I Gede Widhiantara1* , Putu Angga Wiradana1
, Putu Angga Wiradana1 , Anak Agung Ayu Putri Permatasari1
, Anak Agung Ayu Putri Permatasari1 , Ni Kadek Yunita Sari1
, Ni Kadek Yunita Sari1 , I Wayan Rosiana1
, I Wayan Rosiana1 , I Made Gde Sudyadnyana Sandhika1
, I Made Gde Sudyadnyana Sandhika1 , Ni Putu Widya Astuti2
, Ni Putu Widya Astuti2 , Luh Putu Widiastini3
, Luh Putu Widiastini3 , I Made Jawi4
, I Made Jawi4 and I Wayan Putu Sutirta Yasa5
and I Wayan Putu Sutirta Yasa5
1Research Group of Biological Health, Study Program of Biology, Faculty of Health, Science, and Technology, Universitas Dhyana Pura, Jalan Raya Padangluwih, Dalung, North Kuta, Badung Regency, Bali Province, Indonesia.
2Study Program of Public Health, Faculty of Health, Science, and Technology, Universitas Dhyana Pura, Jalan Raya Padangluwih, Dalung, North Kuta, Badung Regency, Bali Province, Indonesia.
3Study Program of Midwifery, Institute of Health Science-Bina Usadha, Jalan Raya Padangluwih, Badung Regency, Bali Province, Indonesia.
4Department of Pharmacology, Faculty of Medicine, Udayana University, Jalan P.B. Sudirman, Dangin Puri Klod, Denpasar City, Bali Province, Indonesia.
5Department of Clinical Pathology, Faculty of Medicine, Udayana University, Jalan P.B. Sudirman, Dangin Puri Klod, Denpasar City, Bali Province, Indonesia.
Corresponding Author E-mail: widhiantara@undhirabali.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2724
Abstract
High cholesterol levels can increase lipid peroxidation in tissues that are potentially toxic to reproductive organ cells, especially the Leydig cells that produce the testosterone hormone. Blumea balsamifera leaf extract (BBLE) has the main content in the form of flavonoid compounds with antihypercholesterolemic activity. This study aimed to determine the effect of BBLE administration on MDA levels, StAR mRNA expression, Leydig cell counts, and testosterone levels in hypercholesterolemic rats. A posttest-only control group design was utilized in this research. For 50 days, 36 male Wistar rats had been separated into two groups: 1) the control group (HCD + 1 ml/day sterile aquadest) and 2) the BBLE group (HCD + 4 mg/bb rats per day). After treatment, MDA testicular tissue levels, StAR mRNA expression, Leydig cell count, and testosterone levels were measured in both groups. The data collected were statistically examined using the Independent T-test and path analysis. The results indicated that the MDA level was lower in the BBLE group, though StAR gene expression, Leydig cell count, and testosterone levels were significantly greater (p0.05). StAR mRNA expression had a significant direct effect on testosterone levels. Administration of BBLE had been shown to improve testosterone hormone secretion in hypercholesterolemic rats by preventing oxidative stress in testicular tissue with the signs of lower MDA levels, up-regulation of the StAR mRNA, and Leydig cell regeneration.
Keywords
Blumea balsamifera; hypercholesterolemia; hormone secretion; lipid peroxidase; oxidative stress; StAR gene
Download this article as:| Copy the following to cite this article: Widhiantara I. G, Wiradana P. A, Permatasari A. A. A. P, Sari N. K. Y, Rosiana I. W, Sandhika I. M. G. S, Astuti N. P. W, Widiastini L. P, Jawi I. M, Yasa I. W. P. S. Blumea balsamifera Leaf Extract Maintain Testosterone Levels in Hypercholesterolemic Rats Through Antioxidant Mechanism and Upregulation of StAR Gene Expression. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Widhiantara I. G, Wiradana P. A, Permatasari A. A. A. P, Sari N. K. Y, Rosiana I. W, Sandhika I. M. G. S, Astuti N. P. W, Widiastini L. P, Jawi I. M, Yasa I. W. P. S. Blumea balsamifera Leaf Extract Maintain Testosterone Levels in Hypercholesterolemic Rats Through Antioxidant Mechanism and Upregulation of StAR Gene Expression. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/484xF2L |
Introduction
Hypercholesterolemic factors have an essential role in enhancing the creation of free radicals and causing the incorrect formation of lipid peroxide at the tissue level, which may contribute to oxidative stress1,2. Hypercholesterolemia causes an increase in the activity of NADPH oxidase, which in turn causes an increase in the production of superoxide anion, which is one of the reactive oxygen species (ROS) that causes oxidative stress. Hypercholesterolemia also raises blood pressure3. On the other hand, ROS are potentially toxic to cells in the reproductive system such as spermatogenic and Leydig cells4,5. A previous study in hypercholesterolemic rats found a significant reduction in plasma testosterone levels. This decline was caused by a disturbance in the hypothalamic-pituitary-testicular axis, Leydig cell deterioration, a decrease in Leydig cell nuclear diameter, or a decrease in LH levels and testicular activity of 17-hydroxysteroid dehydrogenase6,7. The same thing happened to rats fed a diet high in cholesterol for 50 days, and the total number of Leydig cells and spermatogonium A cells significantly decreased8,9.
