Subhayan Sur1* and Ratna B. Ray2
and Ratna B. Ray2
1Cancer and Translational Research Centre, Dr. D Y Patil Biotechnology and Bioinformatics Institute, Dr. D Y Patil Vidyapeeth (DPU), Pimpri, Pune, India.
2Department of Pathology and Cancer Center, Saint Louis University, MO, USA.
Corresponding Author E-mail: subhayan.sur@dpu.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2767
Abstract
Worldwide, cancer incidence and mortality are rising quickly. Cancer remains the biggest cause of death despite advances in therapy. Plants produce bioactive phytochemicals, and as a result, the bioactive elements have long been the focus of cancer research, both for medication discovery and for the discovery of alternative chemo-preventive methods. The medicinal plant Momordica charantia or bitter melon contains a wide variety of phytochemicals, such as triterpenoids, triterpene glycosides, phenolic acids, flavonoids, lectins, sterols, and proteins. In many pre-clinical systems, the Momordica charantia extract exhibits an anti-cancer action against various malignancies. The bioactive components of the extract play a significant role in its anti-cancer properties. With an emphasis on underlying molecular pathways, we address the roles of Momordica's known bioactive components in several cancer models in this review. Through the inhibition of cancer cell proliferation and induction of cell death, several of active ingredients exhibit cancer prevention and therapeutic effects, at least in in-vitro models. Mechanistically examining the active components in pre-clinical systems may reveal a novel approach to cancer treatment.
Keywords
Bitter Melon; Cancer; Cancer Prevention; Cancer Therapy; Momordica charantia; Phytochemical
Download this article as:| Copy the following to cite this article: Sur S, Ray R. B. Emerging Potential of Momordica’s Bioactive Phytochemicals in Cancer Prevention and Therapy. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Sur S, Ray R. B. Emerging Potential of Momordica’s Bioactive Phytochemicals in Cancer Prevention and Therapy. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3udJXGG |
Introduction
Cancer is a major cause of death in both developed and developing countries apart from stroke, coronary heart disease, and the COVID-19 pandemic. The overall cancer incidence and mortality are growing rapidly in the world. According to the GLOBOCAN 2020, there were around 10.0 million new cases of cancer (excluding non-melanoma skin cancer) and 19.3 million cancer deaths (excluding non-melanoma skin cancer) in the world1. The GLOBOCAN 2020 predicts a 47% increase in global cancer incidence from the year 2020 to 2040. In the United States, the projected incidence of new cancer was 1,958,310 with 609,820 deaths to occur in 20232. The most prevalent cancers in men are prostate, lung, and colorectal cancers which account for 48% of all new incidents with prostate cancer alone accounting for 29% in the USA. In women, breast, lung, and colorectal cancers account for 52% while breast cancer alone accounts for 31%2. In addition to spontaneous mutation, external causes like smoking, drinking, viral or bacterial infection, and eating a poor diet increase the risk of uncontrolled cell proliferation3. Despite significant improvements in therapies and technologies, cancer is the first or second leading cause of death in the majority of countries throughout the world, and it is the second highest cause of death in the United States1,2. The best ways to manage diseases are early detection and prevention, which can be accomplished by lowering the risk from environmental and lifestyle variables. By removing these external risk factors, over 80% of human malignancies could be prevented4,5. For example, the results of the Pap test and HPV vaccines against cervical cancer are exceptional achievements that highlight the value of early detection and prevention in treating the illness across a large population. 6. The major challenges in cancer therapy are tumor heterogeneity, acquired resistance, and metastasis7. Significant advancements in targeted therapies have been made in the past few years; however, those methods show limited success with several side effects and are sometimes expensive. To effectively manage the disease, it is crucial to comprehend the disease process and find better therapeutic and preventive approaches. This will allow for the provision of high-quality cancer care that is both accessible and inexpensive.
For a very long period, dietary phytochemicals have been a focus of cancer research, including Leucovorin in the year 1950, Carzinophilin in 1954, Vincristine in 1963, and Actinomycin D in 1964 8,9. Plants can produce a large number of bioactive phytochemicals and thus, for many decades, the natural sources provided the basis for drug discovery. Half of all cancer drugs and antibiotics were discovered from natural sources 10. For example, the medications podophyllotoxin and its derivatives (topotecan, irinotecan), vinca alkaloids (vinblastine, vincristine, vindesine, vinorelbine), taxanes (paclitaxel, docetaxel), and anthracyclines (doxorubicin, daunorubicin, epirubicin, and idarubicin) are all produced from either plant or microbial sources.11.A recent report showed that out of a total of 1881 approved drugs in the last four decades (year 1981- 2019), 929 drugs (anticancer, antibacterial, antifungal, antiviral and antiparasitic) originated from natural sources; whereas others are synthetic drugs with natural pharmacophores or synthetic analogs of natural product9. Thus, research with bioactive phytochemicals is an emerging field in drug discovery and disease management. Many pre-clinical and clinical investigations with newly identified bioactive components or their analogs are now underway. All of these studies show the importance of phytochemicals as a single agent or in combination with conventional therapy in the treatment of various cancer types.
In the present review, we have focused on the bioactive components of Momordica charantia which is commonly known as bitter melon, in cancer prevention and therapy. The Momordica charantia is a tropical and sub-tropical vine and widely cultivated in Asia, Africa, and South America. The plant is popular due to its medicinal value since ancient ages for the treatment of diabetes, diarrhea, and toothache 12. In many pre-clinical models of cancer, such as those of the blood, breast, colon, head and neck (or oral), kidney, liver, lung, ovary, pancreatic, prostate, skin, stomach, and uterine cervix, crude extracts of the fruit, leaves, or seeds of Momordica charantia have shown potential anti-cancer actions13-19. The plant includes more than 30 therapeutic compounds and has the most significant nutritional content of all cucurbits13. In the present review, we have focused on the active phytochemicals of Momordica in cancer prevention and therapy with recent evidence. Thus, this review may be helpful for basic researchers and pharmacologists for attracting and/or designing future mechanistic pre-clinical and clinical studies, and drug development using pure components against different types of cancer.
Methods
In this review, we have discussed the role of active phytochemicals of Momordica charantia or bitter melon in cancer prevention and therapy. Based on available studies from “PubMed,” “PubMed Central,” “Google Scholar,” “Science Direct,” and “Semantic Scholar,” we have summarized the effects of every compound separately, identified from the year 1980 to the present, their isolation methods, source in the plant, chemical composition, metabolism, bioavailability, toxicity, chemical modifications, specific mode of action, and future research direction in various cancer models. ‘Bitter melon’, ‘Momordica charantia’, ‘cancer’, ‘cancer prevention’, and ‘anticancer’ are the search terms utilized. The data included in this study are from research using in-vitro or in-vivo cancer models; while the information on opinions, conference proceedings, news stories, or material specific to a certain profession are excluded.
Momordica charantia and its phytochemicals:
The herbaceous plant Momordica charantia has a bitter flavor and slender stems, tendrils, bright yellow blooms, and light green fruits (Figure 1A). The plant contains a large number of phytochemicals and many of those showed potential biological roles. Depending on the varieties of extraction methods, researchers reported varying amounts of chemical constituents in the plant extract. The plant contains 94% water, 3.7% carbohydrate, 1% proteins, 0.17% fat, fibers, vitamins (A, B-complex, and C); minerals including Ca, Zn, Mg, K, Fe, and P; free amino acids like aspartic acid, alanine, γ-amino butyric acid, glutamic acid, serine, and threonine; fatty acids including linoleic acid, linolenic acid, palmitic acid, oleic acid, stearic acid, and arachidonic acid; essential oils (α and β pinene, octanal, 1, 8-cineole, β-phellandrene, c-dihydrocarveol, carvone, safrole, methyl- eugenol, germacrene D, β-selinene, α-selinene, and myristicin) (Figure 1B) 14,18. The leaf and fruit contain higher amounts of carbohydrates than the seeds. Whereas, the seeds contain a high amount of fat and fibers; the protein content is almost comparable in different plant parts 20. Some of the identified and well-characterized proteins are α- and β-momocharin or momordica antiviral protein 30kDa (MAP30), 14 kDa Ribonucleases (RNase MC2), polypeptide-k, and marmorin13,17,18. Other constituents are phenolic acids and flavonoids which include gallic acid, tannic acid, (+)-catechin, caffeic acid, p-coumaric, gentisic acid, chlorogenic acid, and epicatechin 13.
The cucurbitane-type triterpenoids and cucurbitane-type triterpene glycoside are major phytochemicals in the plant and responsible for the bitterness of the plant 13,17,18. A total of 28 secondary metabolites were found in the water extract of fruits (without seeds) after being subjected to liquid chromatography high-resolution electrospray ionization mass spectrometry analysis (LC- HRESIMS). Of these, 4 metabolites belonged to the cucurbitane-type triterpenoids and 20 to the cucurbitane triterpene glycosides21. The cucurbitane-type triterpenoids were momordicine I (M-I); 7, 23-dihydroxy-3-O-malonylcucurbita-5, 24-dien-19-al; (23E)-cucurbita-5,23,25-triene-3,7-dione (Figure 1C). A new malonylcucurbita-trien-19-al derivative of cucurbitane-type triterpenoid was also identified as having a molecular formula of C33H48O721. Other cucurbitane-type triterpenoids reported in other studies are charantin, momordicine II and III, karavilagenin A, B, C, D and E, and kuguacins A-S 13,17,18(Figure 1C).
