Somaya Y. Shalaby1, Mohamed A. Akela2 , Mustafa Karhib3, Youssef Hussein4
, Mustafa Karhib3, Youssef Hussein4 , Mohamed Rabea1
, Mohamed Rabea1 and Ehab Tousson5*
and Ehab Tousson5*
1Department of Zoology, Faculty of Science, Menoufia University, Egypt
2Department of Biology, College of Science and Humanities, Prince Sattam bin Abdulaziz University, Al-Kharj 11942, Saudi Arabia
3Department of Medical Laboratory Techniques, Al-Mustaqbal University College, 51001 Hillah, Babylon, Iraq
4Departmentof Anatomy, Histology and Emberology, Faculty of Medicine, Mutah University, Al-Karak, Jordan
5Department of Zoology, Faculty of Science, Tanta University, Egypt.
Corresponding Author E-mail: ehabtousson@science.tanta.edu.eg
DOI : https://dx.doi.org/10.13005/bpj/2786
Abstract
A broad spectrum carbamate fungicide called carbendazim (Carb) that is very crucial for preventing plant diseases. and is one of the most pervasive environmental pollutants with serious implications for both human and animal health. In the current study, the role of Coriandrum sativum seeds extract (CSE) on carbendazim induced rats renal toxicity. Carb induced significant elevation in serum creatinine, urea, potassium, kidney malondialdehyde (MDA), nitric oxide (NO), renal injury, PCNA and significant depletion in serum sodium, calcium, renal glutathione (GSH). Treatments of Carb with CSE (CSE+Carb and/or Carb+CSE) improved these parameters and reduced renal toxicity with best results for Carb+CSE than CSE+Carb. Serum phosphorus revealed no significant changes between different groups. The above findings support the hypothesis that the antioxidant characteristics of one or more of CSE constituents can reduce the testicular toxicity of Carb.
Keywords
Antioxidant; Carbendazim; Coriandrum sativum; Kidney
Download this article as:| Copy the following to cite this article: Shalaby S. Y, Akela M. A, Karhib M, Hussein Y, Rabea M, Tousson E. Prophylactic Effects of Coriander Aqueous Extract on Carbendazim -Induced Renal Toxicity in Male Rats. Biomed Pharmacol J 2023;16(4). |
| Copy the following to cite this URL: Shalaby S. Y, Akela M. A, Karhib M, Hussein Y, Rabea M, Tousson E. Prophylactic Effects of Coriander Aqueous Extract on Carbendazim -Induced Renal Toxicity in Male Rats. Biomed Pharmacol J 2023;16(4). Available from: https://bit.ly/3QD7BE0 |
Introduction
One of the most prevalent environmental contaminants, carbendazim (Carb) is a systemic benzimidazole fungicide. It is detrimental to both human and animal health and is essential for reducing plant diseases.1,2 Carb is a “Toxic Substance” according to the World Health Organisation (WHO)3, and long-term exposure to carbs has been linked to a lower rate of human survival.4 In order to interrupt the production of microtubules and meiotic cell division, the fungicidal activity of carbendazim targets tubulin.2,5 Chronic low dosage administration of carbs may induce oxidative stress, which can lead to reproductive and endocrine damage.6,7 Although previous research has shown that carbohydrates can be toxic due to the production of reactive oxygen species (ROS), there have been few attempts to investigate the role of antioxidants in reducing ROS-induced oxidative stress.7-9
A variety of diseases can be treated using chemical compounds that are created by medicinal plants.10-13 Herbal medicines have special qualities compared to modern pharmaceutical therapy, which is based on the production of several active molecules from the combination of unprocessed medicinal components, each of which has its own pharmacological effects. 14,15
The dried seeds of coriander (Coriandrum sativum), a Mediterranean native also known as Chinese parsley, have been utilised for food and fragrance purposes for almost 7000 years in the Middle East.16 In some traditional spice mixtures, especially those used in “Indian Curry,” a significant number of coriander seeds are utilised. There is no liquid essential oil mixture in substitute of this spice mixture. Coriander seeds are frequently used to season curries, puddings, breads, sausages, liqueurs, cakes, and spicy sauces.17,18 The coriander plant has been demonstrated to have a significant role in the etiology of several illnesses thanks to the discovery of certain polyphenolics and antioxidant components contained in it.19 Its antioxidant, antibacterial, antifungal, and digestive qualities make it useful in medicine.18,20
According to pharmacological research conducted on animals, coriander exhibits anti-diabetic, anti-cancer, and hypolipidemic properties.17,21 Antioxidants from food may be helpful in the fight against tissue damage. Therefore, there is a global quest for effective antioxidants to treat liver and stomach illnesses.22,18 Future usage of coriander seeds as a free radical scavenger to stop oxidative food degradation is possible.20 This study looked at the renal toxicity of carbendazim (Carb) and the potential therapeutic value of Coriandrum sativum seed extracts (CSE) in albino rats.
