Manuscript accepted on :16-10-2023
Published online on: 27-11-2023
Plagiarism Check: Yes
Reviewed by: Dr. Victor Oti
Second Review by: Dr. M Mohan Varma
Final Approval by: Dr. Patorn Piromchai
Ahmed Sadek1 , Soheir Mansy2
, Soheir Mansy2 , Ahmed M Abd El Hady3, Olfat Hammam3
, Ahmed M Abd El Hady3, Olfat Hammam3 , Afaf A Abdel Hady4
, Afaf A Abdel Hady4 , Wafaa Wafy5, Eman R. Youness6*
, Wafaa Wafy5, Eman R. Youness6*
1Department of Hepatology and Gastroenterology, Theodor Bilharz Research Institute. Imbaba Giza, Egypt.
2Department of Electron microiscopy, Theodor Bilharz Research Institute. Imbaba Giza, Egypt.
3Department of Pathology, Theodor Bilharz Research Institute. Imbaba Giza, Egypt.
4 Department of Clinical Chemistry, Theodor Bilharz Research Institute. Imbaba Giza, Egypt.
5 Department of Public Health, Theodor Bilharz Research Institute, Imbaba Giza, Egypt.
6 Department of Medical Biochemistry, Medical Researches and Clinical Studies Institute, National Research Centre Cairo, Egypt.
Corresponding Author E-mail:hoctober2000@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2815
Abstract
We aimed to evaluate the histopathological and ultrastructural changes detected by light and electron microscopy, induced in patients having chronic hepatitis C receiving ozone/oxygen gas treatment. Twenty six patients with chronic hepatitis C who were at different stages of liver fibrosis, non-responders to interferon plus ribavirin therapy (n = 9), had contraindications (n = 2), or were not compliant (n = 15) were included. At baseline and 12 weeks after administering the ozone/oxygen gas mixture, liver biopsies were carried out utilizing both the major rectal insufflations and auto-hemotherapy along with clinical evaluation, kidney and liver function assessments, liver biopsies and abdominal ultrasonography. Before and 12 weeks after the treatment of ozone, quantitative PCR was performed. Two pathologists evaluated the histological activity index (HAI) using Ishak's score while working in blind settings, taking into account the degree of inflammation and the stage of fibrosis. Electron microscopy was done for all cases before and after treatment. Significant improvements in liver enzymes in hepatic fibrosis and inflammatory activity based on Ishak scoring system were detected. The mean grade of inflammation dropped from 10.3 to 8.4 and the mean stage of fibrosis dropped from 2.3 to 2.0 both with P value < 0.001 and < 0.05 respectively. Mean PCR values showed significant increase after 12 weeks of treatment from 17059 to 218736 with P value <0.05. As regards electron microscopy findings, the ultra-structural manifestations of HCV infection were disclosed in liver specimens exposed or not to ozone treatment. Stellate cells were often encountered in unexposed samples to ozone, meanwhile they disappeared after ozone exposure. Apoptotic hepatocytes which were frequently encountered before treatment are rarely seen in specimens after treatment with ozone. Signs of cellular regeneration in the form of binucleated cells, RER enveloping mitochondria, hepatocyte progenitors insinuating between the cells at the sinusoid pole are seen after treatment. Circulating inflammatory cells in the sinusoids and infilterating the lobule were decreased after treatment. Peroxisomes were increased after exposure to ozone with longitudinal orientation of mitochondrial cristae thus increasing the antioxidative activity of hepatocytes. No single significant complication was recorded in a total of >1000 settings of ozone therapy. In conclusion, ozone oxygen gas mixture is a direct anti-fibrotic and anti-inflammatory agent in treatment of chronic hepatitis C patients without improving viral PCR as evidenced by histopathology, electron microscopy and quantitative PCR.
Keywords
Chronic liver disease; Electron microscopy; Hepatitis C; Liver fibrosis; Ozone/oxygen gas mixture
Download this article as:| Copy the following to cite this article: Sadek A, Mansy S, El-Hady A. M. A, Hammam O, Hady A. A. A, Wafy W, Youness E. R. Electron Microscopic Studying the Impact of Ozone on Chronic Hepatitis C Patients as Antifibrotic. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Sadek A, Mansy S, El-Hady A. M. A, Hammam O, Hady A. A. A, Wafy W, Youness E. R. Electron Microscopic Studying the Impact of Ozone on Chronic Hepatitis C Patients as Antifibrotic. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/47HpVCW |
Introduction
Hepatitis C virus is one of the greatest mutual reasons of chronic liver disease and a leading suggestion for liver transplantation. Because there isn’t a rodent model of enduring HCV infection, the pathophysiology of HCV-induced liver fibrosis is poorly known 1. Hepatocyte infection by HCV results in oxidative stress and the attraction of inflammatory cells after HCV avoids detection by the HLA-II-directed immune response. Both factors cause collagen formation and HSC activation. Additionally, a number of HCV proteins actively promote HSCs’ inflammatory and fibrogenic actions 2,3. Hepatitis C virus core protein causes mitochondrial damage, oxidative stress, and the expression of genes related to anti-oxidants.4.