The cucurbitane-type triterpene glycosides in the water extract of fruit identified by LC-HRESIMS were momordicoside-B, C, K, L, N, O, V; momorcharaside-A and B, Karaviloside-II, III, X, XI; Goyaglycoside A, C, F; and charantosides-I, IV, V, VII21 (Figure 1C). Additionally, some other studies reported charantosides I-VIII, andkuguaglycoside13,17,18. Moreover, three monoterpenoid glycosides (vomifoliol β-D-glucopyranoside, sacranoside A and myrtenyl O-β-D-glucopyranoside) and one oleanane-type triterpene saponin (goyasaponin III) were identified in the extracts by the LC- HRESIMS21. Interestingly many of the compounds are not well characterized and have unknown biological activity.
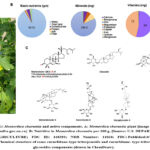 |
Figure 1: Momordica charantia and active components. A: Momordica charantia plant [image source: www.omafra.gov.on.ca]. B: Nutritive in Momordica charantia per 100 g. |
Momordica charantia extract and cancer
The Momordica charantia or bitter melon crude extract (BME) isolated from fruit, leaf,or seed was studied in different cancer models. Many studies show that crude extract or a mixture of compounds acts better than individual pure compounds in an in-vivo system. The crude extract can be made using a variety of solvents, including water, acetone, methanol, ethanol, and n-butanol. The BME demonstrated potential anti-cancer properties against a variety of cancer types, including malignancies of the blood, breast, colon, head and neck, kidney, liver, pancreas, ovary, skin, stomach, and uterine cervix (Figure 2).
Momordica charantia‘s putative anti-tumor properties were initially noted in a mouse lymphoma xenograft model, where the extract’s ammonium acetate precipitates inhibited tumor growth and stimulated immune response 22. The cells were prior exposed to the extract in-vitro before animal injection. The study reported no toxic side effects of the dose to normal mice (22). Similar to this, the water extract of fruit inhibited breast cancer cell proliferation in both in vitro and in vivo models, with superior results against a triple-negative breast cancer model23-25. The BME dose was non-toxic to normal mice or cell lines. The extract caused G2/ M phase cell cycle arrest, enhanced p53, p21, pChk1/2, inhibited cyclin B1 and cyclin D1 expression and induced apoptosis by increasing PARP cleavage and caspase activation in MCF-7 and MDA-MB-231 cells [23]. In breast cancer syngeneic and xenograft mice models, the extract could increase both apoptosis and autophagy through modulation of AMPK/ mTOR pathways24. Further in the TNBC model, the extract inhibited the accumulation of esterified cholesterol by inhibiting acyl-CoA: cholesterol acyltransferase 1 (ACAT- 1), sterol regulatory element-binding proteins-(SREBP-1 and -2), and FASN expression of lipid metabolism25.
A diet containing 0.01% – 1% Momordica charantia seed oil prevented azoxymethane-induced rat colon cancer development by enhancing peroxisome proliferator-activated receptor- gamma (PPARγ)proteinexpression26. Furthermore, a rise in the lipid component of CLA (c9, t11-18:2) was seen in the colonic mucosa and liver. In the human colon cancer cell line (HCT1116), the water extract of seeds showed potential anti-cancer effects by activation of PARP cleavage 27. The methanolic extract of whole fruit showed a better anticancer effect as compared to only skin extract on colon cancer cell lines28. The whole fruit extract effectively inhibited proliferation, colony formation, sphere formation, induced S-phase arrest and autophagic cell death in colon cancer cells 28.
A diet containing Momordica‘s fruit extract prevented benzo-(a)-pyrene-induced forestomach papillomagenesis in Swiss albino mice29. In addition, the methanol extract of the leaf inhibited proliferation and induced apoptosis in different human cancer cell lines, including nasopharyngeal carcinoma cells (Hone-1), gastric adenocarcinoma cells (AGS), colorectal carcinoma cells (HCT- 116), and lung adenocarcinoma cells (CL1-0) in a dose-dependent way30.
In head and neck cancer models, the aqueous extract of fruit suppressed tumor growth in vitro and in vivo by reducing the expression of c-Met and its downstream signaling components phospho-Stat3, c-Myc, and Mcl-131. The BME also induced T-regulatory cell activation and stimulated NK cell- mediated cytotoxicity of head and neck cancer cells32. Furthermore, regular oral drinking protected carcinogen-induced tongue squamous cell cancer development in a mouse model while having no harmful effect in normal animals33. RNA sequence analysis followed by subsequent validation showed that the BME inhibits pro-inflammatory genes s100a9, IL23a, IL1β and immunological checkpoint gene PDCD1/PD1 during cancer prevention33. In addition, the extract could modulate glucose metabolism by significantly reducing pyruvate and lactate levels by inhibiting key regulatory genes of the glycolysis pathway (GLUT-1, PFKP, LDHA, PKM, and PDK3)34. Also, in lipid metabolism, the BME significantly reduced membrane phospholipids- phosphatidylcholine, phosphatidylethanolamine and plasmenylethanolamine, and inhibited calcium-independent Phospholipase A2 (iPLA2) as well as fatty acid biogenesis genes (ACLY, ACC1, and FASN), which resulted in the endoplasmic reticulum (ER) stress and reactive oxygen species (ROS) associated cell death34.
Pre- and post-treatment of methanolic extract of bitter melon prevented diethyl-nitrosamine (DENA) and carbon tetrachloride (CCl4) induced rat hepatocellular-carcinoma (HCC) development by decreasing Cox-2, VEGF, HDAC and MMP-2,-9 and increasing expression of Caspase- 3, and 831. The water and methanol extract of the leaf inhibited human non-small cell lung cells A549 and lung adenocarcinoma cells CL1 in a dose-dependent manner respectively32,33. The anticancer mechanism was linked to increased apoptosis, ROS production, and activation of Src and FAK, which reduced the expression of downstream Akt, β-catenin, and MMPs.
The water extract of fruit inhibited proliferation, induced apoptosis and activated AMPK in human pancreatic carcinoma cells (BxPC-3, MiaPaCa-2, AsPC-1 and Capan-2) and xenograft model in nude mice38. The extract demonstrated potential efficacy against gemcitabine-resistant pancreatic cancer cells, as well as decreased Akt and ERK1/2 phosphorylation39. Furthermore, in pancreatic cancer models, bitter melon extract suppressed sphere formation and decreased cancer stem cell-related genes SOX2, OCT4, NANOG, and CD4440. In the pancreatic carcinoma model, bitter melon extract also modulated glucose metabolism and lactate export by blocking the GLUT1 and MCT4 transporters41. Interestingly, the combinatorial treatment of BME with gemcitabine significantly reduced pancreatic cancer patient-derived xenograft tumor growth in mice 34.
Bitter melon extract inhibited prostate cancer cell PC3 and LNCaP proliferation in vitro, and induced S-phase cell cycle arrest by modifying expression of Cyclin D1, Cyclin E, and p21, and increased apoptosis by increasing Bax and PARP cleavage43. In TRAMP (transgenic adenocarcinoma of mouse prostate) mice, oral gavage of bitter melon extract as a dietary component prevented the progression of high-grade prostatic intraepithelial neoplasia43. Bitter melon leaf extract lowered MMP-2 and MMP-9 levels in a rat prostate cancer cell line and enhanced rat survival in a prostate cancer metastasis model44. A diet containing bitter melon leaf extract (1% and 5%) inhibited PC3 xenograft development by 63% and 57%, respectively, with no deleterious effect on mice’s body weight45. The aqueous extract suppressed rat prostatic adenocarcinoma by inhibiting the G2/M phase of the cell cycle and cyclic GMP46.
In a DMBA-induced skin tumorigenesis model, mice given Momordica fruit and leaf extracts at doses of 500 and 1000 mg/ kg body weight for 30 days had a longer life span and tumor volume was significantly reduced when compared to control values47. Similarly, oral administration of the fruit extract protected against the formation of skin tumors and enhanced life expectancy by reducing lipid peroxidation in the liver and DNA damage in lymphocytes48. Moreover, the fruit extract was observed to strongly stimulate the glutathione-S-transferase, glutathione peroxidase, and catalase during cancer prevention. Similarly, the BME showed a potential cytotoxic effect against adrenocortical cancer cells 35, ovarian cancer cells 36 and cervical cancer cells37.
Most of the studies reported the non-toxic effect of the extract either in normal cells or normal animal models. Different Momordica charantia cultivars are geographically available. A comparative study reported the similar anticancer effects of different Momordica charantia varieties on pancreatic cancer pre-clinical models38. As shown in Figure 2, the mechanism of cancer therapy and prevention against many cancer types is more or less comparable. Pre- or post-initiation stages of carcinogenesis determine the therapeutic or preventative effects of the extracts.
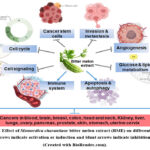 |
Figure 2: Effect of Momordica charantiaor bitter melon extract (BME) on different cancers. |
Momordica charantia bioactive phytochemicals in cancers
The Momordica charantia plant includes a variety of phytochemicals, and the crude extract’s biological activity is influenced by the interaction of these bioactive substances. The next section and Table 1 provide an overview of some bioactive phytochemicals’ anti-cancer properties.
