Akhter Ahmed Ahmed , Pakhshan Abdulla Hassan
, Pakhshan Abdulla Hassan , Abdulilah Saleh Ismaeil
, Abdulilah Saleh Ismaeil  and Shahnaz Burhan Ali
and Shahnaz Burhan Ali
Biology Department, College of Science, Salahaddin University, Kirkuk Road, Erbil, Kurdistan Region, Iraq.
Corresponding Author E-mail: akhter.ahmed@su.edu.krd
DOI : https://dx.doi.org/10.13005/bpj/2740
Abstract
Objective(s): Salmonella typhi, is a serious global health threat because it causes typhoid fever, a severe systemic infection. According to the World Health Organization, millions of cases of typhoid are recorded annually, and thousands of people die from it. To combat this pathogen, new medications are required. The current study aims to study the ability of medicinal plants (thyme and cinnamon) to modulate the properties of Salmonella typhi isolates instead of killing them. Materials and Methods: The plants were extracted with the help of solvents (ethanol and ethyl acetate) and to find out the minimum inhibitory concentration, the different concentrations were used. The biofilm and expression of genes (invA & fliC) of the bacterium were studied when exposed to sub-inhibitory concentrations of the plant extracts. Results: MIC values ranging between 20-25 mg/ml and 10-15 mg/ml for ethanol and ethyl acetate extracts of Thyme respectively. While the MIC values of cinnamon were 18-25 and 10-15 mg/ml for both ethanol and ethyl acetate extracts respectively. The examinations revealed a significant decrease in the composition of biofilms by isolates when treated with SICs from plant extracts. The transcription expression profile of invasion (invA) and flagellar (fliC) genes were downregulated when treated with the plant extracts. Conclusion: The findings indicate that both thyme and cinnamon extracts may have promising activity against the biofilm and virulence of S. typhi. Thus, they could be used as potential as an antibacterial drug.
Keywords
Biofilm; Cinnamon; Gene expression; Salmonella typhi; Thyme
Download this article as:| Copy the following to cite this article: Ahmed A. A, Hassan P. A, Ismaeil A. S, Ali S. B. Weakening of Virulence Factors and Biofilm in Salmonella Typhi by Medicinal Plants Extracts. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Ahmed A. A, Hassan P. A, Ismaeil A. S, Ali S. B. Weakening of Virulence Factors and Biofilm in Salmonella Typhi by Medicinal Plants Extracts. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3ZoZyyD |
Introduction
Salmonella typhi (S. Typhi), the Gram-negative, facultative intracellular pathogen, is a major health concern around the world that causes a severe systemic infection, typhoid fever1. Every year, according to estimates by the World Health Organization (WHO), 11-20 million cases of typhoid are reported globally, and between 128,000–161,000 deaths occur2. An individual may carry the typhoid bacteria asymptomatically for days to years without experiencing any symptoms associated with typhoid fever. Acute or chronic carriers of typhoid can pass the disease to others through the fecal-oral route3. Salmonella pathogenesis requires a large number of virulence genes, which can be found on several parts of the bacterial genome, including plasmids, chromosomes, integrated bacteriophage DNA, Salmonella genomic islands (SGIs), and Salmonella pathogenicity islands (SPIs)4,5. Salmonella spp. biomarker pathogenicity has been widely researched.