Low doses of ozone augment antioxidant endogenous systems as catalase, superoxide dismutase and glutathione 5-6. After CCl4 poisoning, animals can maintain hepatocellular integrity by receiving ozone in atoxic dosages repeatedly. This allows for the induction of an adaptation to oxidative stress. 7 . Ozone in a clinical histopathological study on 141 chronic hepatitis C patients proved to have a potent antifibrotic and anti-inflammatory activity without affecting the virus itself 8 .
Aim of the work
The objective of the study is to evaluate the ultrastructural changes in liver biopsy sections compared with the histopathologic results in chronic hepatitis C patients given ozone for 12 weeks.
Subjects and Procedures
Designing study
This study is a
clinical phase II trial. Enrolled patients were given ozone/oxygen gas mixture for
12 weeks. Each patient gave their informed consent. The electron microscopy and
histopathology were performed in Theodor Bilharz Research Institute in Egypt.
Subjects Selection
Age 19–65 years was
required for enrollment, irrespective to the presence of positive hepatitis C
virus blood indicators and sex for at least 6 months before enrollment in whom
interferon plus ribavirin treatment is not possible (Contra-indicated, failure
of treatment or lack of compliance), unusual value of alanine transaminase
(ALT) in serum within 6 months prior enrollment or evidence of hepatitis by
ultrasound (lymph nodes in porta-hepatis); negative hepatitis B virus (HBsAg)
and; liver biopsy examination immediately showing the degree of hepatic
fibrosis prior to enrollment from 0 to 6 according to Ishak modification of
Knodell score, No previous history of administering the following medications:
antiviral medications, immunoregulating medications, and other antifibrotic
agents; a promise to refrain from receiving additional systemic antiviral
medications, cytotoxic medications, immunoregulators, medications that can
lower serum enzyme activity and bilirubin levels, as well as herbal remedies including
DDB, etc. The following scenarios should be avoided: individuals having
HIV-positive testing results; elevated creatinine in serum 1.5 times higher
than normal; antinuclear antibody (ANA) titer more than a 1:160 dilution;
indicative of autoimmune disorders.; uncompensated liver diseases; bone marrow
inhibition; concurrence of other related diseases that might impact the current
treatment as unstable diabetes, pancreatitis, obvious manifestations of
neurosis, alcoholic liver disease, disability of absorption, drug abuser,
psychosis, malignant disease, unstable angina pectoris, renal insufficiency,
and epilepsy, and so on; taking another medication in a clinical trial within
30 days of the initial dose.
Treatment methods and medications
Afterwards selection and assessment, suitable candidates were given ozone/oxygen gas mixture daily for 10 days in the form of major autohemotherapy (One hundred ml. ozone/oxygen gas mixture at a concentration of 25 μg/ml. were mixed with 50 ml. blood aliquot) together with 150 ml. ozone oxygen gas mixture by rectal insufflation in the same concentration. Then the same treatment was given twice weekly for 12 weeks. Everyone underwent follow-up once weekly during treatment and were followed up at out-patient department for 12 weeks after treatment.
Assessment of blood and urine tests, together with relevant liver function indices
These were evaluated immediately before and 12 weeks once the treatment has begun.
Analysis of PCR for HCV RNA
Quantitative PCR was done immediately before treatment and 12 weeks from the start of treatment.
Imaging examination (B mode ultrasound)
Abdominal ultrasound was done immediately before and after 12 weeks (end of treatment). The detection included the following indexes: maximal oblique radius of right liver lobe, left lobe axis in middle line, main trunk diameter of portal vein, long axis of the spleen, liver surface, parenchymal echopattern and evidence of portal tract thickening for associated shistosomiasis together with the presence or absence of lymph nodes in porta-hepatis. The ultrasound examination was carried out by fixed operators and on fixed equipment.
Histopathological Assessment:
Needle liver biopsy specimens were obtained from all patients upon their written consent using trucut needle. Each specimen was preserved in 10% buffered neutral formalin right away. delivered to the Pathology Department where embed it into paraffin wax blocks, it was treated in various grades of alcohol. Paraffin sections (4mm thick) were cut; 3 from each specimen to be stained with Haematoxylin & Eosin and Masson Trichrom stain for histopathological evaluation and identification of the different grades of inflammatory activity and different stages of chronic hepatic lesions based on the Ishak and Knodellscoring system 9-10. Histopathological samples were viewed and evaluated independently. They were examined by a different pathologist who was not involved in the project from the start based on the observed results. The two pathologists’ mutual consistency was satisfactory.