Momordicine-I (M-I)
The Momordicine-I or M-I (C30H48O4) is a cucurbitane-type triterpene. The secondary metabolite was initially discovered in plants’ leaves and vines39. The fruit extracts in ethanol, n-butanol, and water have all been found to contain the same substance 21,40,41. The M-I was discovered to be effective in promoting insulin secretion from pancreatic beta cell lines 41 and reducing diabetes- related cardiac fibrosis in rats42.
The anticancer role of M-I was studied in oral cancer (head and neck) models. The M-I inhibited human oral cancer cells in a dose-dependent way (IC50 dose at 48 hr= 7µg/mL in Cal27, 17 µg/ mL in JHU022, and 6.5 µg/ mL in JHU029 cells) 21. Mechanistically the M-I inhibited the c-Met expression resulting reduction of downstream signaling molecules c-Myc, STAT3, survivin, and cyclin D1 (Figure 3). In the oral cancer xenograft model, daily IP injection of M-I could regress tumor growth in nude mice by inhibiting c-Met signaling 21. Oral cancer is frequently characterized by increased expression of c-Met signaling which is linked to metastasis and a poor prognosis 43. The Momordica crude extract also has a similar function to M-I in the inhibition of c-Met signaling in the prevention of oral cancer 44. Thus, it appears that one of the main factors influencing Momordica‘s anti-cancer effectiveness may be the M-I.
A pharmacokinetic study showed that the M-I is quite stable in mouse blood and maximum peak concentration is achieved at 1 hr post-IP and oral treatment (PO) with mean clearances at 30.8 mL/ min/ kg (IP) and 534.8 mL/ min/ kg (PO) 21. A drug permeation study using human intestinal epithelial cell monolayers (Caco-2) showed permeation across the basolateral side with an apparent permeability coefficient at 8.12 × 10(-6) 40. In addition, a small amount of the M-I was found to be absorbed inside the Caco-2 cells. The substance is not toxic to mice as evidenced by the lack of alterations in body weight and blood parameters associated with liver and kidney functions after routine IP administration21. Normal oral keratinocytes (NOK) are the least affected by the substance. Thus, M-I may be an important therapeutic agent in the treatment of oral cancer. However, more pre- clinical studies are needed in the presence of intact immune systems in this regard. Also, the role of M-I in other cancers is not known and needs to be evaluated.
 |
Figure 3: Mode of action of Momordicine-I (M-I) in inhibition of c-Met signaling in oral cancer prevention (created with BioRender.com). |
Momordica antiviral protein 30 kd (MAP30) or β momorcharin
The MAP30 or β momorcharin is one of the well-studied phytochemicals of Momordica charantia which is highly present in mature fruit and seeds45. It is a 30 kDa molecular weight type I single- chain ribosome-inactivating protein. It has an immune-modulatory function and has antiviral properties against the herpes simplex virus (HSV) and the human immunodeficiency virus (HIV)46. Additionally, MAP30 has anti-cancer properties against several malignancies.
The MAP30 showed potential anticancer effects in different in vitro cancer models including acute myeloid leukemia47, glioma 48,breast cancer 49, hepatocellular carcinoma 46, lung adenocarcinoma 50, ovarian and prostate cancers in a time-dependent and dose-dependent way by inhibiting cellular proliferation, migration, invasion and inducing S-phase cell cycle arrest and apoptosis51,52. In ovarian cancer cells, the MAP30 was found to induce cisplatin sensitivity upon combinatorial treatment 52. Importantly, the MAP30 dose was found to be non-toxic in normal cells 51,52.
In-vivo anti-cancer effect of MAP30 was studied in various pre-clinical models. The MAP30 treatment effectively prevented xenograft tumor growth of breast, liver, and prostate cancer, ovarian cancer ascites in a mouse model, and increased mice survival 46,49,51,52. It is worth noting that the MAP30 showed no toxic effect on liver and kidney function-related serum parameters in the treated mice 52.
The MAP30 protein was modified by mono-PEGylation and the modified MAP30 was seen as similar cytotoxic to the A549 cells 53. Liposomal MAP30 showed a better cytotoxic effect on bladder cancer cells T24 54. As the Momordica charantia plant contains traces of MAP30, researchers have cloned the gene, expressed it in bacterial culture, and generated recombinant MAP30. The recombinant MAP30 also inhibited cell proliferation and migration, and induced apoptosis in human bladder cancer cell T24 54, colorectal carcinoma LoVo55, and uterine cervical cancer cell HeLa 56 in a time-dependent and dose-dependent manner. In an animal model, the recombinant MAP30 inhibited bladder cancer xenograft tumor growth in mice 54. It significantly increased the level of reactive oxygen species (ROS), but reduced glutathione levels and activities of catalase and glutathione peroxidase in the prevention of bladder cancer. However, histological analysis showed mild changes in the liver and kidney following MAP30 treatment in the mice 54.
Mechanistically, the MAP30 is a ribosome-inactivating protein 57 (Figure 4). It binds both ribosomal RNA and the HIV-1 long-terminal repeat. Apart from that, it acts as a potent inhibitor of histone deacetylase-1 (HDAC-1) resulting in modulation in gene expression 51. The MAP30 inhibited the self-renewal Wnt pathway by reducing the expression of effector molecule β-catenin and its downstream genes c-Myc and Cyclin D1 in glioma and prostate cancer models 48,51. The MAP30 also inhibited the expression of G-protein-coupled receptor 5 (LGR5), NF-kB, JNK and MMP2 and induced expression of PTEN and AMPK signaling 48,51,52,54. The MAP30 has a positive role in the induction of the expression of apoptosis genes Caspase 3, 8, and 9 46,54. It was observed that the MAP30 induces intracellular Ca2+ ion concentration, ROS level, and ROS-mediated apoptosis and ferroptosis52,54. Thus, MAP30 is one of the important contributors to the biological activity of Momordica and it may be a potential therapeutic agent against different cancers (Figure 4).
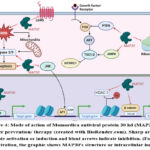 |
Figure 4: Mode of action of Momordica antiviral protein 30 kd (MAP30) in |
Alpha-eleostearic acid (αESA)
The αESA or (9Z,11E,13E)-octadeca-9,11,13-trienoic acid (C18H30O2), is an essential fatty acid present in Momordica charantia seed oil which contains 60% of αESA58. The ESA was found to be converted to conjugated linoleic acid (CLA; 9,11-18:2) in the liver and plasma in the rat model 59.
The potential anti-cancer effect of αESA was seen in human leukemia cells HL6060. The αESA inhibited proliferation, colony formation, induced G2/M cell cycle arrest, and apoptosis in breast cancer cells 58,61,62. Mechanistically, the αESA induced expression of PPARγ, p21, Bax, p53, and caspase-3 in the breast cancer cells. The αESA reduced mitochondrial membrane potential and induced translocation of apoptosis-inducing factor (AIF) and endonuclease G from the mitochondria to the nucleus resulting in apoptosis in breast cancer cells 58. In another study of a breast cancer model, the αESA was found to reduce the expression of HER2/ HER3 and increase the expression of PTEN resulting in reduced expression of activated Akt and its downstream signaling molecules GSK-3β and BAD proteins 62.
In addition, the αESA could decrease proliferation and induce DNA fragmentation in the human colon cancer cells Caco-2 and HT-2960,63. On the other hand, β-eleostearic acid showed a better effect in Caco-2 cells than the αESA64. The αESA also inhibited proliferation and induced both apoptosis and autophagy in human cervical cancer cell HeLa65. It inhibited the expression of phospho-AKT and phospho-P70S6K, increased phospho- ERK1/2 expression and conversion of LC3 I to LC3 II resulting in autophagy-mediated HeLa cell death. The αESA was also found to induce ferroptosis in diverse cancer cells66. The αESA was found to incorporate into cellular lipids and promote lipid peroxidation and cell death mediated by acyl-CoA synthetase long-chain isoform 166. The αESA increased the total antioxidant capacity in plasma and successfully maintained the RBC membrane integrity against stress indicating its protecting role 67. Thus, αESA may be a potential therapeutic agent in many cancers. However, one study reported the toxic effect of the compound on normal human fibroblast cell WI3860. Also, the effect of αESA in in-vivo cancer models is scarce. In addition, its bio-availability, stability, and toxicity information are not known clearly.
Kuguacin j (KuJ)
The KuJ (C30H46O3) is a cucurbitane-type triterpenoid present in Momordica charantia leaves and vines. The biological activity of KuJ was studied in in-vitro cancer cells. The compound was isolated from the methanol extract of leaves 68.
Similar to the crude extract from the leaf, the KuJ inhibited the proliferation of human prostate cancer cells LNCaP and PC3 in a time-dependent and dose-dependent manner 68,69. Both the leaf extract and KuJ showed minimum toxicity in the normal prostate cell line, PNT1A. It induced G1/S cell cycle arrest and apoptosis in the cells. KuJ effectively reduced the expression of cell cycle genes Cyclin D1, Cyclin E1, Cdk2, Cdk4, and PCNA, and induced the expression of p21 and p27 68,69. In apoptosis pathways, it induced Caspase-3 and PARP cleavage, Bax/ Bcl-2, and Bad/ Bcl-xL ratio and reduced survivin expression. In addition, it reduced migration and invasion-associated molecules MMP-2, MMP-9 and uPAin PC3 cells69.