The invasion gene (invA), a biomarker for the recognition of Salmonella spp., has been extensively investigated for its capacity to increase pathogenicity6, and the fliC gene encodes for the synthesis of H (flagellar) antigen, that serves as the basis for Salmonella classification under the Kauffman-White scheme7. However, the ability to treat and even prevent infections due to Salmonella has remained underdeveloped due to antibiotic resistance. Cell-to-cell communication in bacteria known as quorum sensing (QS) system is a mechanism involving various cellular functions, especially those related to bacterial virulence, such as adhesion, invasion, biofilm formation, and bacterial motility. As a result, inhibiting the communication system may be a novel treatment tactic for Salmonella infection that is not dependent on antibiotics8. In response, the scientific community is working hard to find natural antimicrobial drug replacement sources that, ideally, do not promote the emergence of resistance. In this perspective, plants are viewed as an essentially unlimited supply of bioactive components, and various methods have been used to utilize their use as antibacterial agents9. Amongst these, thyme (Thymus vulgaris) has been well-researched for its antibacterial, antioxidant, and anti-inflammatory effects and is one of the most promising nature-identical substances that has already been approved as a food addition10. Additionally, in recent years, cinnamon (Cinnamomum verum) and its compounds, primarily cinnamaldehyde, have been studied for their capacity to inhibit microbial biofilm against a variety of bacteria. It could be used in place of antibiotics to treat infections brought on by biofilms11,12. In this regard, this experimental study aimed to examine the effect of sub-inhibitory concentrations of ethanol and ethyl acetate extracts of thyme and cinnamon on invA and fliC genes expression using Real-time PCR and to study their effect as anti-biofilms on S. typhi strains.
Materials and Methods
Plant extraction process
Thymus vulgaris (thyme) leaves were brought from Akre farms in Kurdistan, Iraq, and the Cinnamomum verum (cinnamon) barks were purchased from the market in Erbil city, Iraq. Both plants were identified by the Herbarium of the Department of Biology at the College of Science, Salahaddin University-Erbil, Iraq. Methods described previously by13were used to extract the plant materials. In short, the extracting of the powder of the plant was by the method of maceration with the help of solvents (ethyl acetate and ethanol). The powder of plant (10.0g) was extracted by stirring three times at regular intervals using 100ml of the solvents over three days at RT after being filtered through a dual layer of muslin material and filter paper (Whatman no. 1). To obtain the crude material of each fraction vacuum evaporator was used to remove the chemical solvents. The extracted fractions were then kept at -20°C and dissolved in dimethyl sulphoxide (10% DMSO, Merck, Germany) and sterilized by membrane filter (0.45 μm) before use. To prevent their effect, solvents were used as control and blanks in all experiments of this study.
Samples sources and Specimens collection
Five non-duplicate isolates have been collected from S. Typhi from blood samples of patients who suffering from typhoid fever and were transferred to the General Hospital in Iraq. The samples were firstly inoculated onto MacConkey and Salmonella Shigella agar media (acuemedia, Neogen, USA) and incubated at 37 oC overnight. The distinct colonies were identified as S. typhi through various biochemical and conventional diagnostic tests as described by Tille14. The VITEK 2 automatic system (Biomerieux, France) was used for further identification of isolates. The susceptibility of the tested bacteria to different antimicrobials (Ceftazidime, Cefepime, Amikacin, Gentamicin, Piperacillin, Piperacillin/ Tazobactam, Aztreonam, Ciprofloxacin, Levofloxacin, Imipenem, Meropenem, Netilmicin, Tobramycin, Tigecycline, Tetracycline, Trimethoprim/ Sulfamethoxazole) was determined and the most two resistant isolates were selected for the experimentations through the current study. The individual colonies were stored in one mlTryptic Soy Broth (TSB) (Oxoid) containing 30% glycerol at -70oC for additional study. An ATCC strain of S. typhi (6539) was bought from Medya Diagnostic Center to be used as a control throughout the study.
Minimum Inhibitory Concentrations and Sub Inhibitory Concentrations(MICs& SICs)
The broth microdilution method was applied to determine the MICs of the plants extracts against multidrug-resistant (MDR) S. typhi isolates15. Ten µL of S. typhi cells at stationary phase adjusted to OD550 0.5 and transferred to 100µL NB supplemented with a range (1–30 mg ml-1) of extracts studies in the wells of a polystyrene microtitre plate (MTP). After one day (24 hrs.) of incubation at 37 oC, the MIC was calculated as the lowermost concentration at no observation of growth occurred. The concentration below MICs were considered sub-inhibitory and were used to study the anti-virulence and anti-biofilm activity in the isolated S. typhi strains. Three biological samples were examined separately.