Ultrastructure assessment by electron microscopy (EM):
Fifty two biopsies taken from 26 patients before and after treatment with ozone were subjected to (EM) examination.Liver biopsies were prepared by fixing them in gluteraldehyde, washing them in equal parts of 0.3 M cacodylate and 0.4 M sucrose, postfixing them in 2% osmium tetroxide, dehydrating them in escalating alcohol concentrations, embedding them in epoxy resin, and polymerizing them. Semithin liver sections stained with methylene blue azur II were examined using light microscope for the identification of the site for the performance of ultrathin sections. In this study, the midzonal area of the liver lobule was the site examined at the level of electron microscopy. Leica Ultracut R, an ultramicrotome, was used to create ultrathin sections. They were inspected with a Philips EM 208 S and stained with uranyl acetate and lead citrate. Examination of the following subcellular criteria was performed: Signs of cellular regeneration and degeneration of apoptotic changes, disorganized lobular architecture, changes in cellular organelles mitochondria, endoplasmic reticulum, free and attached ribosomes, lysosomes, peroxisomes, fat droplets, glycogen rosettes…), nuclear changes, changes of bile canaliculi and canaliuloductular junction, inflammatory cellular infilterate in the lobule of or circulating in blood sinusoids, presence or absence of kupffer cells, stellate cells, presence or absence of collagen in the Disse’s space intercellularly or intracellularly.
Therapeutic effects evaluation
This was based on Ishak modification of knoldell score of the liver biopsy. The patient was considered a responder to treatment when the grade of inflammation decreased 2 or more points and stage of hepatic fibrosis decreased at least 1 point at the end of the treatment course, compared with that before treatment.
Indexes of non-invasive tests assessment
In terms of clinical symptoms, blood liver function tests, and ultrasound detection data, these indices underwent thorough evaluation.
Safety evaluation
The unusual clinical symptoms and results from any tests performed during treatment were noted.
Statistical analysis
Statistical analysis was done via SPSS.9 software computer program. Results were expressed as means ± standard deviation of the means (SD). ANOVA test was used for multiple group comparison. Data were summarized also using cross tabulation. p value = p< 0.05was used to determine statistical significance.
Ethical consent
When the patient was still aware that the study would keep his or her medical records private at all times, informed consent was obtained from the patient’s family members or from the patient himself. When carrying out this research on humans, the World Medical Association’s Declaration of Helsinki, its code of ethics, was adhered to. (FWA 00010609).
Results
Selected patients
26 patients in all met the selection criteria, 11 females (42 %) and 15 males (58 %) (Table 1). All patients had a second liver biopsy at the end of treatment course (12 weeks).
Table 1: Mean values of laboratory data of the study group (26 patients).
|
|
Before |
After |
P value |
|
Hemoglobin (g/dl) |
13.64 ± 1.58 |
12.93 ± 2.24 |
NS |
|
WBCs (109/l) |
6.75 ± 1.85 |
6.24 ± 2.0 |
NS |
|
RBCs (1012/l) |
4.69 ± 0.63 |
4.84 ± 0.80 |
NS |
|
HCt. |
40.20 ± 4.98 |
36.0 ± 9.25 |
< 0.01 |
|
Platelet count (106/l) |
243.79 ± 70.45 |
218.91 ± 80.92 |
NS |
|
PT (sec.) (up to 11.7) |
12.31 ± 0.92 |
12.56 ± 0.99 |
NS |
|
Prothrombin conc. (%) (80-100%) |
86.76 ± 10.84 |
82.66 ± 13.92 |
NS |
|
PTT |
33.77 ± 3.68 (23) |
31.73 ± 2.57 |
NS |
|
AST (U/l) (5-38) |
52.0 ± 31.74 (24) |
46.64 ± 23.72 |
NS |
|
ALT (U/l) (5-41) |
57.75 ± 33.27 |
38.64 ± 29.78 |
< 0.05 |
|
T. bilirubin (mg/dl) (up to 1.1) |
0.58 ± 0.62 |
0.45 ± 0.13 |
NS |
|
Alk. Phosphatase (40-129) |
87.29 ± 28.20 (24) |
62.64 ± 15.94 |
< 0.01 |
|
Serum cholesterol |
155.58 ± 45.79 |
142.82 ± 31.74 |
NS |
|
Serum triglycerides |
151.58 ± 72.37 |
128.36 ± 37.64 |
NS |
|
HDL |
43.10 ± 15.01 |
32.82 ± 5.83 |
< 0.01 |
|
LDL |
84.13 ± 46.08 |
87.82 ± 33.42 |
NS |
|
Albumin (g/dl) (3.5-5) |
4.18 ± 0.44 |
3.83 ± 0.76 |
< 0.05 |
|
Total proteins (g/dl) (6.4-8.2) |
7.83 ± 1.66 |
7.75 ± 0.37 |
NS |
|
Globulin () () |
3.95 ± 0.73 |
3.92 ± 0.95 |
NS |
|
A/G ratio (%) |
1.10 ± 0.26 |
1.04 ± 0.31 |
NS |
|
Urea (mg/dl) (10-50) |
26.92 ± 8.63 |
27.6 ± 5.68 |
NS |
|
Serum creatinine ( up to 1.5 mg/dl) |
0.66 ± 0.13 |
0.75 ± 0.15 |
< 0.05 |
|
Serum Uric acid (3.4-7.0 mg/dl) |
4.41 ± 1.40 |
4.6 ± 1.71 |
NS |
|
INR |
1.10 ± 0.09 |
1.14 ± 0.12 |
NS |
|
PCR |
17059.25±30370.31 (24) |
218736.18±545950.34 (17) |
< 0.05 |
|
Grade of inflammation |
10.33 ± 3.32 |
8.42 ± 4.21 |
< 0.001 |
|
Stage of fibrosis |
2.33 ± 1.66 |
2.0 ± 1.67 |
< 0.05 |
Data were expressed as mean ± standard deviation.