The KuJ inhibited human ovarian cancer cells (SKOV3 and A2780) 70. Interestingly, co-treatment of KuJ with cisplatin or paclitaxel increased cytotoxicity in both cells. In addition, it induced apoptosis by increasing Caspase-3 and PARP cleavage and reducing the expression of survivin.
The KuJ also increased the sensitivity of vinblastine and paclitaxel in the human cervical carcinoma cell line (KB-V1)71. Mechanistically, the KuJ directly interacts with the drug-substrate-binding site on P-glycoprotein (P-gp) and thus inhibits the function of P-gp in the KB-V1 cells. The P-gp is a type of ABC drug transporter that induces efflux of chemotherapeutic drugs from cancer cells. However, induction of cisplatin or paclitaxel sensitivity in ovarian cancer cells by the KuJ was P-gp independent 70. Thus, KuJ may have a potential therapeutic role. However, further mechanistic studies in different cancer pre-clinical models are needed.
Momordica charantia lectin (MCL)
The MCL is a carbohydrate-conjugated protein isolated from Momordica charantia seeds. It is a type II ribosome-inactivating protein and shows some degree of sequence homology to β-momorcharin from Momordica charantia and to lectins from Cucurbita maxima, Cucurbita argyrosperma, Sambucusnigra and Ricinuscommunis72. MCL is an important contributor to the anti-viral effect and hypoglycemic effect of Momordica charantia extract 73.
The MCL inhibited protein and subsequently DNA synthesis in human peripheral blood lymphocytes of leukemia patients 74. The MCL inhibited the viability of HCC cells (HepG2 and PLC/PRF/5) in a dose-dependent and time-dependent way75. It induced G2/M phase cell cycle arrest, DNA fragmentation, mitochondrial injury, autophagy and apoptosis in the HCC cells. The MCL induced p38/ MAPK kinase pathway, Bid activation, PARP cleavage and expression of Caspase 8 and 9. In addition, MCL treatment prevented HCC xenograft growth in mice. The MCL in combination with sorafenib showed a better therapeutic effect against the pre-clinical HCC model without showing toxic side effects.
The MCL treatment inhibited proliferation in nasopharyngeal carcinoma (NPC) cell lines CNE-1 and CNE-2 without affecting normal cells NP69 76. Mechanistically, the MCL induced G1-phase cell cycle arrest, DNA fragmentation, mitochondrial injury and apoptosis in the NPC cells by modulating Cyclin D1 expression, RB and p38/ MAPK, JNK, and ERK phosphorylation. In addition, it induced cytochrome-c release, Caspases- 3, 8, 9 and PARP cleavage. Intraperitoneal administration of MCL effectively prevented CNE-2 xenograft tumor growth in nude mice 76. A combination of MCL with sorafenib showed a better therapeutic effect than the individual effect against the in-vivo NPC growth. No toxic side effect was seen during the MCL treatment in the mice. Thus, MCL may be a potential therapeutic agent.
Alpha momorcharin (α-MMC)
Alpha momorcharin (α-MMC) is another ribosome‑inactivating protein isolated from Momordica charantia seeds. The effect of α-MMC was studied on the human lung adenocarcinoma epithelial cell A549. The α-MMC inhibited cell proliferation in a dose‑dependent and time‑dependent manner and induced S-phase cell cycle arrest and apoptosis on the cells50. The α-MMC also inhibited proliferation and induced apoptosis in human breast cancer cells MDA-MB-231 and MCF-7 and xenograft tumor growth in mice77. However, α-MMC has severe in-vivo hepatotoxicity and stimulates inflammatory responses in human monocytes78,79. To reduce its immunogenicity and toxicity and to increase stability, α-MMC was PEGylated. The PEGylated α-MMC could preserve the anti-tumor effect against lung cancer cell A54953, in-vivo murine breast cancer cell (EMT-6) and human breast cancer cell (MDA-MB-231) transplanted mouse tumor model 80. Interestingly, the PEGylation increased the plasma half-life in rats and reduced its non-specific toxicity 80.
Other components
BG-4 Is a novel and low-molecular weight (4 kDa) protease inhibitor, isolated from Momordica charantia seeds. BG-4 showed a cytotoxic effect and induced apoptosis in human colon cancer cells HCT-116 and HT-29 81. The BG-4 reduced expression of Bcl-2 and increased expression of p21, Bax and Caspase-3 in the cells.
Two cucurbitane-type triterpene glycosides isolated from fruits: charantagenins-D and goyaglycoside-d with an –(OMe) substitution in side chain exhibited significant cytotoxic effects against lung cancer cell line A549, glioblastoma cell line U87, and hepatoma carcinoma cell line Hep3B 82.
Karaviloside-III is a cucurbitane-type triterpene glycoside isolated from immature fruit. It showed a potent cytotoxic activity against activated murine hepatic stellate cells (t-HSC/Cl-6) and human HCC cells Hep3B and HepG2 suggesting its potential role against hepatic fibrosis and cancer 83.
Kuguaglycoside C is a triterpene glycoside isolated from the leaves of Momordica charantia.
The Kuguaglycoside C showed cytotoxic effects against human neuroblastoma IMR-32 cells and induced caspase-independent cell death 84.
RNase MC2 is a 14-kDa unique ribonuclease isolated and purified from Momordica charantia seeds85. It showed RNase activity on tRNA in baker’s yeast, calf liver, and rRNAs from breast cancer cell MCF-7 85. The RNase MC2 inhibited cell proliferation and induced apoptosis in the MCF-7 and HCC cell HepG2 85,86. It induced phosphorylation of Akt, p38, JNK, and ERK, and increased Caspases 8, 9 and PARP cleavage 85,86. In addition, RNase MC2 prevented HepG2 xenograft tumor growth and induced apoptosis in nude mice suggesting its potential anti-cancer effect 86.
Plumericin, an iridoid lactone, isolated Momordica charantia vine, showed antibacterial and anti-proliferative activities 87. The compound potentially inhibited proliferation, and induced G2/M cell cycle arrest and apoptosis in leukemia cells NB4 and K562. It also inhibited proliferation and induced G2/M arrest in HCC cells Hep3B and HepG2 88. It significantly decreased expressions of COX 2 and VEGF in the cells.
A triterpenoid compound, 3β, 7β, 25-trihydroxycucurbita-5, 23(E)-dien-19-al (TCD), isolated from the whole plant, inhibited proliferation and induced autophagy in breast cancer cells MCF-7 and MDA-MB-23189. Mechanistically, the TCD down-regulated Akt-NF-κB signaling, induced p38/ MAPK, p53, inhibited expression of histone deacetylases, and increased ROS generation in the cells 89.
A similar compound, known as triterpenoid, 3β,7β-dihydroxy-25-methoxycucurbita-5,23-dien- 19-al, inhibited proliferation and induced apoptosis in breast cancer cells 90. Mechanistically, it modulated the expression of PPARγ and mTOR-p70S6K signaling molecules Cyclin D1, CDK6, Bcl-2, XIAP, COX-2, NF-κB, ERα, and Akt, activated AMPK and induced endoplasmic reticulum (ER) stress 90.
A series of cucurbitane-type triterpene glycosides isolated from methanol extract of the fruits were studied for anti-viral and anti-cancer effects 91. Among those, two compounds: (19R,23E)- 5β,19-epoxy-19-methoxy- cucurbita-6,23,25-trien-3β-ol and (19R,23E)-5β,19-epoxy-19,25- dimethoxy- cucurbita-6,23-dien-3β-ol showed inhibitory effects in dimethylbenz[a]anthracene (DMBA)- and peroxynitrite induced mouse skin carcinogenesis. In another study, 15 cucurbitane- type triterpene glycosides were isolated from ethanol extract of fruits including kuguaosides A-D, charantoside A, momordicosides I, goyaglycosides-b and -d, 7β,25-dihydroxycucurbita-5,23(E)- dien-19-al 3-O-β-d-allopyranoside, and 25-hydroxy-5β,19-epoxycucurbita-6,23-dien-19-on-3β-ol3- O-β-d-glucopyranoside. Many of those compounds showed anti-proliferative effects against MCF-7 (human breast adenocarcinoma), Doay (human medulloblastoma), HEp-2 (human laryngeal carcinoma), and WiDr (human colon adenocarcinoma) with IC50 values ranging from 10–20 μg/mL for 72 hrtreatment92.