Malondialdehyde (MDA) is a metabolite with high reactivity with several studies showing its role in carcinogenesis, liver and kidney disease, diabetes mellitus, cardiovascular, neurovascular, and various effects on cellular levels 10. MDA levels can be used as a biomarker of changes in lipid oxidation in tissues. On the other hand, MDA is not only produced in the process of lipid peroxidation but it is a common by-product of eicosanoid synthesis in small amounts 11.
In addition to the influence of MDA levels, the testosterone that is produced by changing cholesterol through the process of steroidogenesis initiated by the protein Steroidogenic Acute Regulatory Protein (StAR) has an important role in the functioning of the male reproductive system 12,13. The steroidogenesis process can be blocked by inhibiting StAR protein function due to disturbance of the hypothalamic-pituitary-testicular axis, causing decreased LH production due to aging and free radical damage14,15. Several experiments on mice given a high-fat diet showed a decrease in LH hormone secretion and decreased steroidogenesis enzyme activity including StAR activity 16–18.
Hypercholesterolemic medicines were created and distributed in the market during the previous several decades; nevertheless, the adverse effects made these treatments unsuitable for long-term usage. On the other hand, these side effects are the main cause of more than 24 million deaths expected by 2030 19. A diet with the tendency to consume high-fat and high-cholesterol foods is at risk of causing hypercholesterolemia.
The use of natural ingredients as antihypercholesterolemic agents such as herbal is highlighted 20. Blumea balsamifera, often known as Sembung, is a plant having a high antioxidant capacity21. Balinese in Indonesia generally use B. balsamifera leaves as a traditional drink called “Loloh”. This traditional plant may reduce blood cholesterol levels because of its high antioxidant content and several main secondary metabolites such as flavonoids, phenols, tannins, and alkaloids 21,22. As antioxidants, flavonoid compounds found in sembung leaf extract are reported to work in cell membranes by capturing unsaturated fatty acid-free radicals and converting them into hydroperoxy polyunsaturated fatty acids (PUFA-OOH) which are not free radicals 23. Ring B in flavonoid compounds has a hydroxy group that can produce hydrogen so that it stabilizes free radicals 24. Flavonoid compounds in sembung leaf extract also act as anti-hypercholesterolemic by reducing body LDL and increasing the LDL receptor density in the liver and binding to apolipoprotein B 25.
The effectiveness of B. balsamifera leaf extract (BBLE) on the reproductive function of male Wistar rats treated with a high-fat diet showed a significant increase in the diameter of the seminiferous tubules, spermatogonium-A cells, pachytene spermatocytes, and spermatid-16 after administration of BBLE at a dose of 4 mg/day for 50 days21. This demonstrated the potential of BBLE to preserve the HPT axis in balance and minimize the damaging impacts of free radicals caused by high-cholesterol diets. However, further investigation is needed regarding the impact of oxidative stress by high cholesterol diets and its prevention by direct BBLE on the testicular cells as a site for testosterone production and spermatogenesis.
Materials and methods
Ethical clearance
The Research Ethics Committee of the Faculty of Medicine, Udayana University (UNUD) / Central General Hospital (RSUP) Sanglah, Denpasar has permitted for this research with ethical approval with Protocol No. 2021.03.1.0276.