Material and Methods
Carbendazim (Carb)
The carb was obtained from Pesticides & Chemicals Company in Kafr Elzayat, Gharbia, It was freshly made with maize oil and given orally.
Coriander seeds extracts (CSE) production
Coriander seeds (CS) were ground into a powder, steeped in boiling water for 24 hours, extracted, and then kept at -30°C in the dark until usage18.
Animals and Experimental groups
The experiments were performed on 50 male albino rats (Rattus norvigicus) weighing (140 g ± 10g). Rats were kept in the laboratory for one week before the experimental work and maintained on a standard rodent diet and water was available ad libitum. The rats were randomly and equally divided into five groups (10 rats each).
Gp1: Control Gp served as a negative control and were not given any treatments.
Gp2: Coriandrum Gp (CSE) where rats treated orally (50 mg/kg body weight) for 3 days weekly for 8 weeks.
Gp 3: carbendazim Gp (Carb) where rats treated orally with Carb (100 mg/kg body weight) for 3 days weekly for 8 weeks.23
Gp 4: Co treated CSE with Carb (CSE + Carb) for 8 weeks.
Gp 5: Post treated Carb with CSE (Carb+CSE) where rats treated with Carb for 8 weeks and then treated with CSE for another 8 weeks.
Blood and serum samples
Rats were then slaughtered after being given sodium pentobarbital anesthesia at the conclusion of the research. Blood samples were drawn aseptically via a venipuncture and placed in a dry, clean, and sterile tube without the use of any anticoagulants, allowing the blood to clot. Blood samples were centrifuged for 5 minutes at 4000 rpm after being allowed to stand for 20 minutes at 4 oC to allow for coagulation. The obtained serum was stored at -18 °C until a blood parameter was determined.
Assessment of serum kidney function and electrolytes
Kidney functions as Urea, Creatinine were assayed using a commercial kit (BIOSYSTEMS from Barcelona, Spain).24 Electrolytes estimation were surveyed to measure the levels of potassium, calcium, sodium, and phosphorous using marketable kits of Indian Sensa-core electrolyte. 25
Biochemical assesses
Using a Potter Elvenhjem tissue homogenizer, each piece of kidney tissue was weighed and processed independently. The undiluted tissue homogenate was then whirled in a cold centrifuge for fifteen minutes at a speed of 11.739 rcf. The resultant supernatant was then used to various estimates.
Enzymatic and non-enzymatic antioxidant assays
Malondialdehyde (MDA) levels in kidney tissue homogenate via using Biodiagnostic kits, Egypt.2 After treating kidney homogenates with 5.5′-dithiobis(2-nitrobenzoic acid), GSH concentration was determined using the Tipple and Rogers method and expressed as mol GSH/ mg protein.27 Nitric oxide (NO) activities were estimated in kidney homogenate as its stable metabolites, nitrate and nitrite. Nitrate was first reduced by nitrate reductase to nitrite and then nitrite was determined spectrophotometrically.28
Histological processing
After necropsy, the kidney was quickly removed and immersed in a 10% neutral buffered formalin solution to preserve it for 24-48 hours. The specimens are dehydrated, cleared, and paraffin embedded. Serial sections that were 5 thick were cut into slices and stained with hematoxylin and eosin using a rotary microtome (Litz, Wetzlar, Germany). 29
Proliferating cell nuclear antigen (PCNA)
The detection of PCNA expression used the avidin-biotin complex method. Using Image J’s colour thresholding for quantitation feature, the stained cells’ brown hue was restored.30
Statistical Assessment
Data were reported as the one-way ANOVA was used to examine the significance of difference. *and# significant difference from control and from EST group respectively. Values are expressed as means SE at p< 0.05.