Sub-MIC effect of plant extracts on the biofilm of S. typhi isolates
PCB (Polyvinyl Chloride Biofilm) formation method was used to quantify the biofilm in the bacterial isolates exposed to the SICs of the plant extracts. Overnight cultures of S. typhi were re-suspended in a sterile NB media in the presence and absence of SICs of the studied extracts and incubated at 37 °C in a stationary state for about 24 hours. Then the liquid cultures were removed, and the wells were washed three times with phosphate buffer saline (PBS), dried out and stained with a violet crystal suspension (1%). The excess dye was washed off with distilled water and the amount of dye adherent to the solubility in ethanol was determined(95%). The adhesion ability of the abiotic surface was measured by reading the absorption of the colored suspension by the ELISA reader (Epson, Biotek, UK) with a wavelength of 490 Nm16. Separate analyses of three biological samples were conducted, and the standard error was determined.
RNA extraction and quantification of virulence-related genes
Real-time PCR was used to estimate the effect of the plants extracts at SICs value at the level of expression of virulence genes (invA & fliC). Total RNA was extracted from both untreated bacteria which were used as control and bacteria exposed to various plants extracts according to the instructions provided by the manufacturer (total RNA kit, Favorgen Biotech, Taiwan). c-DNA was synthesized through reverse transcription of the isolated RNA using AddScript cDNA synthesis kit afforded by the manufacturer protocol (addbio,Koria). RT-PCR reactions were performed using RealQ Plus 2x Master Mix Green (Ampliqon, Denmark) in the PCRmax Eco 48 RT-PCR system. The primers used for virulence genes quantification were as follows (sense and antisense): fliC‑d: 5’ actcaggcttcccgtaac gc3’&5’ggctatatgtccttatcgg3’17; and invA, 5’ GTGAAATTATCGCCACGTTCGGGCAA3’ and 5’ TCATCGCACCGT CAAAGGAACC3’18. The candidate genes were analyzed by qPCR and ΔΔCt method19 to calculate the results.
Statistical analysis
GraphPad Prism 8.0 software was used to analyze the obtained results. The two-way contrast analysis (ANOVA) method was used for multiple comparisons. Data presented as the mean±SE.
Results
Different concentrations of ethanol and ethylacetate extracts of both thyme and cinnamon were examined on S. typhi isolates, as shown in Table 1, the MIC for ethanol extracts of thyme was 20 and 25 mg / mL versus different isolates while the MIC for ethyl acetate thyme extracts was 25 mg / mL for the same isolates. The MIC for ethanol extracts of cinnamon was 18 and 25 mg / mL and the MIC for cinnamon extracts of ethyl acetate extract was 10 and 14 mg / mL as shown in Table 2. Data below the MICs are considered SICs and used for biofilm and expression experiments.
Table 1: Minimum Inhibitory Concentrations & Sub-MICs of Thymus vulgaris extracts against MDR S. typhi isolate
|
Bacterial |
MIC (mg/ml) |
Sub-MIC (mg/ml) |
||
|
Ethanol |
Ethyl Acetate Extract |
Ethanol Extract |
Ethyl Acetate Extract |
|
|
ATCC |
18 |
10 |
10 |
5 |
|
S1 |
25 |
14 |
15 |
10 |
|
S2 |
25 |
14 |
15 |
10 |
Table 2: Minimum Inhibitory Concentrations & Sub-MICs of Cinnamomum verumextracts against MDR S. typhi isolates.
|
Bacterial |
MIC (mg/ml) |
Sub-MIC (mg/ml) |
||
|
Ethanol |
Ethyl |
Ethanol |
Ethyl |
|
|
ATCC |
20 |
25 |
10 |
15 |
|
S1 |
25 |
25 |
15 |
15 |
|
S2 |
25 |
25 |
15 |
15 |
The plant extracts have a role in decreasing biofilm formation in S. typhi isolates after treating isolates with SIC of thyme ethanol extracts the biofilm formation decreased significantly as shown in figure 1. As shown in figure 2 the biofilm formation decreased significantly by treating the S. typhi isolates with SIC of cinnamon extracts.