NS= not significant; p< 0.05= significant; p< 0.01= highly significant; p< 0.001= very highly significant.
Analysis of observed indexes
Liver function (tab. 1, Fig 1):
The serum markers of inflammation (ALT, AST) and alkaline phosphatase (ALP) have improved after 12 weeks of treatment where the mean AST decreased from 52 to 46 (NS) while the ALT and ALP showed more decrease, where ALT dropped from 58 to 39 (P<0.05) and the ALP from 87 to 63 (P<0.001). Changes in other parameters of liver function tests, coagulation profile, kidney functions and lipid profile were all within the normal range.
Liver function tests (n=26)
 |
Figure 1: Mean values of liver function tests before and after treatment. *p < 0.05; **p< 0.001 relative to before treatment. |
Evaluation of results of PCR: Tab. (1) Fig. (2)
Follow up of viral kinetics by monitoring PCR count before and 12 weeks after the start of treatment showed increase of count, where after 12 weeks (end of treatment) the PCR raised from 17.059 to 218.736 (P<0.01).
 |
Figure 2: Mean PCR values before and after treatment. P< 0.01 relative to before treatment. |
Liver histological examination: Tab (1), Fig. 3,4.
Evaluation of hepatic fibrosis based on Ishak modification of Knodell’s score has shown marked improvement in hepatic fibrosis and inflammatory activity. The mean grade of inflammation dropped from 10.33 to 8.4 (P < 0.001) and the mean stage of fibrosis dropped from 2.33 to 2.0 (P value < 0.05).
 |
Figure 3: Grade of inflammation. |
 |
Figure 4: Stage of fibrosis. |
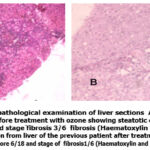 |
Figure 5: Histopathological examination of liver sections A: Section from patient liver before treatment with ozone showing steatotic changes Activity score 12/18 and stage fibrosis 3/6 fibrosis (Haematoxylin and Eosin stain, X200). |
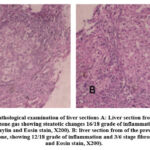 |
ِFigure 6: Histopathological examination of liver sections A: Liver section from patient before treatment with ozone gas showing steatotic changes 16/18 grade of inflammation and 4/6 stage fibrosis (Haematoxylin and Eosin stain, X200). |
Results of electron microscopy (EM)
 |
Figure 7 |
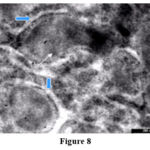 |
Figure 8 |
Ultrastuctural manifestations of HCV infection was disclosed in liver specimens exposed or not to ozone treatment. HCV infection was characterized by the significant dilatation or hypertrophy of the cysternae of rough endoplasmic reticulum. These cysternae were laded with electron dense granular materials (Fig.7).
Also, stage 4,5 and 6 liver samples the tubular configuration of the rough endoplasmic reticulum showed blind ballooned end filled with very fine electron dense proteinacious material or wrapping the mitochondria indicating that the cell was engaged in active protein synthesis. (Fig.8). Moreover electron dence C virus core proteins crowned the outer surface of the RER membrane especially in stage 5 and 6 (Fig 8).
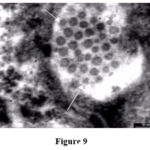 |
Figure 9 |
 |
Figure 10 |
Virus like particles of about 30-40 nm in diameter were often disclosed in the examined sections (Fig.9).
