of the GABA-ergic inhibitory processes in the central nervous system. The strychnine convulsions model reproduces conditions similar to primary generalized convulsions in humans 21. The anticonvulsant activity of compounds in this model can be associated with both direct activation of glycine-sensitive receptors and concomitant potentiation of glycine- and GABA-ergic activity22. Isoniazid drug blocks the enzyme glutamate decarboxylase, resulting in reduced GABA biosynthesis 23. As a result of the elimination of GABAergic inhibition, hyperexcitability of neurons and convulsions develop.
Analysis of statistics
The collected data were subjected to one-way analysis of variance using the standard software program BIOSTAT 2009 and variation statistics using the paired Student’s test, along with a determination of the significance of the indicators (Mean±Std error). Variations between the groups under comparison were deemed noteworthy when the 95% p-value was less than 0.05.
Results
Chemical characterization of 4-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-aniline (compound 2)
In order to obtain annulated heterocyclic compound, we for the first time carried out the heterocyclization of 4-amino-3-(4-aminophenyl)-1H-1,2,4-triazole-5(4H)-thione (1), which can easily exist in protic solutions in the thiol form – 4-amino-5-(4-aminophenyl)-4H-1,2,4-triazole-3-thiol (1a) with phenacyl bromide. Condensation of thiol (1a) was carried out by heating equimolar amounts of reagents in ethanol at the boiling point of the solvent for 5 hours. As a result, a heterocyclization product, 4-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)aniline (2), with a high ( 92%) yield as a yellow powder (Fig.1.):
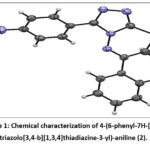 |
Figure 1: Chemical characterization of 4-(6-phenyl-7H-[1,2,4] triazolo[3,4-b][1,3,4]thiadiazine-3-yl)-aniline (2). |
Earlier [24], compound 2 was obtained starting from potassium salts of N1-acyl-N2-dithiocarbazates and hydrazine hydrate, followed by heterocyclization of the resulting substituted triazole (1a) with phenacyl bromide in ethanol. However, the yield of the product is 59%. According to the method developed by us, the yield is 30% higher than in the literature 24.
Detection in the 1H NMR spectrum of a new singlet signal of methylene group protons (S-CH2) at 4.04 ppm. and the disappearance of the proton signal of the amino group of the triazole ring (NH2) at 5.63 ppm, as well as the absence of C=O signals in the IR spectrum in the region of 1645 cm–1 and the presence of absorption of the C=N group at 1628 cm–1 for at C- 8 indicate the closure of the cycle with the receipt of a new bicyclic product 2.
Its 13C NMR spectrum shows the presence of signals in the region of a weak field of carbon atoms at 116.7 (C-1′), 117.4 (C-3′,5′), 128.6 (C-1”), 130.1 (C-2′ ,6′), 131.2 (C-2”,4”), 133.1 (C-3”), 134.6 (C-6”), 144 (C-8), 148.4 (C-4′, 5”), 153.7 (C-3), 157.2 (C-5) ppm, which are complemented by one signal in a strong field at 23.5 (C-7) ppm. It should be noted that the signal of the carbon atom at C-3 is significantly shifted to a stronger field (153.7 ppm) compared to the indicator (181.5 ppm) of the analogous carbon atom at 4-amino-3-(4- aminophenyl)-1H-1,2,4-triazole-5(4H)-thione (1). These data unambiguously confirm the structure of compound 2.
Synthesis of 4-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)-aniline (2)
A mixture of 0.5 g (2.4 mmol) 4-amino-3-(4-aminophenyl)-1H-1,2,4-triazole-5(4H)-thione (1) and 0.48 g (2.4 mmol) phenacyl bromide in ethanol (10 ml) was boiled for 5 hours. After the solvent was distilled off, the residue was treated with water, the yellow precipitate formed was filtered off, dried in air, and a powdered product, 4-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)aniline (2). Yield 0.68 g (92%), yellow powder, mp. 250–2510С, Rf=0.73 (chloroform:ethanol – 10:1). IR spectrum, ν, cm–1: 3314 (NH2-Ar), 1628 (C=N). UV spectrum, λmax: 276 nm. 1H NMR spectrum (DMSO-d6, δ (ppm), J (Hz)): 4.41 (2H, s, S-CH2), 5.69 (2H, br.s , NH2-Ar), 6.69 (2Н, d, J=8.5, H-3′,5′), 7.61 (3H, dd, J=6.0, 6.7, Н-2”,3”,4” ), 7.72 (2H, d, J=8.5, H-2′,6′), 8.21 (2H, d, J=6.5, H-1”,5”). 13С NMR (CD3COOD, δ (ppm), J (Hz)): 23.5 (С-7), 116.7 (С-1′), 117.4 (С-3′, 5′), 128.6 (C-1”), 130.1 (C-2′, 6′), 131.2 (C-2”,4”), 133.1 (C-3”), 134.6 (C-6”), 144 (C-8), 148.4 (C-4′, 5”), 153.7 (C-3), 157.2 (C-5) (Fig.2.).