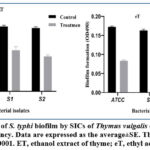 |
Figure 1: Decrease of S. typhi biofilm by SICs of Thymus vulgalis extracts measuring at 490 nm absorbency. Data are expressed as the average±SE. |
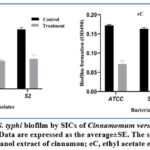 |
Figure 2: Decrease of S. typhi biofilm by SICs of Cinnamomum verum extracts measuring at490 nm absorbency. Data are expressed as the average±SE. |
The expression of flagellar (fliC) gene of S. typhi isolates were measured by RT_PCR as shown in figure 3 all plant extracts down regulated fliC-d gene in different ratio.
 |
Figure 3: Transcriptional profiles of fliC gene expression from isolates of S. typhi isolates exposed to SICs from Thymus vulgaris and Cinnamomum verum extracts. |
Figure 4 shows the folds of invasion (invA) gene expression change after treatment of S. typhi isolates with plant extracts, the results indicate that all plant extracts have the downregulating effect against S. typhi isolates.
 |
Figure 4: Transcriptional profiles of invA gene expression of isolates of S. typhi isolates exposed to SICs of Thymus vulgaris and Cinnamomum verum extracts. |
Discussion
Infections due to bacteria have been recognized as significant contributors to the aetiology of a variety of human diseases. The advent of MDR organisms20 has sparked research into quorum-sensing modulation strategies as an alternative to conventional antibiotic therapies for attenuating pathogenicity21.
S. typhi infections have grown to be a dangerous problem in hospital-acquired infection, especially in individuals with weakened immune systems22. This bacterium is one of the top priority pathogens worldwide according to WHO. Therefore, a broad range of approaches is now being explored in order to generate distinct anti-infective strategies16.
This study proves the impact of thyme and cinnamon extracts on the invA and fliC expression and the development of microbial biofilms in S. typhi strains recovered from typhoid fever patients. All of the extracts tested pose a significant antimicrobial activity by retarding or minimizing Salmonella strains biofilm formation by decreasing virulence gene expression when in vitro analyzed. However, the sensitivity of the strains has changed mainly depending on the plant and the type of extracts.
The observed inhibitory activity (Table 1 and Table 2) was indicated by extracts of thyme and cinnamon in different concentrations. The data showed variation in the MIC among plant extracts; the ethanol and ethyl acetate extracts of cinnamon showed the lowest MIC values (18 and 10 mg/ml) respectively against the ATCC strain, together with the ethyl acetate extract of cinnamon (14 mg/ml), against the S1 and S2 strains.
The outcomes are in line with23,24, thus according to Mostafa et al. (2018), the diversity in plant extracts’ chemical composition and the volatile nature of those components is what causes the variability in MIC25.
The tested Salmonella strains produced modest biofilms, in line with earlier researches26-28which also observed a decline in the bacterial biofilm under the presence of sub-inhibitory concentrations of thyme and cinnamon extracts, the quantitative biofilm measurements were significantly reduced to weak or no biofilm formation.
It has been demonstrated that several plants can effectively stop the development of biofilms in a variety of bacteria, including S. typhi29. The present study’s findings suggested that cinnamon ethyl acetate extract may possess the potential to inhibit the development of biofilms and change their phenotype from moderate to weak and negative biofilms (Fig. 2). Complex mechanisms affect bacteria pathogenic by changing cell wall bacterial permeability, leading to osmotic shock and cytoplasm leakage. The antimicrobial mechanism of extracting thyme and cinnamon, based on the main constituents of essential oils, such as thymol, carvacrol, and cinnamaldehyde, depends on their ability to inhibit bacterial activity by damaging the cell membrane, change the profile of lipids. Inhibition of ATPases, cell division, membrane reservoirs, motility, and biofilm formation, via anti-quorum sensing effects30,31.In particular, these components disintegrate the outer membrane of bacteria (Gram-negative), which release lipopolysaccharides that increase the permeability of the cytoplasmic membrane to ATP12. The bacteria of Gram-negative that still presents a significant human public health and economic problems is Salmonella spp.32,33.