Condensation of cytoplasmic organelles around the nucleus was a common picture. The cytoplasm was occupied by electron dense granular proteinaciuous materials, polyribosomes, glycogen rosettes, and lipid deposits. Accumulated lipids appeared mainly as diffuse homogenous electron lucent deposits which look like hyaline material or it may take the form of circumscribed fat droplets (Fig. 10).
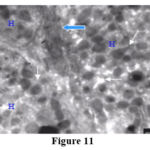 |
Figure 11 |
 |
Figure 12 |
In cases classified at the level of light microscopy stage 4,5 and 6, wide intercellular spaces filled with homogenous electron lucent materials with intervening intercellular microvilli was mainly seen (Fig. 11).
Moreover bile canaliculi with stunted or lost microvilli was a common finding (Fig. 12).
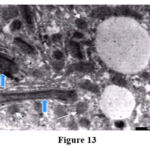 |
Figure 13 |
 |
Figure 14 |
Considerable glycogen deposits, collagen in the Disse’s spaces, inter or intracellularly were detected in stage 5 and 6 (Fig. 13).
On the other hand liver tissue exposed to ozone treatment revealed increase in peroxisomes and longitudinal orientation of mitochondrial cristae most probably engendered by increase exposure to oxygen (Fig. 13).
Moreover, stellate cells with intracytoplasmic fat droplets are not seen in the examined liver sections taken from the patients after ozone exposure. Meanwhile, stellate cells were often encountered in unexposed samples to ozone especially in cases classified as stage 1 and 2. (Fig.14).
 |
Figure 15 |
Signs of cellular regeneration in the form of binucleated cells, RER enveloping the mitochondria, and hepatocyte progenitors seen insinuating between the cells at the sinusoid pole are seen (Fig. 15). Also apoptotic hepatocytes are rarely seen.Circulating inflammatory cells in the sinusoids and infiltrating the lobule were decreased in four cases after ozone exposure.
Adverse effects
No clinically significant complication or side effect detected in a total number of settings exceeding 1000.
Discussion
Several chronic liver disease patients have severe and ongoing liver damage led to hepatic fibrosis, a precursor to cirrhosis, which is characterized by an unusual accumulation of extracellular matrix. 11, 12, 13,14. Liver fibrosis denotes a chief universal healthcare burden. Current rehabilitation is inadequate to eliminate the causative cause. This approach has been effective in some diseases, mainly in chronic viral hepatitis and haemochromatosis 2,15,16,17. While other patients seeking medical attention were in an advanced stage of fibrosis, many patients’ conditions rendered treatment impossible 18,19. The lack of liver transplant donors has prompted researchers to look for broadly applicable antifibrotic treatments 20.
Novel treatments for liver fibrosis are thus highly necessary. Over the past ten years, enormous advancements in our understanding of hepatic fibrosis have been made. Based on their capacity to undergo activation following liver injury from any cause, hepatic stellate cells play a crucial role 13, 21, 22, 23. It has been established that the majority of the excess extracellular matrix seen in chronic liver fibrosis is formed by hepatic stellate cells. The rational development of innovative antifibrotic medicines has been made possible by the thorough hepatic stellate cell biology knowledge 23. Based on the fundamental principles underlying the fibrogenic process, an effective treatment for hepatic fibrogenesis would likely also involve a number of other factors 24, 25, 26, 27.
Antifibrotic drugs should work on several aspects of the formation and genesis of hepatic fibrosis because it is currently believed that treating antihepatic fibrosis and hepatic fibrosis are two dissimilar thoughts. Additionally, liver injury and the activation of hepatic stellate cells are caused by significant mechanisms such as oxygen stress and lipid peroxidation. Therefore, lipid peroxidation inhibition was an important strategy of antihepatic fibrosis 22, 24,.
Oxidative stress is a key factor in liver damage. ROS including superoxide anions, hydroxyethyl radicals, hydroxyl radicals and hydrogen peroxide are generated from a numerous of insults such as alcohol metabolism, ischemia/reperfusion and drug/toxin metabolites. ROS were implicated in apoptosis and necrosis of hepatocytes, and share in HSC activation 3, 8. Numerous major classes of free radical scavengers, as glutathione peroxidase (Gpx), catalase and superoxide dismutase (SOD), as well as SOD mimics, were investigated in many forms of liver injury, and they provided the tissue with efficient defense against oxidative damage 29,30. The therapeutic response achieved by ozone, after repeated administration is through the oxidative stress induced in sub-injurious levels that can cause a preconditioning effect which is able to re-equilibrate the redox system changed by pathogenic stimuli.
LOPs can function as physiological messengers that can reactivate a disrupted biological system at submicromolar concentrations 6. All organs can be affected by LOPs in trace amounts, especially the CNS and bone marrow, notifying definite cell receptors of a calculated and minimal oxidative stress eliciting the adaptive response.