 |
Figure 2: Synthesis of 4-(6-phenyl-7H-[1,2,4]triazolo [3,4-b][1,3,4]thiadiazin-3-yl)-aniline (2). |
Pharmacological studies of 4-(6-phenyl-7h-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)aniline
Study of acute toxicity parameters of 4-(6-phenyl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazin-3-yl)aniline (compound 2).
It has been established that 4-(6-phenyl-7H-(1,2,4)-triazolo[3,4-b]-(1,3,4)-thiadiazin-3-yl)-aniline (2) causes animals the same picture of poisoning. With the introduction of the substance in doses from 500 to 1000 mg/kg, it did not cause significant changes in the general condition and behavior of intact animals, with the exception of a short-term increase in breathing. With an increase in the dose (starting from a dose of 1500 mg/kg), depression of the general condition and limitation of motor activity were noted. The drug at higher doses caused tremor, tail reaction, prolonged tonic-clonic convulsions and death of experimental animals within 3-7 hours. The average lethal dose for mice is 2150 mg/kg. Based on the above, it can be concluded that the substance is of low toxicity (class IV), LD50 was 2150 mg/kg. Details regarding the toxicity of carbamazepine are as follows: oral LD50 (rats): 1957 mg/kg; LD50 (mice): 1920 mg/kg [6]. Details regarding the toxicity of carbamazepine are as follows: oral LD50 (rats): 1957 mg/kg; LD50 (mice): 1920 mg/kg 7.
Locomotor activity (LA) in mice on the background of administration of compound 2 at doses of 10 and 30 mg/kg was higher than in control mice.
LA in white mice was monitored for hours by oral administration. At 10 mg/kg, compound 2’s activity was 80 and verticalization was 193% compared to the control group; at 30 mg/kg, it was 55 and 38% (P≤0.05) and 80 and 69% in the control groups, respectively. Compound 2’s impact on research activities (RA) and LA in the “open field” test, per C. Hall 25.
With a single administration of compound 2 was tested in doses of 3; 10; 30 and 60 mg/kg orally. LA was assessed by the number of intersections of the lines of the squares, and research activity was estimated by the number of peeps into the gaps of minks. Compound 2 increased LA and exploratory activity at all doses when compared to the control group, as Table 1 illustrates. An oral dose of 60 mg/kg showed a tendency to reduce both LA and RA. It is evident that the number of minks inspected matched the motor activity indicators (see tab. 1).
Table 1: The effect of compound 2 on LA and research activity with a single administration of n = 10
|
Doses of compound 2 |
Number of line intersections |
Number of mink studies |
|
Control (sal.s-n) |
7,2±1,92 |
6,8±1,68 |
|
3 mg/kg р.о. |
12,2±1,92* |
10±1,68* |
|
10 mg/kg р.о. |
14,83±3,01* |
12,2±0,43* |
|
30 mg/kg р.о. |
15,83±2,58* |
17,1±1,29* |
|
60 mg/kg р.о. |
9,2±1,92* |
10±1,68* |
Note. * p≤0.05 when compared to the matching control
Study of the anti-anxiety activity of compound 2.
The experiment used compounds 2 doses 3; 10; 30 and 60 mg/kg orally. PTZ is considered anxiogenic in this dose, causing the sensation to increase excitement and fear. The effect of the above-mentioned doses of the compound studied on the alleviation of anxiety and fear reactions that occur in white mice has been studied. The results are shown in tab. 2, where it can be seen that all dosages of substance 2—up to four times as much as the control group—increase the “anti-anxiety index” K. The most effective dose, however, was 30 mg/kg.