Results
Impacts of CSE on kidney functions and electrolytes
Table 1 exposed that; Carb induced a significant elevation in serum creatinine, urea, potassium, and significant depletion in the level of calcium and sodium ions, as compared control and CSE groups. Treatment of Carb with CSE as co-treatment group (CSE+Carb) or post treatment group (Carb+CSE) revealed a significant depletion in the level of creatinine, urea, potassium, and significant elevation in the level of calcium and sodium ions as compared to Carb group with best results for Carb+CSE. Serum phosphorus revealed no significant changes between different groups.
Table 1: Modifications in the levels of kidney functions & electrolytes.
|
Carb+CSE |
CSE+Carb |
Carb |
CSE |
Control |
|
|
38.30# ± 2.37 |
47.9*# ± 3.60 |
72.5* ± 4.19 |
35.4# ± 2.23 |
35.7# ± 2.81 |
Creatinine (mg/dl) |
|
0.92# ± 0.04 |
1.14*# ± 0.04 |
1.54* ± 0.08 |
0.81# ± 0.06 |
0.85# ± 0.02 |
Urea (mg/dl) |
|
131.9*# ± 6.20 |
127.6*# ± 8.53 |
124.9* ± 6.55 |
136.6# ± 7.32 |
134.1# ± 5.39 |
Na+ (mEq/L) |
|
3.65# ± 0.16 |
3.97*# ± 0.23 |
4.24* ± 0.19 |
3.58# ± 0.13 |
3.61# ± 0.17 |
K+ (mEq/L) |
|
4.36 ± 0.19 |
4.37 ± 0.35 |
4.38 ± 0.11 |
4.38 ± 0.12 |
4.37 ± 0.65 |
Ph (mEq/L) |
|
9.41# ± 0.37 |
9.04*# ± 0.41 |
8.80* ± 0.24 |
9.58# ± 0.30 |
9.60# ± 0.28 |
Ca++ (mEq/L) |
The significance of modification was analyzed by one – way ANOVA. Values are expressed as means ± SE. *and # significant deviation from the Carb group and the control, respectively at p< 0.01.
Oxidative stress and antioxidant parameters in kidney homogenates
Table 2 demonstrated a statistically significant elevation in the level of MDA and NO while a significant depletion in GSH in kidney homogenates in the Carb group compared to the control and CSE groups. In distinction; when Carb were treated with CSE (CSE+Carb or as Carb+CSE), kidney homogenates showed a significant elevation in GSH and a significant depletion in MDA and NO with best results for Carb+CSE.
Table 2: Activities of malondialdehyde (MDA; nmol/g protein), glutathione (GSH; µmol/g tissue), and nitric oxide (NO; µmol\L) in kidney tissue of male treated rats.
|
Carb+CSE |
CSE+Carb |
Carb |
CSE |
Control |
|
|
8.83# ± 0.55 |
13.44*# ± 0.65 |
20.05* ± 1.19 |
4.96# ± 0.32 |
5.81# ± 0.26 |
MDA |
|
0.473# ± 0.046 |
0.953*# ± 0.023 |
2.151* ± 0.058 |
0.375# ± 0.061 |
0.414# ± 0.029 |
NO |
|
1.698# ± 0.033 |
1.271*# ± 0.108 |
1.169* ± 0.124 |
1.769# ± 0.0177 |
1.853# ± 0.029 |
GSH |
The significance of modification was analyzed by one – way ANOVA. Values are expressed as means ± SE. *and # significant deviation from the Carb group and the control, respectively at p< 0.01.
Histological observations
Impact of CSE in kidney structure
Glomruli and tubules in the cortical and medullary parts of the kidneys in the control and CSE groups had normal histological features (Figure 1A&B). Contrarily, the kidney sections of the rats treated with Carb revealed marked tubular cell and glomerular atrophy, vaculation, and distinct necrotic tubular cells (Figure 1C&D). As opposed to that; kidney sections in CSE+Carb revealed moderate necrosis, atrophy of the renal corpuscles and mild inflammatory cells (Figure 1E). Kidney sections in Carb+CSE showed significant improvement in the glomruli with mild renal tubules atrophy (Figure 1F).