In this study EM showed that liver tissue exposed to ozone treatment revealed increase in peroxisomes. On an average, human hepatocytes (liver cells) show ~1,000 peroxisomes. Half-life of peroxisomes is only 5 days. Enzymes for oxidation are generally found in peroxisomes. Their key enzymes are peroxidase and catalase; further they have superoxide-dismutase, uratoxidase, and D-amino acid oxidase. Inducing an adaptation to oxidative stress through repeated administration of ozone in atoxic dosages enables mice to maintain hepatocellular integrity after CCl4 poisoning. Low doses of ozone increased antioxidant endogenous systems such as catalase, superoxide dismutase and glutathione 5,6,9 .Ultra-structural manifestations of HCV infection was disclosed in liver specimens exposed or not to ozone treatment. This coincides with our results as we have disclosed that mean PCR increased with ozone treatment in a period of 12 weeks from 17.059 to 218.736. This also coincides with the results of Sadek 8 where viral kinetics showed gradual increase of mean PCR after 10 and 20 days and 12 weeks. Circulating inflammatory cells in the sinusoids and infilterating the lobule were decreased in four cases after ozone exposure as seen on EM. This explains the significant decrease of liver enzymes in this study as well as in the study of Sadek 8 where ALT, AST and ALK.Ph Showed marked improvement. The grade of inflammation also improved on histopathology from 10.33 to 8.42 by Ishak’s score in this study compared to decrease from 10.08 to 7.94 in the study of Sadek 8.
Stellate cells with intracytoplasmic fat droplets are not seen in the examined liver sections taken from the patients after ozone exposure on EM examination. Meanwhile stellate cells were often encountered in unexposed samples to ozone especially in cases classified as stage 1 and 2. Signs of cellular regeneration in the form of binucleated cells, RER enveloping mitochondria, hepatocyte progenitors seen insinuating between the cells at the sinusoid pole. Also apoptotic hepatocytes are rarely seen on the contrary of their common existence in specimens before treatment. Hepatic fibrogenesis and inflammatory reactions are triggered by the chronic hepatocellular mortality caused by necrosis and/or apoptosis. Hepatocytes should ideally renew and replace dying cells; however, the imbalance of growth factors and the altered liver architecture and circulation frequently prevent hepatocellular regeneration in the case of chronic liver injury. Cellular apoptotic bodies and debris accumulate and cause inflammatory reactions, which may form a self-amplifying loop that further delay recovery from injury, and stimulates the fibrogenic process 31. In vitro, HSC might engulf the apoptotic bodies, and apoptotic bodies may also be phagocytosed by quiescent cells. Myofibroblast phenotypic transdifferentiation is made easier by HSC 32. . Hepatic biopsy samples from HCV infection and a rat model of bile-duct ligation both showed active HSC with engulfed apoptotic bodies. 33 . This explains the improvement of liver fibrosis after 12 weeks of ozone treatment where the mean stage of fibrosis dropped from 2.3 to 2.0 coinciding with the results of Sadek 8 where the mean stage of fibrosis dropped from 1.98 to 1.41 in a similar period of time of ozone treatment. The improvement of liver inflammation and fibrosis scores despite elevation of the mean PCR count is similar to the effect of treatment of fibrosis using interleukin-10. Interleukin-10 medication for 12 months reduced intrahepatic inflammation and fibrosis in individuals with chronic HCV infection-mediated fibrosis who had not responded to antiviral therapy (mean change from 5 + 0.2 – 4.5 + 0.3 P‹0.05) 21,34. During therapy, serum HCV RNA levels increased (mean HCV RNA at day 0, 12.3 + 3 mEq/ml; at 12 months 38 mEq/ml; P‹0.05).
Also there was an obvious shift of lymphocyte response towards a Th2 predominant phenotype. Many lines of evidence seem to support the idea that HCV infection is non cytopathic, including histologic examination of liver biopsies and the fact that the many HCV carriers have high titer virus expression and have normal liver enzymes and little or no damage in the liver 35,36,37, 38,39,40,41. Ozone is well known to affect the immune system, and induces production of interleukin-10 in the body on treatment using the major autohemotherapy or rectal insufflation routes 40- 43.
Conclusion
We concluded that Ozone oxygen gas mixture is a direct anti-fibrotic and anti-inflammatory agent in treatment of chronic hepatitis C patients without improving viral PCR as evidenced by histopathology, electron microscopy and quantitative PCR.
Acknowledgment
We thanks all the participants.
Conflicts of Interest
The authors declare that there is no conflict of interest.
Funding Sources
There is no funding Sources.
References
- Dienstag J L. Sexual and perinatal transmission of hepatitis C. Hepatology. 1997;26:665-705.