Table 2: The effect of compound 2 on the feeling of anxiety a single administration of n = 10.
|
Doses of compound 2 |
Time location in light compartments in seconds |
Time location in dark compartments in seconds |
The number of transitions from camera to camera in seconds |
The relationship of time spent in a light and dark camera |
|
Control (PTZ 25 mg/kg s/c) |
47±3.6 |
73±4.34 |
7.6±0.24 |
0.64 |
|
3 mg/kg + PTZ |
68±4.8* |
52±2.89* |
10.4±0.12* |
1.3 |
|
10 mg/kg + PTZ |
76±4.34* |
44±4.09* |
12.1±0.45* |
1.73 |
|
30 mg/kg + PTZ |
87±4.58* |
33±3.37* |
14.3±0.48* |
2.64 |
|
60 mg/kg + PTZ |
60±2.6* |
60±3.85* |
9.2±0.96* |
1.0 |
Note. * p≤0.05 when compared to the matching control
Study of the anticonvulsant activity of compound 2 of the seizure threshold in the model of strychnine seizures.
After the introduction of strychnine in 100% of the control animals, there was evidence of the onset of tonic-clonic seizures. In the experimental groups of animals showed anticonvulsant activity. The results obtained are reflected in tab. 3.
Table 3: Effect of compound 2 and Carbamazepine on survival and latency of strychnine-induced convulsions (1.2 mg/kg, s.c) (n = 10).
|
Substances |
Mean latency to clonic seizures, in sec. |
Duration of seizures, in sec. |
Survival in % |
|
Control (strychnine) |
10/10a 360±43.38 |
108±26.03 |
0 |
|
Compound 2 3 mg/kg + strychnine |
6/10 a 705±86.76* |
258±14.46* |
50 |
|
Compound 2 10 mg/kg + strychnine |
6/10 a 615±28.92* |
148±19.28* |
50 |
|
Compound 2 30 mg/kg + strychnine |
5/10 a 438±14.46* |
78±16.87* |
80 |
|
Compound 2 60 mg/kg + strychnine |
10/10 a 552±28.92* |
66±12.05* |
30 |
|
Carbamazepine 20 mg/kg.+ strychnine |
5/10 a 480±28.92* |
60±14.46* |
70 |
|
Carbamazepine 50 mg/kg.+ strychnine |
6/10 420±43.38* |
438±28.92* |
50 |
Note. * p≤0.05 when compared to the matching control
a – how many of the group’s mice experienced seizures
In the control group of animals, the average duration of the latent period of seizures was 360±43,38 sec. Against the background of the introduction of compound 2 at doses of 3; 10; 30 and 60 mg/kg, the latent period increased and amounted to 705±86,76, 615±28,92, 438±14,46 and 552±28,92 sec., respectively, the survival rate was up to 80%. The reference drug carbamazepine slightly increased the latent period, but the survival rate was up to 70% in contrast to the control cohort (p<0.05).
The absence of a statistically significant change in the duration of the latent period of seizures caused by subcutaneous administration of strychnine in animals treated with compound 2 allows us to conclude that the studied compound has a significant effect on the glycinergic system.
Study of the anticonvulsant activity of compound 2 of the seizure threshold in a model of bicuculin convulsions.
Studies are carried out on male mice, white, outbred, weighing 18–24 g. Each dose is tested on 10 animals. Bicuculin is a direct GABAA blocker. Bicuculline is administered at a dose that causes convulsions in 97% of animals (usually a dose of 2.7 mg/kg), subcutaneously in the cervical region of the back. Animals are observed for 30-60 minutes after bicuculline injection. Test compound 2 is administered in doses of 3; 10; 30 and 60 mg/kg orally, taking into account the peak of its maximum effect, before the introduction of bicuculline. As a criterion for evaluating the anticonvulsant effect of a new substance, its ability to suppress the development of repetitive clonic convulsions of the fore and/or hind limbs lasting more than 3 s without loss of the rollover reflex is used (see Table 4.). The search for new effective antiepileptic drugs is carried out quite widely and in different directions. Much attention continues to be paid to the GABA system. Active selective and irreversible inhibitors of GABA-transaminase, which penetrate well into the brain, substances of direct GABAA mimetic action, have been created.