Table 1: The anticancer effect of Momordica’s bioactive phytochemicals
|
Compound |
Compound type |
Isolat ed from |
Cancer type |
Cance r model |
Mechanism |
Phenotypic changes |
References |
|
Momordicin e-I (M-I) |
Cucurbitan e-type triterpene |
Fruit |
Head and neck cancer |
In- vitro and in- vivo |
Inhibits c-Met signaling |
Inhibits tumor growth |
(21) |
|
Momordica Antiviral Protein 30 Kd (MAP-30) |
Protein |
Fruit and seeds |
Cancers in breast, glioma, liver, lung, ovary, prostate and leukemia |
In- vitro and in- vivo |
Type I ribosome- inactivating protein;inhibitsHDAC1, Wnt signaling, reduces expression of LGR5, NF- kB, JNK, MMP2 and induces expression of PTEN AMPK, Caspase 3, 8 and 9, and induces ROS generation |
Inhibits cell proliferation, migration, invasion, induces S- phase arrest, apoptosis and chemotherapy sensitivity |
(58-70) |
|
Alpha- eleostearic acid (α-ESA) |
Fatty acid |
Seeds |
Cancers in breast, colon, cervix, and leukemia |
In- vitro |
Inhibits Akt signaling, induces expression of PPARγ, p21, Bax, p53, and Caspase-3 |
Inhibits proliferation, colony formation, induces G2/M cell cycle arrest, apoptosis, and autophagy |
(71- 79) |
|
Kuguacin J |
Cucurbitan e-type triterpenoid |
Leaf |
Prostate, ovary and cervical cancer |
In- vitro |
Inhibits P-glycoprotein, reduces expression Cyclin D1, Cyclin E1, Cdk2, Cdk, PCN, survivin, MMP-2, MMP-9 and uPA, and induces p21, p27, Caspase-3 and PARP cleavage, Bax/Bcl-2 and Bad/Bcl-xL ratio |
Inhibits proliferation, induces G1/S cell cycle arrest, apoptosis and chemotherapy sensitivity |
(45, 81-83) |
|
Momordica |
Carbohydra te conjugated protein |
Seeds |
Leukemia, liver, and naso- pharyngeal carcinoma |
In- vitro and in- vivo |
Type II ribosome inactivating protein, reduces Cyclin D1 expression, modulates RB and p38/ MAPK, JNK, and ERK phosphorylation, induces Bid, cytochrome c release, Caspases- 3, 8, 9 and PARP cleavage |
Inhibits proliferation, induces G1 or G2/M phase arrest, mitochondrial injury, autophagy, apoptosis, and chemotherapy sensitivity |
(86-88) |
|
charantia |
|||||||
|
lectin |
|||||||
|
(MCL) |
|||||||
|
α-momorchari |
Protein |
Seeds |
Brest and lung cancer |
In- vitro and in- vivo |
Ribosome inactivating protein, increases Caspase- 3 |
Inhibits proliferation, induces S- or G2/M phase arrest and apoptosis |
(63, 89) |
|
BG-4 |
Protein |
Seeds |
Colon cancer |
In- vitro |
Protease inhibitor, reduces Bcl-2 and increases expression of p21, Bax and Caspase-3 |
Inhibits cell proliferation and induces apoptosis |
(93) |
|
Charantagen ins-D and Goyaglycosi de-d |
Cucurbitan e-type triterpene glycosides |
Fruit |
Glioblasto ma, lung and liver cancer |
In- vitro |
Not known |
Inhibit proliferation |
(94) |
|
Karaviloside -III |
Cucurbitan e- type triterpene glycoside |
imma ture fruit |
Liver cancer |
In- vitro |
Not known |
Inhibits proliferation |
(95) |
|
Kuguaglycos ide C |
Cucurbitan e- type triterpene glycoside |
Leave s |
Neuroblast oma |
In- vitro |
Caspase independent cell death |
Inhibits proliferation and induced cell death |
(96) |
|
RNase MC2 |
Protein |
Seeds |
Breast and liver cancer |
In- vitro and in- vivo |
Induces phosphorylation of Akt, p38, JNK, and ERK, and increases Caspases 8, 9 and PARP cleavage |
Inhibits proliferation and induces apoptosis |
(97, 98) |
|
Plumericin |
Iridoid lactone |
Vine |
Leukemia and liver cancer |
In- vitro |
Inhibits Cox2 and VEGF expression |
Inhibits proliferation, induces G2/M cell cycle arrest and apoptosis |
(99, 100) |
|
3β,7β,25- trihydroxycu curbita- 5,23(E)-dien- 19-al |
Cucurbitan e- type triterpenoid |
Whol e plant |
Breast cancer |
In- vitro |
Inhibits histone deacetylases, Akt-NF-κB signaling, induces p38/ MAPK, p53, and ROS generation |
Inhibits proliferation and induces autophagy |
(101) |
|
3β,7β- dihydroxy- 25- methoxycucu rbita-5,23- dien-19-al |
Cucurbitan e- type triterpenoid |
Whol e plant |
Breast cancer |
In- vitro |
Inhibits expression of PPARγ, Cyclin D1, CDK6, Bcl-2, XIAP, Cox-2, NF-κB, ERα, and Akt, induces AMPK and ER- stress |
Inhibits proliferation and induces apoptosis |
(102) |
|
(19R,23E)- 5β,19-epoxy- 19-methoxy- cucurbita- 6,23,25- trien-3β-ol and (19R,23E)- 5β,19-epoxy- 19,25- dimethoxy- cucurbita- 6,23-dien-3β- ol |
Cucurbitan |
Fruit |
Skin |
In-vivo |
Not known |
Prevented carcinogenesis |
(103) |
Conclusion
As discussed in this review, Momordica charantia is a medicinal plant and the crude extract of the
whole plant or plant parts (fruit, leaves, or seeds) exhibits effective cancer-preventative and therapeutic activities. The extract contains many bioactive phytochemicals, including triterpenoids, triterpene glycoside, phenolic acids, flavonoids, lectins, sterols, proteins, and saponins. Studies suggested that the combined impact of these bioactive phytochemicals determines the biological activity of the extract. Among the bioactive phytochemicals, Momordicine-I (M-I),
MomordicaAntiviral Protein 30 Kd (MAP30) or Momorcharin, alpha-Eleostearic acid (ESA), kuguacin J (KuJ), Momordica charantia lectin (MCL), RNase MC2, alpha-Momorcharin (-MMC), BG-4, and karaviloside-III show potential anticancer effects. By modifying the gene expression of related pathways, the majority of the compounds prevent the growth of cancer cells, cause cell cycle arrest in either the S or G2/M phase, and promote either apoptotic or autophagic cell death or both of cancer cells. Most of the compounds show minimum side effects at least in in-vitro models. Each compound has specific mechanisms, sometimes targets multiple molecules at a time, and plays potential anti-cancer effects against multiple types of cancer. However, to design prospective studies for interventional therapies, more analysis of active components and in-depth mechanistic study in pre-clinical systems are required.
Future directions
There are several nutrients and bioactive elements in Momordica charantia. In vitro and in vivo cancer models have been used to characterize and assess some of the components. However, to identify a specific target in cancers, detailed mechanistic studies are highly recommended. There is a need for extensive validation or follow-up research as well as effects when combined with traditional therapy. Additionally, for many of the components found, the data on bioavailability, stability, metabolism, and toxicity are not well evaluated. Many components still need to be assessed for their functional characterization.
Complex interactions between tumor cells and the immune microenvironment closely control multistep carcinogenesis. One crucial step in the development of cancer is immune suppression which is accomplished by inflammation in tumor-bearing hosts 93, modulation in natural killer (NK) cells 94,95 and myeloid-derived suppressor cells (MDSCs) 96,suppression and dysfunction of CD8+T-cells, and activation of regulatory T-cells (Tregs) in the microenvironment 97,98. Effective anti-tumor immunity must elicit both innate and adaptive immune responses. Naturally occurring anti- inflammatory or immunomodulatory plant extracts contribute to anticancer effects in modulating immune signaling pathways 99,100. Activation of NK cells treated with Momordica charantia extract enhances killing of cancer cells in vitro and inhibits CD4+FoxP3+T cell populations following the extract treatment in syngeneic head & neck tumor-bearing mice 100,101. Therefore, it will be critical to study the active components of Momordica charantia in regulating the tumor microenvironment.
Conflict of interest
The authors declare that they do not have any Conflicts of Interests.
Funding Sources
This work is supported by the Ramalingaswami Re-entry Fellowship [BT/HRD/35/02/2006], Department of Biotechnology, Govt. of India to S.S. [BT/RLF/Re- entry/47/2021] and research grant R01 DE024942 from the National Institutes of Health to R. B. Ray.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: a cancer journal for clinicians. May 2021;71(3):209-249. doi:10.3322/caac.21660
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA: a cancer journal for clinicians. Jan 2023;73(1):17-48. doi:10.3322/caac.21763
- Wu S, Zhu W, Thompson P, Hannun YA. Evaluating intrinsic and non-intrinsic cancer risk factors. Nature communications. Aug 28 2018;9(1):3490. doi:10.1038/s41467-018-05467-z
- Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nature medicine. Aug 2004;10(8):789-99. doi:10.1038/nm1087
- Venitt S. Mechanisms of spontaneous human cancers. Environ Health Perspect. May 1996;104 Suppl 3:633-7. doi:10.1289/ehp.96104s3633
- The global challenge of cancer. Nature Cancer. 2020/01/01 2020;1(1):1-2. doi:10.1038/s43018-019-0023-9
- Zugazagoitia J, Guedes C, Ponce S, Ferrer I, Molina-Pinelo S, Paz-Ares L. Current Challenges in Cancer Treatment. Clin Ther. Jul 2016;38(7):1551-66. doi:10.1016/j.clinthera.2016.03.026
- Dehelean CA, Marcovici I, Soica C, et al. Plant-Derived Anticancer Compounds as New Perspectives in Drug Discovery and Alternative Therapy. Molecules. Feb 19 2021;26(4)doi:10.3390/molecules26041109
- Newman DJ, Cragg GM. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. Journal of natural products. Mar 27 2020;83(3):770-803. doi:10.1021/acs.jnatprod.9b01285
- Khalifa SAM, Elias N, Farag MA, et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar Drugs. Aug 23 2019;17(9)doi:10.3390/md17090491
- Safarzadeh E, Sandoghchian Shotorbani S, Baradaran B. Herbal medicine as inducers of apoptosis in cancer treatment. Advanced pharmaceutical bulletin. Oct 2014;4(Suppl 1):421-7. doi:10.5681/apb.2014.062
- Jia S, Shen M, Zhang F, Xie J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. International journal of molecular sciences. Nov 28 2017;18(12)doi:10.3390/ijms18122555
- Sur S, Ray RB. Bitter Melon (Momordica Charantia), a Nutraceutical Approach for Cancer Prevention and Therapy. Cancers. Jul 27 2020;12(8)doi:10.3390/cancers12082064
- Sur S, Ray RB. Diverse roles of bitter melon (<i>Momordica charantia</i>) in prevention of oral cancer. Journal of Cancer Metastasis and Treatment. 2021;7:12. doi:10.20517/2394- 4722.2020.126
- Fang EF, Froetscher L, Scheibye-Knudsen M, Bohr VA, Wong JH, Ng TB. Emerging Antitumor Activities of the Bitter Melon (Momordica charantia). Current protein & peptide science. 2019;20(3):296-301. doi:10.2174/1389203719666180622095800
- Farooqi AA, Khalid S, Tahir F, et al. Bitter gourd (Momordica charantia) as a rich source of bioactive components to combat cancer naturally: Are we on the right track to fully unlock its potential as inhibitor of deregulated signaling pathways. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. Sep 2018;119:98-105. doi:10.1016/j.fct.2018.05.024
- Dandawate PR, Subramaniam D, Padhye SB, Anant S. Bitter melon: a panacea for inflammation and cancer. Chinese journal of natural medicines. Feb 2016;14(2):81-100. doi:10.1016/S1875-5364(16)60002-X
- Raina K, Kumar D, Agarwal R. Promise of bitter melon (Momordica charantia) bioactives in cancer prevention and therapy. Seminars in cancer biology. Oct 2016;40-41:116-129. doi:10.1016/j.semcancer.2016.07.002
- Nerurkar P, Ray RB. Bitter melon: antagonist to cancer. Pharmaceutical research. Jun 2010;27(6):1049-53. doi:10.1007/s11095-010-0057-2
- Bakare R, Magbagbeola O, Akinwande A, Okunowo O. Nutritional and chemical evaluation of Momordica charantia. Journal of Medicinal Plants Research. 2010;4:2189-2193.