PCNA expression changes in kidney
Mild reactions in nuclei were presented after PCNA expressions in kidney sections in control and CSE groups (Figure 2A&B). while; a strong reaction was presented in Carb group (Figure 2C&D). As opposed to that; moderate reaction for PCNA was presented in CSE+Carb while mild reaction was detected in Carb+CSE (Figure 2E&F).
 |
Figure 1: Hematoxylin and Eosin-stained photomicrographs of kidney sections from the various experimental groups. |
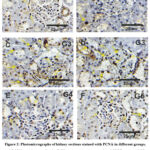 |
Figure 2: Photomicrographs of kidney sections stained with PCNA in different groups. |
Discussion
Various health risks are directly caused by environmental pollutants on living things. Pesticides are mostly to blame for environmental contamination. A pesticide is a chemical or biological agent used to prevent, repel, or eliminate pests that might harm or impair the development and well-being of living things, including both plants and animals. The broad spectrum benzimidazole carbamate fungicide carbendazim (carb), which has systemic action, is used to treat a variety of fungal diseases that affect field crops, fruits, ornamentals, and vegetables. In developing and adult animals, exposure to pesticides causes cytotoxicity, genotoxicity, and embryotoxicity alterations.31 Despite their significant usage and global dissemination, the function of pesticides in the development of illnesses in people is still debatable. Therefore the current study aimed to study the impact of CSE against Carb induced toxicity, oxidative stress and tissue injury in male rat kidney. Creatinine and urea are nitrogenous metabolic byproducts. The main byproduct of dietary protein and tissue protein turnover is urea. Muscle creatine catabolism results in creatinine. Both are bodily water-distributed molecules that are quite tiny. Current results revealed that; Carb induced elevation in urea, creatinine, potassium levels and depletion in sodium, calcium as compared to control and CSE while the treatments with CSE in both cases of co- or/and post treatments improved this modulation. Elevation of serum urea observed in the present study in response to pesticides exposure may be explained by impairment in its synthesis as a result of impaired hepatic function, or for the disturbance in protein metabolism as found in the present results or the decrease in the filtration rate of the kidney. Since urea and creatinine are indicators of renal function, elevations in these levels may be explained by the synergistic effects of carbendazim toxicity.32 which would reduce the kidneys’ ability to eliminate these metabolic wastes. Current results agree with 33, 34 who find that Carb induced renal toxicity. Current results agree with 35 who reported that there was an increase in blood urea due to Carb. Increase in urea content is indicative of an increase in tissue ammonia generation. It is therefore possible that elevation of urea and creatinine levels is the consequence of metabolic changes in rat cells brought about by these pesticides.
Current results agree with 31 who studied the protective effect of CSE on hepato-renal toxicity, also agree with 36 who reported that coriander seeds improved the renal toxicity by carbon tetrachloride. In addition to current results agree with 37 who reported that coriander seeds extract have the ability to improve the hepato-renal toxicity induced by sodium arsenite. Also, current results agree with 38 who reported that; CSE have the ability to improve the renal oxidative stress induced by lead, also; agree with 16 who reported that Coriandrum sativum extract contributes to resistance to oxidative stress via decreases in heavy metal concentrations in the kidney.
Environmental pollution-induced oxidative stress increases the expression of ROS, which in turn weakens the antioxidant defence system39,40; According to 41-43, it has been discovered that many pesticides cause oxidative stress, which produces free radicals and an alternative antioxidant or oxygen free radical scavenging enzyme system. Malonyldialdehyde (MDA), a marker of tissue membrane deterioration that is produced when carbs are consumed, was found to be elevated in the kidneys of the present study’s participants.44 This rise in MDA concentration may also reflect membrane instability and point to oxidative stress. Additionally, our findings indicated a GSH depletion in the kidneys, and CSE therapy modifies these findings. These GSH and NO measurements in the kidney point to an imbalance in the animal’s antioxidant system, which in turn causes increased membrane peroxidation in the organs. Current findings support the claims made 34.44 who find that carbs cause renal oxidative stress in rats by raising ROS levels. Current findings support 45,46 who found that CSE reduced renal tissue damage and oxidative stress brought on by lead nitrate.
Conclusion
Carbamate fungicide (carbendazim; Carb) induced liver toxicity where it changes liver structure and functions. Treatments of carbendazim with Coriandrum sativum seeds extract (Carb+CSE) modulates and improved these parameters with best results for post treatments than co-treatments.
Conflict of Interest
The authors declare no competing interests.
Funding Sources
References
- Rama, E.M., Bortolan, S., Vieira, M.L., Gerardin, D.C.C. and Moreira, E.G. Reproductive and possible hormonal effects of carbendazim. Regulatory Toxicology and Pharmacology, 2014; 69(3), pp.476-486.
CrossRef - Li, G., Li, J., Tan, W., Yang, M., Wang, H. and Wang, X. Effectiveness and mechanisms of the adsorption of carbendazim from wastewater onto commercial activated carbon. Chemosphere, 2022; 304, p.135231.