CrossRef - Schuppan D, Krebs, A., Bauer, M., and Hahn, E.G. Hepatitis C and liver fibrosis. Cell Death Differ. 2003;10 (Suppl. 1):S59-S67
CrossRef - Bataller R, Schwabe RF, Choi YH, Yang L, Paik YH, Lindquist J, Qian T, Achoonhoven R,Hagedorn CH, Lemasters JJ, Brenner DA, NADPH oxidase signal transduces angiotensin II in hepatic stellate cells and is critical in hepatic fibrosis. J Clin Invest 2003; 112: 1383- 1394.
CrossRef - Okuda M, Li K, Beard MR, et al. Mitochondrial injury, oxidative stress and antioxidant gene expression are inducd by hepatitis C virus core protein. Gastroenterology. 2002; 122: 366-375.
CrossRef - Bocci, V., 2002, Oxygen-ozone therapy. A critical evaluation, Kluwer Academic Publisher.
CrossRef - Bocci, V., 2005, Ozone- A new medical drug. Springer academic publisher.
- Candellario-Jalil, E, S Mohammed- Al-Dalain, O S Leon Fernandez, S M Menendez, G Perez-Davidson, N Merino, S Sam and H H Ajamieh. Oxidative Preconditioning Affords Protection Against Carbon Tetrachloride-induced glycogen Depletion and Oxidative Stress in Rats J Appl Toxicol. 2001; 21 () 297-301
CrossRef - Sadek A, Elbasha H , Abd EL Hady A, Lotfy H, Hamed S, Hammam O, Aql S, Alashry M. Effect of ozone therapy with and without Anti-oxidants on viral kinetics and liver histopathology, in hepatitis C patients: A phase III clinical study 1st international ozone congress 16 – 18 June 2006 Istanbul- Turkey.
- Ishak K, Baptista A, Bianchi L et al. Histological grading and staging of chronic hepatitis. J Hepatol 1995; 22: 696-699.
CrossRef - Knodell RG, Ishak KG, Black WC, Chen TS, Criag R, Kaplowitz N, Kiernan TW, Wollman J. Formulation and application of a numerical scoring system for assessing histological activity in asymptomatic chronic active hepatitis. Hepatology. 1981; 1: 43-435.
CrossRef - Albanis E, Friedman SL. Hepatic fibrosis. Pathogenesis and principles of therapy. Clin Liver Dis. 2001; 5: 315-334.
CrossRef - Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J Gastroenterol .1997; 32: 424-430
CrossRef - Li D, Friedman SL. Liver fibrogenesis and the role of hepatic stellate cells: new insights and prospects for therapy. J
Gastroenterol Hepatol. 1999; 14: 618-633
CrossRef - Poli G, Parola M, Oxidative damage and fibrogenesis. Free Radic Biol Med. 1997; 22: 287-305
CrossRef - Yu YY, Wang QH, Zhu LM, Zhang QB, Xu DZ, Guo YB, Wang CQ, Guo SH, Zhou XQ, Zhang LX. A clinical research on oxymatrine for the treatment of chronic hepatitis B. Zhonghua Ganzangbing Zazhi 2002; 10: 280-281
- Dong Y, Xi H, Yu Y, Wang Q, Jiang K, Li L, Effects of oxymatrine on the serum levels of T helper cell 1 and 2 cytokines and the expression of the S gene in hepatitis B virus S gene transgenic mice: a study on the hepatitis B virus mechanism of oxymatrine. J Gastroenterol Hepatol 2002; 17: 1299-1306.
CrossRef - Xie Y, Zhao H, Dai WS, Xu DZ. HBV DNA level and antigen concentration in evaluating liver
- Chen Y, Li J, Zeng M. Lu L, Ou D, Mao Y, Fan Z, Hua J, The inhibitory effect of oxymatrine on hepatitis C virus in vitro. Zhonghua Ganzangbing Zazhi. 2001; 9(Suppl): 12-14.
- Yang W, Zeng M, Fan Z, Mao Y, Song Y, Jia Y, Lu L, Chen CW, Peng YS, Zhu HY. Prophylactic and therapeutic effect of oxymatrine on D-galactosamine-induced rat liver fibrosis. Zhonghua Ganzangbing Zazhi. 2002; 10: 193-196.
- Fallowfield JA, Iredale JP, Reversal of liver fibrosis and cirrhosis – an emerging reality ( SMJ 2003 49(1): 3-6.
CrossRef - Tsukamoto H, Rippe R, Niemela O, Lin M. Roles of oxidative stress in activation of Kupffer and Ito cells in liver fibrogenesis. J Gastroenterol Hepatol. 1995; 10(Suppl 1): S50-53
- Kim KY, Choi I, Kim SS. Progression of hepatic stellate cell activation is associated with the level of oxidative stress
rather than cytokines during CCl4-induced fibrogenesis. Mol Cells. 2000;10: 289-300. - Oakley F, Trim N, Constandinou CM, Ye W, Gray AM, Frantz G, Hillan K, Kendall T, Benyon RC, Mann DA, Iredale JP. Hepatocytes express nerve growth factor during liver injury: evidence for paracrine regulation of hepatic stellate cell apoptosis. Am J Pathol 2003; 163: 1849-1858.