- Sur S, Steele R, Isbell TS, Venkata KN, Rateb ME, Ray RB. Momordicine-I, a Bitter Melon Bioactive Metabolite, Displays Anti-Tumor Activity in Head and Neck Cancer Involving c-Met and Downstream Signaling. Cancers. Mar 21 2021;13(6)doi:10.3390/cancers13061432
- Jilka C, Strifler B, Fortner GW, Hays EF, Takemoto DJ. In vivo antitumor activity of the bitter melon (Momordica charantia). Cancer research. Nov 1983;43(11):5151-5.
- Ray RB, Raychoudhuri A, Steele R, Nerurkar P. Bitter melon (Momordica charantia) extract inhibits breast cancer cell proliferation by modulating cell cycle regulatory genes and promotes apoptosis. Cancer research. Mar 1 2010;70(5):1925-31. doi:10.1158/0008-5472.CAN-09-3438
- Muhammad N, Steele R, Isbell TS, Philips N, Ray RB. Bitter melon extract inhibits breast cancer growth in preclinical model by inducing autophagic cell death. Oncotarget. Sep 12 2017;8(39):66226-66236. doi:10.18632/oncotarget.19887
- Shim SH, Sur S, Steele R, et al. Disrupting cholesterol esterification by bitter melon suppresses triple-negative breast cancer cell growth. Molecular carcinogenesis. Nov 2018;57(11):1599-1607. doi:10.1002/mc.22882
- Kohno H, Yasui Y, Suzuki R, Hosokawa M, Miyashita K, Tanaka T. Dietary seed oil rich in conjugated linolenic acid from bitter melon inhibits azoxymethane-induced rat colon carcinogenesis through elevation of colonic PPARgamma expression and alteration of lipid composition. International journal of cancer. Jul 20 2004;110(6):896-901. doi:10.1002/ijc.20179
- Chipps ES, Jayini R, Ando S, et al. Cytotoxicity analysis of active components in bitter melon (Momordica charantia) seed extracts using human embryonic kidney and colon tumor cells. Natural product communications. Sep 2012;7(9):1203-8.
- Kwatra D, Subramaniam D, Ramamoorthy P, et al. Methanolic extracts of bitter melon inhibit colon cancer stem cells by affecting energy homeostasis and autophagy. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:702869. doi:10.1155/2013/702869
- Deep G, Dasgupta T, Rao AR, Kale RK. Cancer preventive potential of Momordica charantia L. against benzo(a)pyrene induced fore-stomach tumourigenesis in murine model system. Indian journal of experimental biology. Mar 2004;42(3):319-22.
- Li CJ, Tsang SF, Tsai CH, Tsai HY, Chyuan JH, Hsu HY. Momordica charantia Extract Induces Apoptosis in Human Cancer Cells through Caspase- and Mitochondria-Dependent Pathways. Evidence-based complementary and alternative medicine : eCAM. 2012;2012:261971. doi:10.1155/2012/261971
- Ali MM, I HB, Ghanem HM, A HA-H, Mousa FM. The prophylactic and therapeutic effects of Momordica charantia methanol extract through controlling different hallmarks of the hepatocarcinogenesis. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. Feb 2018;98:491-498. doi:10.1016/j.biopha.2017.12.096
- Thiagarajan S, Arapoc DJ, Husna Shafie N, et al. Momordica charantia (Indian and Chinese Bitter Melon) Extracts Inducing Apoptosis in Human Lung Cancer Cell Line A549 via ROS- Mediated Mitochodria Injury. Evidence-based complementary and alternative medicine : eCAM. 2019;2019:2821597. doi:10.1155/2019/2821597
- Hsu HY, Lin JH, Li CJ, et al. Antimigratory Effects of the Methanol Extract from Momordica charantia on Human Lung Adenocarcinoma CL1 Cells. Evidence-based complementary and alternative medicine : eCAM. 2012;2012:819632. doi:10.1155/2012/819632
- Dhar D, Raina K, Kumar D, et al. Bitter melon juice intake with gemcitabine intervention circumvents resistance to gemcitabine in pancreatic patient-derived xenograft tumors. Molecular carcinogenesis. Oct 2020;59(10):1227-1240. doi:10.1002/mc.23251
- Brennan VC, Wang CM, Yang WH. Bitter melon (Momordica charantia) extract suppresses adrenocortical cancer cell proliferation through modulation of the apoptotic pathway, steroidogenesis, and insulin-like growth factor type 1 receptor/RAC-alpha serine/threonine-protein kinase signaling. Journal of medicinal food. Apr 2012;15(4):325-34. doi:10.1089/jmf.2011.0158
- Yung MM, Ross FA, Hardie DG, et al. Bitter Melon (Momordica charantia) Extract Inhibits Tumorigenicity and Overcomes Cisplatin-Resistance in Ovarian Cancer Cells Through Targeting AMPK Signaling Cascade. Integrative cancer therapies. Sep 2016;15(3):376-89. doi:10.1177/1534735415611747
- Limtrakul P, Khantamat O, Pintha K. Inhibition of P-glycoprotein activity and reversal of cancer multidrug resistance by Momordica charantia extract. Cancer Chemother Pharmacol. Dec 2004;54(6):525-30. doi:10.1007/s00280-004-0848-4
- Kandhari K, Paudel S, Raina K, et al. Comparative Pre-clinical Efficacy of Chinese and Indian Cultivars of Bitter Melon (Momordica charantia) against Pancreatic Cancer. Journal of cancer prevention. Dec 30 2021;26(4):266-276. doi:10.15430/JCP.2021.26.4.266
- Yasuda M, Iwamoto M, Okabe H, Yamauchi T. STRUCTURES OF MOMORDICINES I, II AND III, THE BITTER PRINCIPLES IN THE LEAVES AND VINES OF MOMORDICA CHARANTIA L. CHEMICAL & PHARMACEUTICAL BULLETIN. 1984;32(5):2044-2047. doi:10.1248/cpb.32.2044
- Wu SB, Yue GG, To MH, Keller AC, Lau CB, Kennelly EJ. Transport in Caco-2 cell monolayers of antidiabetic cucurbitane triterpenoids from Momordica charantia fruits. Planta Med. Jul 2014;80(11):907-11. doi:10.1055/s-0034-1382837
- Keller AC, Ma J, Kavalier A, He K, Brillantes AM, Kennelly EJ. Saponins from the traditional medicinal plant Momordica charantia stimulate insulin secretion in vitro. Phytomedicine : international journal of phytotherapy and phytopharmacology. Dec 15 2011;19(1):32-7. doi:10.1016/j.phymed.2011.06.019
- Chen PY, Shih NL, Hao WR, Chen CC, Liu JC, Sung LC. Inhibitory Effects of Momordicine I on High-Glucose-Induced Cell Proliferation and Collagen Synthesis in Rat Cardiac Fibroblasts. Oxidative medicine and cellular longevity. 2018;2018:3939714. doi:10.1155/2018/3939714
- Rothenberger NJ, Stabile LP. Hepatocyte Growth Factor/c-Met Signaling in Head and Neck Cancer and Implications for Treatment. Cancers (Basel). Apr 24 2017;9(4)doi:10.3390/cancers9040039
- Rajamoorthi A, Shrivastava S, Steele R, et al. Bitter melon reduces head and neck squamous cell carcinoma growth by targeting c-Met signaling. PloS one. 2013;8(10):e78006. doi:10.1371/journal.pone.0078006
- Lee-Huang S, Huang PL, Nara PL, et al. MAP 30: a new inhibitor of HIV-1 infection and replication. FEBS Lett. Oct 15 1990;272(1-2):12-8. doi:10.1016/0014-5793(90)80438-o
- Fang EF, Zhang CZ, Wong JH, Shen JY, Li CH, Ng TB. The MAP30 protein from bitter gourd (Momordica charantia) seeds promotes apoptosis in liver cancer cells in vitro and in vivo. Cancer letters. Nov 1 2012;324(1):66-74. doi:10.1016/j.canlet.2012.05.005
- Qian S, Sun L, Li J, et al. MAP30 inhibits autophagy through enhancing acetyltransferase p300 and induces apoptosis in acute myeloid leukemia cells. Oncology reports. Jun 2016;35(6):3705-13. doi:10.3892/or.2016.4705
- Jiang Y, Miao J, Wang D, et al. MAP30 promotes apoptosis of U251 and U87 cells by suppressing the LGR5 and Wnt/beta-catenin signaling pathway, and enhancing Smac expression. Oncology letters. Apr 2018;15(4):5833-5840. doi:10.3892/ol.2018.8073
- Lee-Huang S, Huang PL, Sun Y, et al. Inhibition of MDA-MB-231 human breast tumor xenografts and HER2 expression by anti-tumor agents GAP31 and MAP30. Anticancer research. Mar-Apr 2000;20(2A):653-9.