CrossRef - WHO Environmental Health Criteria: Benomyl. Geneva, Switzerland: World Health Organization, 1993; 148.
- Singh, S., Singh, N., Kumar, V., Datta, S., Wani, A.B., Singh, D., Singh, K. and Singh, J., Toxicity, monitoring and biodegradation of the fungicide carbendazim. Environmental chemistry letters, 2016; 14, pp.317-329.
CrossRef - Salimi, F., Asadikaram, G., Abolhassani, M., Pourfarjam, Y., Nejad, H.Z., Abbasi-Jorjandi, M. and Sanjari, M., Organochlorine pesticides induce thyroid tumors through oxidative stress; an in vivo and in silico study. Environmental Science and Pollution Research, 2023; 30(15), pp.45046-45066.
CrossRef - Lu, S.Y., Liao, J.W., Kuo, M.L., Wang, S.C., Hwang, J.S. and Ueng, T.H., Endocrine-disrupting activity in carbendazim-induced reproductive and developmental toxicity in rats. Journal of Toxicology and Environmental Health, Part A, 2004; 67(19), pp.1501-1515.
CrossRef - Rajeswary, S., Mathew, N., Akbarsha, M.A., Kalyanasundram, M. and Kumaran, B., Protective effect of vitamin E against carbendazim-induced testicular toxicity–histopathological evidences and reduced residue levels in testis and serum. Archives of toxicology, 2007; 81, pp.813-821.
CrossRef - Ahmed, H., Hala M, F., Salwa A, M., Hekma A, A.L. and Mostafa A, S., Amelioration of carbendazim-induced testicular dysfunction by vitamin E and pumpkin seed oil in rats. Journal of Drug Research of Egypt. 2010; 31 (1): 9-21.
- Sakr, S.A. and Shalaby, S.Y., Carbendazim-induced testicular damage and oxidative stress in albino rats: ameliorative effect of licorice aqueous extract. Toxicology and Industrial Health, 2014; 30(3), pp.259-267.
CrossRef - Abd Eldaim, M.A., Tousson, E., El Sayed, I.E.T., Abd El, A.E.A.H. and Elsharkawy, H.N., Grape seeds proanthocyanidin extract ameliorates Ehrlich solid tumor induced renal tissue and DNA damage in mice. Biomedicine & Pharmacoth., 2019; 115, p.108908.
CrossRef - Gupta, R., Kannan, G.M., Sharma, M. and Flora, S.J. Therapeutic effects of Moringa oleifera on arsenic-induced toxicity in rats. Environmental toxicology and pharmacology, 2005; 20(3), pp.456-464.
CrossRef - Hasan, A.F., Hameed, H.M., Tousson, E., Massoud, A., Atta. F., Youssef, H. Role of Oral Supplementation of Damiana (Turnera diffusa) Reduces the Renal Toxicity, Apoptosis and DNA Damage Associated with Amitriptyline Administration in Rats. Biomedical and Pharmacol. Journal. 2022;15(3):1245-53.
CrossRef - Elbandrawy, M.M., Sweef, O., Elgamal, D., Mohamed, T.M. and Elgharabawy, R.M., Ellagic acid regulates hyperglycemic state through modulation of pancreatic IL-6 and TNF-α immunoexpression. Saudi Journal of Biological Sciences, 2022; 29(5), pp.3871-3880.
CrossRef - Momin, A.H., Acharya, S.S. and Gajjar, A.V. Coriandrum sativum-review of advances in phytopharmacology. International Journal of Pharmaceutical Sciences and Research, 2012; 3(5), p.1233.
- Mutar, T.F., Tousson, E., Hafez, E., Abo Gazia, M. and Salem, S.B., Ameliorative effects of vitamin B17 on the kidney against Ehrlich ascites carcinoma induced renal toxicity in mice. Environmental Toxicology, 2020; 35(4), pp.528-537.
CrossRef - Nishio, R., Tamano, H., Morioka, H., Takeuchi, A. and Takeda, A. Intake of heated leaf extract of Coriandrum sativum contributes to resistance to oxidative stress via decreases in heavy metal concentrations in the kidney. Plant Foods for Human Nutrition, 2019; 74, pp.204-209.