The transcription levels of virulence genes (invA and fliC), under thyme and cinnamon extracts SIC stress were determined by RT-qPCR analysis in the current work. The bacterial strains showed drastically reduced gene expression (Fig. 3 and 4).
Based on the fold change technique, S. typhi strains treated with SICs of thyme and cinnamon extracts showed down-regulation in the fliC gene (involved in the QSpath for biofilm development) expression and noticeably inhibited to 9-folds in the cinnamon ethyl acetate extracts, and were blocked at S1 and S2 strains in particular. This emphasized that the extracts reduced the Salmonella virulence by suppressing the QS systems activity. Since host compartment-specific flagellar regulation is important to Salmonella virulence. Our results agree with previous researchers’ conclusions34,35.
On the opposite, invA gene expression was observed differentially among the S. typhi strains after exposure to thyme SIC. The upregulation in ethanolic thyme extract was mainly confirmed on strain S2 followed by thyme ethyl acetate extract. While a significant decrease in regulation has been shown by ethanol cinnamon extracts and ethyl acetate in which some strains have been banned
The invA gene is needed for full Salmonella virulence because it improves internalization, which is required for deeper tissue invasion36. Thus, thyme and cinnamon can inhibit biofilm formation by affecting gene transcription, implying that these genes are required for S. typhi strains to infect the host28.
Earlier studies and our findings line up with each other. Since the synergistic interactions between an extract’s active ingredients are one of the prime reasons for its ability to preclude the growth of bacteria37, notably Salmonella38,39.
Considering the natural antibacterial agents in thyme and cinnamon combined with their pharmacokinetics such as anti-inflammatory, antioxidant, antitumor, and neuro-protective properties40. In addition to their topical applications as a constituent of personal hygiene products, which have no cytotoxicity for human consumption. Regardless, excessive long-term use is not advised because current toxicological data show that undesirable side effects may occur at higher doses of thyme and cinnamon that appear in the studies of pharmacological41.
Conclusion
Thyme and cinnamon extracts have shown promising activities against isolates bacteria in both bacterial virulence and the formation ofbiofilm. The results of the biofilm inhibition examination indicated that the studied plant extracts are able to show anti-biofilm activity against S. typhi. Moreover, we concluded that the studyof extracts ofplantsregulates both the invA and fliC genes. Future analysis could be carried out in order to search for the most effective components of the examined plants. Thus thyme and cinnamon are used as potential antimicrobial drugs.
Acknowledgment
This study was supported by the Department of Biology, College of Science, Salahaddin University-Erbil, Iraq.
Conflicts of Interest
No conflict of interests is declared.
References
- Akshay, S. D., Nayak, S., Deekshit, V. K., Rohit, A., & Maiti, B. Differential expression of outer membrane proteins and quinolone resistance determining region mutations can lead to ciprofloxacin resistance in Salmonella Typhi. Archives of Microbiology, 2023; 205: 136. doi:10.1007/s00203-023-03485-0
CrossRef - WHO. Typhoid 2022 [cited 2022 june 18, 2022]. Available from: https://www.who.int/news-room/fact-sheets/detail/typhoid.
- Gonzalez-Escobedo G, Marshall JM, Gunn JS. Chronic and acute infection of the gall bladder by Salmonella Typhi: understanding the carrier state. Nature Reviews Microbiology 2011; 9:9-14.
CrossRef - Card R, Vaughan K, Bagnall M, Spiropoulos J, Cooley W, Strickland T, et al. Virulence Characterisation of Salmonella enterica Isolates of Differing Antimicrobial Resistance Recovered from UK Livestock and Imported Meat Samples. Frontiers in Microbiology 2016; 7.
CrossRef - Bayoumi MA, Griffiths MW. Probiotics down-regulate genes in Salmonella enterica serovar typhimurium pathogenicity islands 1 and 2. J Food Prot 2010; 73:452-460.