- Fan X, He L, Meng Y, Li G, Li L, Meng Y. Alpha-MMC and MAP30, two ribosome- inactivating proteins extracted from Momordica charantia, induce cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Molecular medicine reports. May 2015;11(5):3553-8. doi:10.3892/mmr.2015.3176
- Xiong SD, Yu K, Liu XH, et al. Ribosome-inactivating proteins isolated from dietary bitter melon induce apoptosis and inhibit histone deacetylase-1 selectively in premalignant and malignant prostate cancer cells. International journal of cancer. Aug 15 2009;125(4):774-82. doi:10.1002/ijc.24325
- Chan DW, Yung MM, Chan YS, et al. MAP30 protein from Momordica charantia is therapeutic and has synergic activity with cisplatin against ovarian cancer in vivo by altering metabolism and inducing ferroptosis. Pharmacol Res. Nov 2020;161:105157. doi:10.1016/j.phrs.2020.105157
- Sun Y, Sun F, Li J, et al. Mono-PEGylation of Alpha-MMC and MAP30 from Momordica charantia L.: Production, Identification and Anti-Tumor Activity. Molecules. Oct 31 2016;21(11)doi:10.3390/molecules21111457
- Shi Z, Pang K, Yang W, et al. Antitumor mechanism of MAP30 in bladder cancer T24 cells, and its potential toxic effects in mice. Cell Mol Biol (Noisy-le-grand). Apr 20 2020;66(1):42-48.
- Fan JM, Luo J, Xu J, et al. Effects of recombinant MAP30 on cell proliferation and apoptosis of human colorectal carcinoma LoVo cells. Mol Biotechnol. May 2008;39(1):79-86. doi:10.1007/s12033-008-9034-y
- Lv Q, Yang XZ, Fu LY, et al. Recombinant expression and purification of a MAP30-cell penetrating peptide fusion protein with higher anti-tumor bioactivity. Protein Expr Purif. Jul 2015;111:9-17. doi:10.1016/j.pep.2015.03.008
- Wang YX, Jacob J, Wingfield PT, et al. Anti-HIV and anti-tumor protein MAP30, a 30 kDa single-strand type-I RIP, shares similar secondary structure and beta-sheet topology with the A chain of ricin, a type-II RIP. Protein Sci. Jan 2000;9(1):138-44. doi:10.1110/ps.9.1.138
- Grossmann ME, Mizuno NK, Dammen ML, Schuster T, Ray A, Cleary MP. Eleostearic Acid inhibits breast cancer proliferation by means of an oxidation-dependent mechanism. Cancer prevention research. Oct 2009;2(10):879-86. doi:10.1158/1940-6207.CAPR-09-0088
- Tsuzuki T, Tokuyama Y, Igarashi M, et al. Alpha-eleostearic acid (9Z11E13E-18:3) is quickly converted to conjugated linoleic acid (9Z11E-18:2) in rats. The Journal of nutrition. Oct 2004;134(10):2634-9. doi:10.1093/jn/134.10.2634
- Kobori M, Ohnishi-Kameyama M, Akimoto Y, Yukizaki C, Yoshida M. Alpha-eleostearic acid and its dihydroxy derivative are major apoptosis-inducing components of bitter gourd. Journal of agricultural and food chemistry. Nov 26 2008;56(22):10515-20. doi:10.1021/jf8020877
- Zhang T, Gao Y, Mao Y, et al. Growth inhibition and apoptotic effect of alpha-eleostearic acid on human breast cancer cells. Journal of natural medicines. Jan 2012;66(1):77-84. doi:10.1007/s11418-011-0556-4
- Zhuo RJ, Wang F, Zhang XH, et al. Alpha-eleostearic acid inhibits growth and induces apoptosis in breast cancer cells via HER2/HER3 signaling. Molecular medicine reports. Mar 2014;9(3):993-8. doi:10.3892/mmr.2014.1892
- Yasui Y, Hosokawa M, Kohno H, Tanaka T, Miyashita K. Troglitazone and 9cis,11trans,13trans-conjugated linolenic acid: comparison of their antiproliferative and apoptosis- inducing effects on different colon cancer cell lines. Chemotherapy. 2006;52(5):220-5. doi:10.1159/000094865
- Yasui Y, Hosokawa M, Kohno H, Tanaka T, Miyashita K. Growth inhibition and apoptosis induction by all-trans-conjugated linolenic acids on human colon cancer cells. Anticancer research. May-Jun 2006;26(3A):1855-60.
- Eom JM, Seo MJ, Baek JY, et al. Alpha-eleostearic acid induces autophagy-dependent cell death through targeting AKT/mTOR and ERK1/2 signal together with the generation of reactive oxygen species. Biochem Biophys Res Commun. Jan 1 2010;391(1):903-8. doi:10.1016/j.bbrc.2009.11.161
- Beatty A, Singh T, Tyurina YY, et al. Ferroptotic cell death triggered by conjugated linolenic acids is mediated by ACSL1. Nature communications. Apr 14 2021;12(1):2244. doi:10.1038/s41467- 021-22471-y
- Pal M, Ghosh M. Prophylactic effect of alpha-linolenic acid and alpha-eleostearic acid against MeHg induced oxidative stress, DNA damage and structural changes in RBC membrane. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. Aug 2012;50(8):2811-8. doi:10.1016/j.fct.2012.05.038
- Pitchakarn P, Suzuki S, Ogawa K, et al. Induction of G1 arrest and apoptosis in androgen- dependent human prostate cancer by Kuguacin J, a triterpenoid from Momordica charantia leaf. Cancer letters. Jul 28 2011;306(2):142-50. doi:10.1016/j.canlet.2011.02.041
- Pitchakarn P, Suzuki S, Ogawa K, et al. Kuguacin J, a triterpeniod from Momordica charantia leaf, modulates the progression of androgen-independent human prostate cancer cell line, PC3. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association. Mar 2012;50(3-4):840-7. doi:10.1016/j.fct.2012.01.009
- Pitchakarn P, Umsumarng S, Mapoung S, et al. Kuguacin J isolated from bitter melon leaves modulates paclitaxel sensitivity in drug-resistant human ovarian cancer cells. Journal of natural medicines. Oct 2017;71(4):693-702. doi:10.1007/s11418-017-1099-0
- Pitchakarn P, Ohnuma S, Pintha K, Pompimon W, Ambudkar SV, Limtrakul P. Kuguacin J isolated from Momordica charantia leaves inhibits P-glycoprotein (ABCB1)-mediated multidrug resistance. The Journal of nutritional biochemistry. Jan 2012;23(1):76-84. doi:10.1016/j.jnutbio.2010.11.005
- Wang H, Ng TB. Ribosome inactivating protein and lectin from bitter melon (Momordica charantia) seeds: sequence comparison with related proteins. Biochem Biophys Res Commun. Dec 9 1998;253(1):143-6. doi:10.1006/bbrc.1998.9765
- Saeed F, Afzaal M, Niaz B, et al. Bitter melon (Momordica charantia): a natural healthy vegetable. International Journal of Food Properties. 2018/01/01 2018;21(1):1270-1290. doi:10.1080/10942912.2018.1446023
- Licastro F, Franceschi C, Barbieri L, Stirpe F. Toxicity of Momordica charantia lectin and inhibitor for human normal and leukaemic lymphocytes. Virchows Arch B Cell Pathol Incl Mol Pathol. 1980;33(3):257-65. doi:10.1007/BF02899186
- Zhang CZ, Fang EF, Zhang HT, Liu LL, Yun JP. Momordica charantia lectin exhibits antitumor activity towards hepatocellular carcinoma. Invest New Drugs. Feb 2015;33(1):1-11. doi:10.1007/s10637-014-0156-8
- Fang EF, Zhang CZ, Ng TB, et al. Momordica Charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer prevention research. Jan 2012;5(1):109-21. doi:10.1158/1940-6207.CAPR- 11-0203
- Cao D, Sun Y, Wang L, et al. Alpha-momorcharin (alpha-MMC) exerts effective anti-human breast tumor activities but has a narrow therapeutic window in vivo. Fitoterapia. Jan 2015;100:139- 49. doi:10.1016/j.fitote.2014.11.009
- Wang L, Shen F, Zhang M, et al. Cytotoxicity mechanism of alpha-MMC in normal liver cells through LRP1 mediated endocytosis and JNK activation. Toxicology. May 16 2016;357-358:33- 43. doi:10.1016/j.tox.2016.05.025