Table 4: Effect of compound 2 and carbamazepine on survival and latency of during convulsions caused by bicuculline (2,7 mg/kg, s.c.), (n=10).
|
Substances |
Beginning of convulsions, min. |
Duration convulsions, min. |
Number convulsions |
Survival in % |
|
Control (bicuculline) |
10/10 a 228±12.05 |
108±8.43 |
2 |
30 % |
|
Compound 2 3 mg/kg + bicuculline |
5/10 a 480±10.84* |
492±9.64* |
1 |
80 % |
|
Compound 2 10 mg/kg + bicuculline |
5/10 a 430±9.15* |
420±28.92* |
1 |
90 % |
|
Compound 2 30 mg/kg + bicuculline |
6/10 a 315±21.69* |
315±19.28* |
2.75 |
70 % |
|
Compound 2 60 mg/kg + bicuculline |
5/10 a 438±24.1* |
612±26.51* |
4.3 |
70 % |
|
Carbamazepine 20 mg/kg.+ bicuculline |
5/10 a 456±25.3* |
426±43.38* |
1.67 |
100 % |
|
Carbamazepine 50 mg/kg, + bicuculline |
6/10 a 780±36.15* |
498±33.74* |
2 |
100% |
Note. * p≤0.05 when compared to the matching control
a – how many of the group’s mice experienced seizures
Study of the Anticonvulsant Activity of compound 2 on Seizure Threshold in an Isoniazid Seizure Model.
During the 120-minute observation period, all of the mice in the control group that received isoniazid 300 mg/kg ip developed generalized tonic-clonic seizures, which resulted in 100% of the cases ending in death. The latent period of clonic seizures and the time to death were statistically significantly prolonged by 1.95 and 2.35 times, respectively, at doses of 20 and 50 mg/kg of the reference medication carbamazepine, and the survival rate rose to 136% as a result. Study compound 2 at dose 3; 10; 30 and 60 mg/kg orally prolonged the latent period of clonic seizures and the time to death in 1,35; 1,37; 1.21 and 1.12 times, respectively, along with this, the survival time increased to 79,4% (see tab.5.).
Despite the existing positive bias in the changes in the indicators of convulsive activity after the administration of compound 2 at a dose of 3; 10; 30 and 60 mg/kg, no statistically significant effect on the latent period of isoniazid-induced clonic seizures and animal survival was found. This may be explained by the involvement of other nerve structures in epileptogenesis, in contrast to other models of seizures induced by GABA receptor blockers 25.
Table 5: Effect of compound 2 and carbamazepine on survival and latency of during convulsions caused by isoniazid (300 mg/kg, in.p.), (n=10).
|
Substances |
Beginning of convulsions, min. |
Duration convulsions, min. |
Number convulsions |
Survival time, min |
|
Control (isoniazid) |
10/10 a 29±3.4 |
6.6±1.2 |
1.4±0.2 |
36±3.8 |
|
Compound 2 3 mg/kg + isoniazid |
10/10 a 39.1±6.02* |
7.6±1.12* |
1.8±0.48* |
44.4±5.7* |
|
Compound 2 10 mg/kg + isoniazid |
10/10 a 39.8±6.7* |
7.8±1.1* |
2.2±0.8* |
47.4±5.9* |
|
Compound 2 30 mg/kg + isoniazid |
10/10 a 35±7.1* |
24.4±3.2* |
3.2±0.7* |
59.4±6.7* |
|
Compound 2 60 mg/kg + isoniazid |
10/10 a 32,6±6,8* |
32±4.1* |
3.2±0,8* |
64.6±7.4* |
|
Carbamazepine 20 mg/kg + isoniazid |
8/10 a 50±7,6* |
1±0.06* |
2.25±0.5* |
70.25±8.9* |
|
Carbamazepine 50 mg/kg + isoniazid |
10/10 a 55,2±7,8* |
42.6±6.5* |
5±1.2* |
84.8±8.4* |
Note. * p≤0.05 when compared to the matching control
a – how many of the group’s mice experienced seizures
Study of the anticonvulsant activity of compound 2 of the seizure threshold in the model of PTZ seizures
Animals of the experimental groups were injected intragastrically at a screening dose of 3; 10; 30 and 60 mg/kg of the test compound 2. Animals from the positive control group received carbamazepine at a dose of 20 and 50mg/kg orally. The convulsive state in animals was modeled by a single subcutaneous injection of PTZ into the cervical region at a dose of 90 mg/kg for 60 minutes following the solvent or the investigated substances’ administration. Monitoring was carried out for 30 minutes, followed by the calculation of convulsive phenomena and mortality. Animals that did not experience recurrent clonic convulsions lasting more than 3 seconds after injection of the substance and then PTZ for 30 minutes were considered protected (see Tab. 6).