CrossRef - Poli G. Pathogenesis of liver fibrosis: role of oxidative stress. Mol Aspects Med. 2000; 21: 49-98.
CrossRef - Bjorneboe A, Bjorneboe GE. Antioxidant status and alcohol-related diseases. Alcohol Alcohol. 1993; 28: 111-116.
- Coutinho EM, Barros AF, Barbosa A Jr, Oliveira SA, Silva LM, Araujo RE, Andrade ZA. Host nutritional status as a contributory factor to the remodeling of schistosomal hepatic fibrosis. Mem Inst Oswaldo Cruz. 2003; 98: 919-925.
CrossRef - Perrillo RP. Management of the patient with hepatitis B virus-related cirrhosis. J Hepatol. 2003; 39(Suppl 1): S177-180.
CrossRef - Wu J, Zern MA. Hepatic stellate cells: a target for the treatment of liver fibrosis. J Gastroenterol. 2000; 35: 665-672 damage of patients with chronic hepatitisB. Hepatobiliary Pancreat Dis Int. 2003; 2: 418-422.
CrossRef - Nguyen WD, Kim DH, Alam HB, Provido HS, Kirkpatrick JR. Polyethylene glycol-superoxide dismutase inhibits lipid peroxidation in hepatic ischemia/reperfusion injury. Crit Care .1999; 3: 127-130.
CrossRef - Ferret PJ, Hammoud R, Tulliez M, Tran A, Trebeden H, Jaffray P, Malassagne B, Calmus Y, Weill B, Batteux F. Detoxification of reactive oxygen species by a nonpeptidylmimic of superoxide dismutase cures acetaminophen-induced acute liver failure in the mouse. Hepatology. 2001; 33: 1173-1180.
CrossRef - Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology. 2004; 39: 273-278.
CrossRef - Canbay A, Higuchi H, Bronk SF, Taniai M, Sebo TJ, Gores GJ. Fas enhances fibrogenesis in the bile duct ligated mouse: a link between apoptosis and fibrosis. Gastroenterology. 2002; 123: 1323-1330.
CrossRef - Torok N, Wu J, Zern MA, Halsted C, French S, Friedman SL, Zhan SS. Phagocytosis of apoptotic bodies by hepatic stellate cells occurs in vivo and is an important mechanism in liver fibrogenesis. Digestive Disease Week (DDW), May 14- 19, 2005, Chicago, IL (oral presentation, Abstract No. 80). Gastroenterology. 2005; 125: 4(S2): A15.
- Nelson DR, TU Z, Soldevila-Pico C, et al. Long term interleukin-10 therapy in chronic hepatitis C patients has a proviral and anti-inflammatory effect. Hepatology. 2003;38:859.
CrossRef - Rodriguez-Inigo E, Bartolome J, de Lucas, S et al. Histological damage in chronic hepatitis C is not related to the extent of infection in the liver. Am J Pathol. 1999; 154:1877-81.
CrossRef - Romeo R, Colombo M, Rumi M, et al. Lack of association between type of hepatitis C virus, serum load and severity of liver disease. J Viral Hepat.1996;3:183-90.
CrossRef - Rhee S G, Bae Y S, Lee S R and Kwon J. Hydrogen peroxide a key messenger that modulates protein phosphorylation through cysteine oxidation . Sci STKE. 2000; 10 53 PEI.
CrossRef - Barnes P J, Karin M. Nuclear Factor Kappa B: a pivotal transcription in chronic inflammatory diseases. N Engl J M. 1997; 336: 1066-1071.
CrossRef - Bocci V, Paulesu L. Studies on the biological effects of ozone I. Induction of interferon gamma on human leucocytes. Haematolgica.1990; 57: 510-515.
- Bocci, V 1999a. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Brit. J Biomed. Sci. 56: 54-60.
- Valacci G, bocci V, 2000. Studies on the biological effects of ozone. Release of factors from human endothelial cells. Mediat.Inflamm. 9: 271-276.
CrossRef - Bocci, V 1999. Biological and clinical effects of ozone. Has ozone therapy a future in medicine? Brit. J Biomed. Sci. 56: 54-60.
CrossRef - National Institutes of Health. NIH Consensus Development Conference Statement: Management of Hepatitis C 2002: A Preliminary Draft Statement, June 10-12, 2002. Bethesda, Maryland: NIH Consensus Development Program; 2002.