CrossRef - Yibru, E., Menon, M.K.C, Belayneh, Y., et. al. The effect of coriandrum sativum seed extract on hyperglycemia, lipid profile and renal function in streptozotocin induced type- 2 diabetic Swiss albino mice. Int J Health Sci Res. 2015; 5(7):166-177.
- Moustafa, A.H.A., Ali, E.M.M., Moselhey, S.S., Tousson, E. and El-Said, K.S., Effect of coriander on thioacetamide-induced hepatotoxicity in rats. Toxicology and industrial health, 2014; 30(7), pp.621-629.
CrossRef - Alankooshi A.A., Hasan A. F., Tousson E., El-Atrsh A, Mohamed T.M. Impact of coriander seeds extract against thyroidectomy induced testicular damage and DNA replication in male rats. OnLine Journal of Biological Sciences 2023; 23 (2): 193.201. DOI: 10.3844/ojbsci.2023.193.201
CrossRef - Mechchate, H., Es-Safi, I., Amaghnouje, A., Boukhira, S., A. Alotaibi, A., Al-Zharani, M., A. Nasr, F., M. Noman, O., Conte, R., Amal, E.H.E.Y. and Bekkari, H. Antioxidant, anti-inflammatory and antidiabetic proprieties of LC-MS/MS identified polyphenols from coriander seeds. Molecules, 2021; 26(2), p.487.
CrossRef - Attaallah, A., Elmrazeky, A.R., El-Beltagy, A.E.F.B., Abdelaziz, K.K. and Soliman, M.F. Modulatory role of Coriandrum sativum (coriander) extract against diabetic complications on the gonads of female rats and their offspring. Tissue and Cell, 2023; p.102127.
CrossRef - Xu, J.Y., Zhang, L., Li, Z.P. and Ji, G. Natural products on nonalcoholic fatty liver disease. Current Drug Targets, 2015; 16(12), pp.1347-1355.
CrossRef - Sakr, S.A. and Shalaby, S.Y. Carbendazim-induced testicular damage and oxidative stress in albino rats: ameliorative effect of licorice aqueous extract. Toxicology and Industrial Health, 2014; 30(3), pp.259-267.
CrossRef - Patton, C.J. and Crouch, S.R. Spectrophotometric and kinetics investigation of the Berthelot reaction for the determination of ammonia. Analytical chemistry, 1977; 49(3), pp.464-469.
CrossRef - Tousson, E., El‐Atrsh, A., Mansour, M. and Abdallah, A. Modulatory effects of Saussurea lappa root aqueous extract against ethephon‐induced kidney toxicity in male rats. Environmental Toxicology, 2019; 34(12), pp.1277-1284.
CrossRef - Mesbah, L., Soraya, B., Narimane, S. and Jean, P.F., protective effect of flavonides against the toxicity of vinblastine cyclophosphamide and paracetamol by inhibition of lipid–peroxydation and increase of liver glutathione. Haematol 2004; 7(1): 59-67.
- Saggu, S., Sakeran, M.I., Zidan, N., Tousson, E., Mohan, A. and Rehman, H., 2014. Ameliorating effect of chicory (Chichorium intybus L.) fruit extract against 4-tert-octylphenol induced liver injury and oxidative stress in male rats. Food and chemical toxicology, 72, pp.138-146.
CrossRef - Robbins, R.A. and Grisham, M.B. Nitric oxide. The international journal of biochemistry & cell biology, 1997; 29(6), pp.857-860.
CrossRef - Salama, A.F., Tousson, E., Ibrahim, W. and Hussein, W.M., 2013. Biochemical and histopathological studies of the PTU-induced hypothyroid rat kidney with reference to the ameliorating role of folic acid. Toxicology and industrial health, 29(7), pp.600-608.
CrossRef - Tousson, E., Ali, E.M., Ibrahim, W. and Mansour, M.A. Proliferating cell nuclear antigen as a molecular biomarker for spermatogenesis in PTU-induced hypothyroidism of rats. Reproductive sciences, 2011; 18(7), pp.679-686.
CrossRef - Fahmy, H.A., Shreif, N.H. and Gharib, O.A. The protective effect of Coriandium sativum extract on hepato-renal toxicity induced in irradiated rats. European Journal of Medicinal Plants, 2014; 4(2), p.196.
CrossRef - Abolaji, A.O., Awogbindin, I.O., Adedara, I.A. and Farombi, E.O. Insecticide chlorpyrifos and fungicide carbendazim, common food contaminants mixture, induce hepatic, renal, and splenic oxidative damage in female rats. Human & experimental toxicology, 2017; 36(5), pp.483-493.