- Chen YJ, Zhu JQ, Fu XQ, et al. Ribosome-Inactivating Protein alpha-Momorcharin Derived from Edible Plant Momordica charantia Induces Inflammatory Responses by Activating the NF- kappaB and JNK Pathways. Toxins (Basel). Nov 26 2019;11(12)doi:10.3390/toxins11120694
- Deng NH, Wang L, He QC, et al. PEGylation alleviates the non-specific toxicities of Alpha- Momorcharin and preserves its antitumor efficacy in vivo. Drug Deliv. 2016;23(1):95-100. doi:10.3109/10717544.2014.905652
- Dia VP, Krishnan HB. BG-4, a novel anticancer peptide from bitter gourd (Momordica charantia), promotes apoptosis in human colon cancer cells. Scientific reports. Sep 15 2016;6:33532. doi:10.1038/srep33532
- Wang X, Sun W, Cao J, Qu H, Bi X, Zhao Y. Structures of new triterpenoids and cytotoxicity activities of the isolated major compounds from the fruit of Momordica charantia L. Journal of agricultural and food chemistry. Apr 18 2012;60(15):3927-33. doi:10.1021/jf204208y
- Yue J, Sun Y, Xu J, et al. Cucurbitane triterpenoids from the fruit of Momordica charantia L. and their anti-hepatic fibrosis and anti-hepatoma activities. Phytochemistry. Jan 2019;157:21-27. doi:10.1016/j.phytochem.2018.10.009
- Tabata K, Hamano A, Akihisa T, Suzuki T. Kuguaglycoside C, a constituent of Momordica charantia, induces caspase-independent cell death of neuroblastoma cells. Cancer science. Dec 2012;103(12):2153-8. doi:10.1111/cas.12021
- Fang EF, Zhang CZ, Fong WP, Ng TB. RNase MC2: a new Momordica charantia ribonuclease that induces apoptosis in breast cancer cells associated with activation of MAPKs and induction of caspase pathways. Apoptosis : an international journal on programmed cell death. Apr 2012;17(4):377-87. doi:10.1007/s10495-011-0684-z
- Fang EF, Zhang CZ, Zhang L, Fong WP, Ng TB. In vitro and in vivo anticarcinogenic effects of RNase MC2, a ribonuclease isolated from dietary bitter gourd, toward human liver cancer cells. Int J Biochem Cell Biol. Aug 2012;44(8):1351-60. doi:10.1016/j.biocel.2012.04.013
- Saengsai J, Kongtunjanphuk S, Yoswatthana N, Kummalue T, Jiratchariyakul W. Antibacterial and Antiproliferative Activities of Plumericin, an Iridoid Isolated from Momordica charantia Vine. Evidence-based complementary and alternative medicine : eCAM. 2015;2015:823178. doi:10.1155/2015/823178
- Min J, Cao L, Zhou J, Wu X, Li L. Plumericin inhibits growth of liver carcinoma cells via downregulation of COX-2 and VEGF. Tropical Journal of Pharmaceutical Research. 12/01 2018;17:2387-2392. doi:10.4314/tjpr.v17i12.11
- Bai LY, Chiu CF, Chu PC, Lin WY, Chiu SJ, Weng JR. A triterpenoid from wild bitter gourd inhibits breast cancer cells. Scientific reports. Mar 1 2016;6:22419. doi:10.1038/srep22419
- Weng JR, Bai LY, Chiu CF, Hu JL, Chiu SJ, Wu CY. Cucurbitane Triterpenoid from Momordica charantia Induces Apoptosis and Autophagy in Breast Cancer Cells, in Part, through Peroxisome Proliferator-Activated Receptor gamma Activation. Evidence-based complementary and alternative medicine : eCAM. 2013;2013:935675. doi:10.1155/2013/935675
- Akihisa T, Higo N, Tokuda H, et al. Cucurbitane-type triterpenoids from the fruits of Momordica charantia and their cancer chemopreventive effects. Journal of natural products. Aug 2007;70(8):1233-9. doi:10.1021/np068075p
- Hsiao PC, Liaw CC, Hwang SY, et al. Antiproliferative and hypoglycemic cucurbitane-type glycosides from the fruits of Momordica charantia. Journal of agricultural and food chemistry. Mar 27 2013;61(12):2979-86. doi:10.1021/jf3041116
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. Mar 4 2011;144(5):646-74. doi:10.1016/j.cell.2011.02.013
- Baginska J, Viry E, Berchem G, et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proceedings of the National Academy of Sciences of the United States of America. Oct 22 2013;110(43):17450-5. doi:10.1073/pnas.1304790110
- Moretta L, Pietra G, Montaldo E, et al. Human NK cells: from surface receptors to the therapy of leukemias and solid tumors. Front Immunol. 2014;5:87. doi:10.3389/fimmu.2014.00087
- Singh L, Muise ES, Bhattacharya A, et al. ILT3 (LILRB4) Promotes the Immunosuppressive Function of Tumor-Educated Human Monocytic Myeloid-Derived Suppressor Cells. Mol Cancer Res. Apr 2021;19(4):702-716. doi:10.1158/1541-7786.MCR-20-0622
- Thommen DS, Schumacher TN. T Cell Dysfunction in Cancer. Cancer cell. Apr 9 2018;33(4):547-562. doi:10.1016/j.ccell.2018.03.012
- Curiel TJ. Regulatory T cells and treatment of cancer. Curr Opin Immunol. Apr 2008;20(2):241-6. doi:10.1016/j.coi.2008.04.008
- Aravindaram K, Yang NS. Anti-inflammatory plant natural products for cancer therapy. Planta Med. Aug 2010;76(11):1103-17. doi:10.1055/s-0030-1249859
- Bhattacharya S, Muhammad N, Steele R, Kornbluth J, Ray RB. Bitter Melon Enhances Natural Killer-Mediated Toxicity against Head and Neck Cancer Cells. Cancer prevention research. Jun 2017;10(6):337-344. doi:10.1158/1940-6207.CAPR-17-0046
- Bhattacharya S, Muhammad N, Steele R, Peng G, Ray RB. Immunomodulatory role of bitter melon extract in inhibition of head and neck squamous cell carcinoma growth. Oncotarget. May 31 2016;7(22):33202-9. doi:10.18632/oncotarget.8898
Abbreviation
AIF: Apoptosis-inducing factor, AMPK: AMP-activated protein kinase, BAD: BCL2 associated agonist of cell death, BAX: BCL2 Associated X, Apoptosis Regulator, BCl2: B-cell lymphoma 2,Bid: BH3-interacting domain death agonist, BME: bitter melon extract, CCl4: carbon tetrachloride, Cdk-2/ 4/ 6: Cyclin-dependent kinase 2/ 4/ 6, CLA: Conjugated linoleic acid, c-Met: mesenchymal epithelial transition factor- C, c-Myc: c-MYC proto-oncogene, COX2: Cyclooxygenase-2, DENA: diethyl-nitrosamine, DMBA: 7, 12-Dimethylbenz(a)anthracene, ER: Endoplasmic reticulum, ERK: Extracellular signal-regulated kinase, ER-α: Estrogen receptor alpha, FOXP3: Forkhead box P3, GSK-3β: Glycogen synthase kinase-3β, HCC: Hepatocellular carcinoma, HDAC-1: Histone deacetylase 1, HER2/ HER3: Human epidermal growth factor receptor 2/ 3, HIV: Human immunodeficiency virus, HPV: Human papillomavirus, HSV: Herpes simplex virus, IP: Intraperitoneal, JNK: c-Jun N-terminal kinase, KuJ:kuguacin J,LC-HRESIMS: Liquid chromatography high resolution electrospray ionization mass spectrometry analysis, LGR-5: Leucine-rich repeat-containing G protein-coupled receptor 5, MAP30: Momordica Antiviral Protein 30kD, MAPK: Mitogen-activated protein kinase, MCL: Momordica charantialectin,MDSC: aMyeloid-derived suppressor cell, M-I: momordicine I,MMP-2/ 9: Matrix metalloproteinase-2/ 9, NF- kB: Nuclear factor kappa B, NK- cell: Natural killer cell, NOK: Normal oral keratinocytes, PARP: Poly (ADP-ribose) polymerase, PCNA: Proliferating cell nuclear antigen, P-gp: P-glycoprotein, PPAR-γ: Peroxisome proliferator-activated receptor γ, PTEN: Phosphatase and tensin homolog, RB: Retinoblastoma protein, RBC: Red blood cell, ROS: Reactive oxygen species, STAT3: Signal transducer and activator of transcription 3,T-reg: Regulatory T cells, uPA: Urokinase-type plasminogen activator, VEGF: Vascular endothelial growth factor, XIAP: X-linked inhibitor of apoptosis protein, α-ESA: alpha-eleostearic acid, α-MMC: alpha momorcharin.