Table 6: Effect of Synthetic Compound 2 and Carbamazepine on convulsion threshold in PTZ-induced convulsion models 90 mg/kg s.c. (n=10)
|
Substances and doses |
Mean latency to clonic seizures, in sec. |
Duration of seizures, in sec. |
Survival in % |
|
Control (PTZ 90 mg/kg s.c.) |
10/10 a 108±28.92 |
204±14.46 |
0 |
|
Compound 2 3 mg/kg + PTZ |
6/10 a 264±12.05* |
180±28.92* |
20 |
|
Compound 2 10 mg/kg + PTZ |
6/10 a 315±16.87* |
204±28.92* |
30 |
|
Compound 2 30 mg/kg + PTZ |
5/10 a 360±28.92* |
225±7.23* |
40 |
|
Compound 2 60 mg/kg + PTZ |
10/10 a 252±14.46* |
300±21.69* |
0 |
|
Carbamazepine 20 mg/kg + PTZ |
5/10 a 162±7.23* |
330±24.1* |
0 |
|
Carbamazepine 50 mg/kg + PTZ |
6/10 a 210±18.07* |
582±12.05* |
0 |
Note. * p≤0.05 when compared to the matching control
a – how many of the group’s mice experienced seizures
Thus, compound 2 prolongs the latent period of the seizure and reduces the duration of the seizure. In addition, it has an impact on survival, more than in control groups that received various doses of the well-known drug carbamazepine.
Discussion
In the scientific literature, this group is based on various pharmacological activity of chemical compounds. In addition to the above classes, heterocyclic ring compounds, including triazoles 26, quinazoline-4(3N)-onys 27, xanthonys and isatinys 28, are important for the production of new anticonvulsants. In particular, the broad-spectrum anticonvulsant activity of loreclezol related to (arylalkyl) triazoles is associated with the modulation of GAMK receptors 29. According to the research of a group of scientists, the compound 4-alkyl-5-aryl-1,2,4-triazole-3-tyone was included in the group of promising anticonvulsants30,33. The anticonvulsant activity of these compounds is due to their ability to interact with sodium channels 34. Antiepileptic drugs (AEDs), which are the primary therapy for the treatment of epilepsy, selectively depress the central nervous system, however, the efficacy of these drugs is limited; as nearly 30% of patients on medication still suffer from uncontrolled seizures35. Tiagabine, vigabatrin, lamotrigine, zonisamide, felbamate, oxacarbazine, topiramate, and gabapentine are among the current AEDs belonging to the new generation. Although the pharmacokinetic profile and patient tolerance of newer antiepileptic medications are improved, their efficacy is not appreciably greater than that of older medications. Although there are numerous AEDs available, new compounds are required to improve seizure control and reduce the many side effects, including sedation, ataxia, gastrointestinal disorders, hepatotoxicity, megaloblastic anaemia, and cancer risk36,37. In addition, derivatives of 4-alkyl-5-aryl-1,2,4-triazole-3-thyone demonstrate good pharmacological and toxicological indicators: low neurotoxicity and low toxicity compared to human cells, lack of genotoxic properties, rapid onset of action and persistence, increased valproate activity against seizures38. In studies conducted to study the anticonvulsant activity of compounds 2, doses of 3; 10; 30 and 60 mg/kg were selected as a result of screening experiments and studied on various models of seizures. In the model of seizures caused by strychnine, the test substance showed carbamazepine-like activity at doses of 3 and 10 mg/kg, while at a dose of 30 mg/kg it showed high activity and in models induced using bicuculin and isoniazid, the studied drug showed activity similar to carbamazepine. On the contrary, the model of seizures induced by PTZ showed high activity at doses of 3, 10 and 30 mg/kg. Isoniazid drug blocks the enzyme glutamate decarboxylase, resulting in reduced GABA biosynthesis23. As a result of the elimination of GABAergic inhibition, hyperexcitability of neurons and seizures develop. Compound 2 eliminates and reduces isoniazid convulsions. This may be explained by the involvement of other nerve structures in epileptogenesis, in contrast to other models of seizures induced by GABA receptor blockers39. PTZ is known to block the chloride ionophore GABA-benzodiazepine chlorionophore complex. Compound 2 eliminates and reduces PTZ cramps.