CrossRef - Balasubramanian R, Im J, Lee J-S, Jeon HJ, Mogeni OD, Kim JH, et al. The global burden and epidemiology of invasive non-typhoidal Salmonella infections. Human Vaccines & Immunotherapeutics 2019; 15:1421-1426.
CrossRef - Hirose K, Itoh K-I, Nakajima H, Kurazono T, Yamaguchi M, Moriya K, et al. Selective amplification of tyv (rfbE), prt (rfbS), viaB, and fliC genes by multiplex PCR for identification of Salmonella enterica serovars Typhi and Paratyphi A. Journal of clinical microbiology 2002; 40:633-636.
CrossRef - Zhang X, Liu B, Ding X, Bin P, Yang Y, Zhu G. Regulatory Mechanisms between Quorum Sensing and Virulence in Salmonella. Microorganisms 2022; 10:2211.
CrossRef - Gyawali R, Hayek S, Ibrahim SA. Plant extracts as antimicrobials in food products: Mechanisms of action, extraction methods, and applications. Handbook of natural antimicrobials for food safety and quality 2015; 49:49-62.
CrossRef - Raziyeh M, Mohammad K, Mansour R, Seyed AA. M, Amir M. Antioxidant, Antimicrobial Activities, and Characterization of Phenolic Compounds of Thyme (Thymus vulgaris L.), Sage (Salvia officinalis L.), and Thyme–Sage Mixture Extracts, Journal of Food Quality, 2023; Article ID 2602454, 9 pages, 2023. https://doi.org/10.1155/2023/2602454
CrossRef - Kosari F, Taheri M, Moradi A, Hakimi Alni R, Alikhani MY. Evaluation of cinnamon extract effects on clbB gene expression and biofilm formation in Escherichia coli strains isolated from colon cancer patients. BMC cancer 2020; 20:1-8.
CrossRef - Didehdar M, Chegini Z, Tabaeian SP, Razavi S, Shariati A. Cinnamomum: The New Therapeutic Agents for Inhibition of Bacterial and Fungal Biofilm-Associated Infection. Frontiers in Cellular and Infection Microbiology 2022; 12.
CrossRef - Harborne JB. Phytochemical Methods A guide to modern techniques of plant analysis. Third ed. London: Chapman & Hall; 1998.
- Tille PM. Bailey & Scott’s Diagnostic Microbiology, 15th ed.2021.
- Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat Protoc 2008; 3:163-175.
CrossRef - Ahmed AA, Salih FA. Quercus infectoria gall extracts reduce quorum sensing-controlled virulence factors production and biofilm formation in Pseudomonas aeruginosa recovered from burn wounds. BMC Complementary and Alternative Medicine 2019; 19:177.
CrossRef - Zhou L, Pollard AJ. A novel method of selective removal of human DNA improves PCR sensitivity for detection of Salmonella Typhi in blood samples. BMC Infect Dis 2012; 12:164.
CrossRef - Phumkhachorn P, Rattanachaikunsopon P. Detection of viable Salmonella Typhi by reverse transcription-multiplex polymerase chain reaction. Emirates Journal of Food and Agriculture 2017:1.
CrossRef - Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 2001; 25:402-408.
CrossRef - Pustake M, Giri P, Tambolkar S, Nayak S. Extensively Drug-Resistant Typhoid Fever: A Call to Action. Indian J Community Med 2022; 47:153-154.
CrossRef - Yang Q, Scheie AA, Benneche T, Defoirdt T. Specific quorum sensing-disrupting activity (A QSI) of thiophenones and their therapeutic potential. Sci Rep 2015; 5:18033.
CrossRef - Avershina E, Shapovalova V, Shipulin G. Fighting Antibiotic Resistance in Hospital-Acquired Infections: Current State and Emerging Technologies in Disease Prevention, Diagnostics and Therapy. Frontiers in Microbiology 2021; 12.
CrossRef - Li C, Xu Z, Chen W, Zhou C, Wang C, Wang M, et al. The Use of Star Anise-Cinnamon Essential Oil as an Alternative Antibiotic in Prevention of Salmonella Infections in Yellow Chickens. Antibiotics 2022; 11:1579.