CrossRef - Verma, S.K, Prasad J. Efficacy of fungicides and Trichoderma harzianum against leaf spot of Aloe vera incited by Alternaria alternata. Annals of Plant ProtectionSciences. 2014; 22(2):454-6.
- Nwozo, S.O., Ozegbe, P.C. and Olasehinde, O. Carbendazim alters kidney morphology, kidney function tests, tissue markers of oxidative stress and serum micro-elements in rats fed protein-energy malnourished diet. International Journal of Biological and Chemical Sciences, 2017; 11(3), pp.1046-1055.
CrossRef - Yousef M.I.F., El-Demerdash M., Kamel K.I et al. Changes in some hematological and biochemical inices of rabbits induced by isoflavones and CYP. J. Toxicol. Environ. Health 2003; 189:223-234.
CrossRef - Iqbal, M.J., Butt, M.S., Shehzad, A. and Asghar, M. Evaluating therapeutic potential of coriander seeds and leaves (Coriandrum sativum L.) to mitigate carbon tetrachloride-induced hepatotoxicity in rabbits. Asian Pacific Journal of Tropical Medicine, 2018; 11(3), pp.209-213.
CrossRef - Saeed, M., Amen, A., Fahmi, A., El Garawani, I. and Sayed, S. The possible protective effect of Coriandrum sativum seeds methanolic extract on hepato-renal toxicity induced by sodium arsenite in albino rats. Journal of Applied Pharmaceutical Science, 2014; 4(12), pp.044-051.
CrossRef - Kumar, V.M., Dale, W., Rao, Y.P., Rajanna, S. and Rajanna, B. Protective role of Coriandrum sativum seed extract against lead-induced oxidative stress in rat liver and kidney. Current Trends in Biotechnology and Pharmacy, 2013; 7(2), pp.650-664.
- Wang, K., Chen, H., Fan, R.L., Lin, Z.G., Niu, Q.S., Wang, Z. and Ji, T. Effect of Carbendazim on Honey Bee Health: Assessment of survival, pollen consumption, and gut microbiome composition. Ecotoxicology and Environmental Safety, 2022; 239, p.113648.
CrossRef - Becker, S., Soukup, J.M. and Gallagher, J.E., 2002. Differential particulate air pollution induced oxidant stress in human granulocytes, monocytes and alveolar macrophages. Toxicology in vitro, 16(3), pp.209-218.
CrossRef - Farkhondeh, T., Mehrpour, O., Forouzanfar, F., Roshanravan, B. and Samarghandian, S. Oxidative stress and mitochondrial dysfunction in organophosphate pesticide-induced neurotoxicity and its amelioration: a review. Environmental Science and Pollution Research, 2020; 27, pp.24799-24814.
CrossRef - Yang, C., Lim, W. and Song, G., 2020. Mediation of oxidative stress toxicity induced by pyrethroid pesticides in fish. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 234, p.108758.
CrossRef - Sule, R.O., Condon, L. and Gomes, A.V., 2022. A common feature of pesticides: oxidative stress—the role of oxidative stress in pesticide-induced toxicity. Oxidative medicine and cellular longevity, 2022.
CrossRef - Salimi, F., Asadikaram, G., Abolhassani, M., Pourfarjam, Y., Nejad, H.Z., Abbasi-Jorjandi, M. and Sanjari, M., 2023. Organochlorine pesticides induce thyroid tumors through oxidative stress; an in vivo and in silico study. Environmental Science and Pollution Research, 30(15), pp.45046-45066.
CrossRef - Adedara, I.A., Vaithinathan, S., Jubendradass, R., Mathur, P.P. and Farombi, E.O., Kolaviron prevents carbendazim-induced steroidogenic dysfunction and apoptosis in testes of rats. Environmental toxicology and pharmacology, 2013;.35(3), pp.444-453.
CrossRef - Kansal, L., Sharma, V., Sharma, A., Lodi, S. and Sharma, S.H., 2011. Protective role of coriandrum sativum (coriander) extracts against lead nitrate induced oxidative stress and tissue damage in the liver and kidney in male mice.2011.
- Sharma, V. and Kansal, L. The protective effect of Rubia cordifolia against lead nitrate-induced immune response impairment and kidney oxidative damage. Indian journal of pharmacology, 2011; 43(4), p.441.
CrossRef