Conclusions
In terms of toxicity, synthetic compound 2 belongs to low-toxic substances, has a psychosedative, anxiolytic effect, increases motor and exploratory activity and has a pronounced anticonvulsant effect due to the prolongation of the latent period of seizures and survival in some models of seizures. Based on the conducted studies, we can suggest that this substance is a promising anticonvulsant, as it performs comparably to carbamazepine.
Acknowledgment
The authors’ acknowledgements go to the Institute of Chemistry of Plant Substances named after Academician S.Yu. Yunusov of the Academy of Sciences of the Republic of Uzbekistan for supporting this work.
Conflict of Interest
The authors declare no conflict of interest.
Funding Sources
The work financial supported by the Ministry of Higher Education, Science and Innovation of Republic of Uzbekistan (Grant № F-FA-2021-408 “Study of the laws of introduction of pharmacophore fragments into the molecule on the basis of modern cross-coupling and heterocyclization reactions” and Grant № ALM-2023031533-02 “Creation of anticonvulsant medicinal substance from a number of heterocyclic compounds synthesized on the basis of domestic raw materials”).
References
- www.who.ru According to the World Health Organization.
- Paruch K, Kaproń B, Łuszczki JJ, Paneth A, Plech T. Effect of Linker Elongation on the VGSC Affinity and Anticonvulsant Activity among 4-Alkyl-5-aryl-1,2,4-triazole-3-thione Derivatives. Molecules. 2023; 28(13):5287. https://doi.org/10.3390/molecules28135287
CrossRef - Shabanov P.D. Psychopharmacology. St. Petersburg: N-L, 2008. 368 p.
- Denisenko P.P., Tarasenko A.A. Chloropropoxy derivatives of 7-hydroxycoumarin, which have a tranquilizing and hepatoprotective effect // Russian Federation Patent No. 2452732 dated 06/10/2012. BI №16.
- Kharkevich D. A. Pharmacology: textbook. 12th ed., corrected and additional M.: GEOTAR-Media, 2017. 760 p.
- Soderstrom J, Murray L, Little M, Daly FF. Toxicology case of the month: Carbamazepine overdose. Emerg Med J. 2006 Nov;23(11):869-71. doi: 10.1136/emj.2006.034884.
CrossRef - Tolou-Ghamari Z, Zare M, Habibabadi JM, Najafi MR: A quick review of carbamazepine pharmacokinetics in epilepsy from 1953 to 2012. J Res Med Sci. 2013 Mar;18 (Suppl 1): S81-5.
- Barrons R, Roberts N. The role of carbamazepine and oxcarbazepine in alcohol withdrawal syndrome. J Clin Pharm Ther. 2010 Apr;35(2):153-67. doi: 10.1111/j.1365-2710.2009.01098.x.
CrossRef - Aurora RN, Kristo DA, Bista SR, Rowley JA, Zak RS, Casey KR, Lamm CI, Tracy SL, Rosenberg RS: The treatment of restless legs syndrome and periodic limb movement disorder in adults–an update for 2012: practice parameters with an evidence-based systematic review and meta-analyses: an American Academy of Sleep Medicine Clinical Practice Guideline. Sleep. 2012 Aug 1;35(8):1039-62. doi: 10.5665/sleep.1988.
CrossRef - Sobhi M.G., Sayed M.R. Synthesis of triazolo[4,3-b][1,2,4,5]tetrazines and triazolo[3,4-b] [1,3,4] thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation, ARKIVOC, 2009, xi, 58-68.