CrossRef - Paudel SK, Bhargava K, Kotturi H. Antimicrobial activity of cinnamon oil nanoemulsion against Listeria monocytogenes and Salmonella spp. on melons. LWT 2019; 111:682-687.
CrossRef - Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci 2018; 25:361-366.
CrossRef - Boskovic M, Djordjevic J, Ivanovic J, Janjic J, Zdravkovic N, Glisic M, et al. Inhibition of Salmonella by thyme essential oil and its effect on microbiological and sensory properties of minced pork meat packaged under vacuum and modified atmosphere. International Journal of Food Microbiology 2017; 258:58-67.
CrossRef - Noorbakhsh F, Rahmati P. Effects of Thymus vulgaris and Cinnamomum verum Essential Oils on bap and ica Gene Expression in Staphylococcus aureus. Archives of Clinical Infectious Diseases 2022; 17.
CrossRef - Morshdy AEM, El-Tahlawy AS, Qari SH, Qumsani AT, Bay DH, Sami R, et al. Anti-Biofilms’ Activity of Garlic and Thyme Essential Oils against Salmonella typhimurium. Molecules 2022; 27:2182.
CrossRef - Noorbakhsh F, Rahmati P. Effects of Thymus vulgaris and Cinnamomum verum Essential Oils on bap and ica Gene Expression in Staphylococcus aureus. 2022; 17:e122410.
CrossRef - Nazzaro F, Fratianni F, De Martino L, Coppola R, De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals (Basel) 2013; 6:1451-1474.
CrossRef - Vasconcelos NG, Croda J, Simionatto S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb Pathog 2018; 120:198-203.
CrossRef - Zgurskaya HI, Rybenkov VV. Permeability barriers of Gram-negative pathogens. Ann N Y Acad Sci 2020; 1459:5-18.
CrossRef - Barreto-Santamaria A, Arevalo-Pinzon G, Patarroyo MA, Patarroyo ME. How to Combat Gram-Negative Bacteria Using Antimicrobial Peptides: A Challenge or an Unattainable Goal? Antibiotics (Basel) 2021; 10.
CrossRef - Chubiz JE, Golubeva YA, Lin D, Miller LD, Slauch JM. FliZ regulates expression of the Salmonella pathogenicity island 1 invasion locus by controlling HilD protein activity in Salmonella enterica serovar typhimurium. J Bacteriol 2010; 192:6261-6270.
CrossRef - Choi J, Shin D, Kim M, Park J, Lim S, Ryu S. LsrR-mediated quorum sensing controls invasiveness of Salmonella typhimurium by regulating SPI-1 and flagella genes. PLoS One 2012; 7:e37059.
CrossRef - Khan AA, Nawaz MS, Khan SA, Cerniglia CE. Detection of multidrug-resistant Salmonella typhimurium DT104 by multiplex polymerase chain reaction. FEMS Microbiology Letters 2000; 182:355-360.
CrossRef - Wagner H, Ulrich-Merzenich G. Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 2009; 16:97-110.
CrossRef - Aswathanarayan JB, Vittal RR. Inhibition of biofilm formation and quorum sensing mediated phenotypes by berberine in Pseudomonas aeruginosa and Salmonella typhimurium. RSC Advances 2018; 8:36133-36141.
CrossRef - Qi Y, Zhao W, Wang T, Pei F, Yue M, Li F, et al. Proteomic analysis of the antimicrobial effects of sublethal concentrations of thymol on Salmonella enterica serovar Typhimurium. Applied Microbiology and Biotechnology 2020; 104:3493-3505.
CrossRef - Parham S, Kharazi AZ, Bakhsheshi-Rad HR, Nur H, Ismail AF, Sharif S, et al. Antioxidant, Antimicrobial and Antiviral Properties of Herbal Materials. Antioxidants (Basel) 2020; 9.
CrossRef - Sharifi-Rad J, Dey A, Koirala N, Shaheen S, El Omari N, Salehi B, et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front Pharmacol 2021; 12:600139.
CrossRef