CrossRef - Mashooq A.B., Abdul A.K., Shahanavaj K., Abdullah AD. Synthesis of New [1,2,4]Triazolo[3,4-b][1,3,4]thiadiazines and Study of Their Anti-Candidal and Cytotoxic Activities, Journal of Chemistry, Volume 2014, Article ID 897141, 7 pages. http://dx.doi.org/ 10.1155/2014/897141
CrossRef - Swagatika Gh., Amita V., Alok M., Milan K.M. Synthesis, characterization and antimicrobial evaluation of some novel 1,2,4-triazolo[3,4-b][1,3,4]thiadiazine bearing substituted phenylquinolin-2-one moiety. Arabian Journal of Chemistry, 2019, 12, 3046-3053.
CrossRef - Strzelecka M, Świątek P. 1,2,4-Triazoles as Important Antibacterial Agents. Pharmaceuticals. 2021; 14(3):224. https://doi.org/10.3390/ph14030224
CrossRef - Palma E, Tilocca B, Roncada P. Antimicrobial Resistance in Veterinary Medicine: An Overview. Int J Mol Sci. 2020 Mar 11;21(6):1914. doi: 10.3390/ijms21061914.
CrossRef - Donadu MG, Trong Le N, Viet Ho D, Quoc Doan T, Tuan Le A, Raal A, Usai M, Marchetti M, Sanna G, Madeddu S, Rappelli P, Diaz N, Molicotti P, Carta A, Piras S, Usai D, Thi Nguyen H, Cappuccinelli P, Zanetti S. Phytochemical Compositions and Biological Activities of Essential Oils from the Leaves, Rhizomes and Whole Plant of Hornstedtia bella Škorničk. Antibiotics (Basel). 2020 Jun 18;9(6):334. doi: 10.3390/antibiotics9060334.
CrossRef - Shivananda W., Airody V.A., Nalilu S.K. Antimicrobial and Antiinflammatory Studies on Some 1,2,4-Triazolo[3,4-b][1,3,4]thiadiazines and 1,2,4- Triazolo[3,4-b][1,3,4]thiadiazoles Containing Quinoxaline, Asian Journal of Chemistry, 2008, Vol. 20, No. 1, 629-641.
- Sui X.C., John A.D., Zhang HZ., Shailaja K., Gisela C., Nilantha S.S., William E.K. 3-Aryl-6-aryl-7H-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazines and analogs as activators of caspases and inducers of apoptosis and the use thereof, Feb. 21, 2008. US 2008/0045514 A1.
- Aggarwal, R.; Sumran, G. An insight on medicinal attributes of 1,2,4-triazoles, European Journal of Medicinal Chemistry, Volume 205, 2020, 112652, https://doi.org/10.1016/j.ejmech.2020.112652.
CrossRef - Wang Y., Liu D. An Important Potential Anti-Epileptic/Anticonvulsant Active Group: A Review of 1,2,4-Triazole Groups and Their Action. Drug Res. 2022, 72, 131–138. DOI: 10.1055 / a-1670-6992
CrossRef - Kilfoil T., Michel A., Montgomery D., Whiting R. / Effect of anxyolyic and anxyogenic drugs on exploratory activity a simple model of anxiety in mice // Psychopharmacology, 1989, v. 28, No 9, p. 901-905
CrossRef - Li M. Anticonvulsant activity of B2, an adenosine analog, on chemical convulsant-induced seizures / M. Li, R. Kang, J. Shi [et al.] // PLoS One. – 2013. – Т. 8. – №. 6. – С. e67060.
CrossRef - Khatoon H. Evaluation of anticonvulsant and neuroprotective effects of camel milk in strychnine-induced seizure model / H. Khatoon, R. Najam, T. Mirza [et al.] // Asian Pacific Journal of Tropical Disease. – 2015. – Т. 5. – №. 10. – С. 817-820.
CrossRef - Carta M. Isoniazid-induced reduction in GABAergic neurotransmission alters the function of the cerebellar cortical circuit / M. Carta, L. Murru, E. Barabino [et al.] // Neuroscience. – 2008. – Т. 154. – №. 2. – С. 710-719.
CrossRef - El-Dawy MA, Omar AM, Ismail AM, Hazzaa AA. Potential broad spectrum anthelmintics IV: design, synthesis, and antiparasitic screening of certain 3,6-disubstituted-(7H)-s-triazolo-[3,4-b][1,3,4]thiadiazine derivatives. J Pharm Sci. 1983 Jan;72(1):45-50. doi: 10.1002/jps.2600720111. PMID: 6827463.
CrossRef - Hall C. / The relationship between emotionality and ambulatory activity. // J.Comp. Psychol., 1936, 22, 345-452.
CrossRef - Ayati A., Emami S., Foroumadi A. The importance of triazole scaffold in the development of anticonvulsant agents. Eur. J. Med. Chem. 109 (2016) 380–392.
CrossRef - Ugale VG, Bari SB. Quinazolines: new horizons in anticonvulsant therapy. Eur J Med Chem. 2014 Jun 10;80:447-501. doi: 10.1016/j.ejmech.2014.04.072.
CrossRef - Malawska K., Rak A., Gryzło B. et al. Search for new potential anticonvulsants with anxiolytic and antidepressant properties among derivatives of 4,4-diphenylpyrrolidin-2-one. Pharmacol. Rep 69, 105–111 (2017). https://doi.org/10.1016/j.pharep.2016.09.020
CrossRef - Wingrove P.B., Wafford K.A., Bain C., Whiting P. J. The modulatory action of loreclezole at the gamma-aminobutyric acid type A receptor is determined by a single amino acid in the beta 2 and beta 3 subunit. Proc. Natl. Acad. Sci. U.S.A. 91 (1994) 4569–4573.
CrossRef - Plech T., Łuszczki J.J., Wujec M., Flieger J., Pizoń M. Synthesis, characterization and preliminary anticonvulsant evaluation of some 4-alkyl-1,2,4-triazoles. Eur J Med Chem 2013, 60, 208-215.
CrossRef - Plech T., Kaproń B., Łuszczki J.J., at. all. Studies on the anticonvulsant activity of 4-alkyl-1,2,4-triazole-3-thiones and their effect on gabaergic system. Eur J Med Chem 2014, 86, 690-699.
CrossRef - Kaproń B., Łuszczki J., Paneth, A., at. all. Molecular mechanism of action and safety of 5-(3-chlorophenyl)-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione – a novel anticonvulsant drug candidate. Int J Med Sci 2017, 14, 741-749.
CrossRef - Kaproń B., Łuszczki J.J., Płazińska A., at. al. Development of the 1,2,4-triazole-based anticonvulsant drug candidates acting on the voltage-gated sodium channels. Insights from in-vivo, in-vitro, and in-silico studies. Eur J Pharm Sci. 2019, 129, 42-57.
CrossRef - Kaproń B., Łuszczki J., Paneth A., at. all. Molecular mechanism of action and safety of 5-(3-chlorophenyl)-4-hexyl-2,4-dihydro-3H-1,2,4-triazole-3-thione – a novel anticonvulsant drug candidate. Int J Med Sci 2017, 14, 741-749.
CrossRef - Mioramalala S.A., Bruand, P.E., Ratsimbasoa A., Rafanomezantsoa R.M., Raharinivo, M.M. Vincent C., Preux P.M., Boumédiène F. Raharivelo A. Effects of an educational comic book on epilepsy-related knowledge, attitudes and practices among schoolchildren in Madagascar. Epilepsy Research, Volume 176, 2021, 106737, https://doi.org/10.1016/j.eplepsyres.2021.106737.
CrossRef - Hu T., Zhang J., Wang J., Sha L., Xia Y., Ortyl T.C., Tian X., Chen L. Advances in Epilepsy: mechanisms clinical trials and drug therapies J. Med. Chem., 66 (7) (2023), pp. 4434-4467, 10.1021/acs.jmedchem.2c01975
CrossRef - Ozbek O., Gürdere M.B. A review on the synthesis and applications of molecules as anticonvulsant drug agent candidates Med. Chem. Res., 29 (2020), pp. 1553-1578, 10.1007/s00044020-02595-4
CrossRef - Łuszczki J.J., Marzęda P., Gut-Lepiech A., at. al. New derivative of 1,2,4-triazole-3-thione (TP427) potentiates the anticonvulsant action of valproate, but not that of carbamazepine, phenytoin or phenobarbital in the mouse tonic-clonic seizure model. Pharmacol Rep. 2019, 71, 299-305.
CrossRef - Miller J.W. Functional anatomy of pentylenetetrazol and electroshock seizures in the rat brainstem / J.W. Miller A.C. McKeon J.A. Ferrendelli // Annals of neurology. – 1987. – Т. 22. – №. 5. – С. 615-621.
CrossRef