Research design
This experimental research used a Randomized Posttest-Only Control Group Design. For 50 days, 36 adult male Wistar rats (Rattus norvegicus) were randomly divided into two groups: Control (HCD + sterile distilled water) and BBLE (HCD + BBLE 4 mg/mL/BW rats orally)20.
Preparation of BBLE
Fresh B. balsamifera leaves of medium size and green were collected from plantations in Luwus Village, Tabanan that were previously determined as species at the National Research and Innovation Agency (BRIN), Bali Botanical Garden, Tabanan, Bali. Using a grinding machine, washed, dried, and crushed B balsamifera leaves. B. balsamifera leaf powder was weighed ± 250 and macerated in 70 % ethanol for 24 hours furthermore filtered. The collected filtrate was then evaporated with a vacuum rotary evaporator with a temperature of 50℃, speed of 80 rpm, and pressure of 80 kPa to obtain crude extract. The crude extract standards were adjusted to the Indonesian Herbal Pharmacopeia II Edition 2017, namely yield not less than 10.6%, the compound identity of flavonoids (+), water content not more than 14%, and total ash not more than 6.7% 26.
Rats models and treatment
The model animals were male Wistar rats that met the inclusion requirements (aged 12-14 weeks, body weight ranged from 150-200 grams). Wistar rats were randomly selected and acclimatized for 1 week. Rats were fed a regular diet that includes protein (20-25%), fat (5%), starch (45-50%), crude fiber (5%), ash (4%), and vitamins and minerals during the acclimation period. Furthermore, the high-cholesterol diet (HCD) contains a combination of 10% (100 gr) lard oil, 5% (50 gr) duck egg yolk, and up to 1,000 gr of standard feed. Ad libitum feeding of up to 20 gr/day.
The control group was given an HCD and distilled water. Whereas the BBLE group acquired up to 4 mg/mL/body weight per day of B. balsamifera leaf extract, each rat was given 1 mL containing 4 mg of extract orally for 50 days16. After 50 days, the testicular organs were surgically taken for assay of tissue MDA levels, StAR mRNA expression, and Leydig cell number. While serum testosterone was examined through blood taken from the orbital sinus.
Malondialdehyde (MDA)
Tissue MDA was measured using the QuantiChrom TBARS Assay Kit (BioAssay Systems, USA) (Cat. No. DTBA010). As much as 20 mg of mashed testicular tissue samples were taken and put into a test tube. Add 1 mL of chilled 20% TCA then vortexed and centrifuged at 3,500 rpm for 10 minutes. The supernatant was taken and put into another test tube that already contained 2 mL of 0.67% TBA. All tubes were put into the tube rack, then put in a water bath at 100°C for 10 minutes. After that, it was taken out and cooled in a vessel filled with ice water. The result of the reaction was taken as much as 1 ml and put in the cuvette. The absorbance of the sample was then measured with a spectrophotometer at the maximum wavelength (OD max = 532 nm). The number that appears on the screen was converted into a formula to get the MDA level (µmol/mL). The TBARS concentration of the sample was calculated using the following equation:
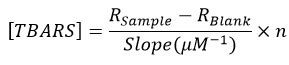
R sample and R blank were OD 535 nm or fluorescence intensity values of sample and H2O blank (standard), n was the sample dilution factor (n = 3 for proteinate sample).
StAR mRNA Expression
Examination of StAR mRNA expression was carried out in several stages including mRNA extraction (RNeasy Mini Kit Qiagen, USA), Reverse Transcriptase (RNA to cDNA), Primary optimization and Running Samples, and StAR gene Amplification.
Testosterone levels
Testosterone levels were measured using the Enzyme Linked Immunosorbent Assay (ELISA) with the Rat Testosterone BT-Lab Kit (BioAssay, USA) (Cat.No. E0259Ra). Briefly, all reagents, samples, and standards were prepared. After adding the sample, and standard antigen, and biotinylated it, it was incubated for 60 minutes at 37°C before being aspirated and washed 5 times before being incubated for 60 minutes at 37°C with avidin-HRP. After that, aspirate and wash 5 times before adding substrate solution A and substrate solution B and incubating for 10 minutes at 37°C in dark conditions. After adding a stop solution, the OD value was measured at 450 nm in 15 minutes.
Histopathology of Testis
Rat testes were fixed in 10% formaldehyde solution and passed through a series of ethanol baths and then cleaned using toluene, and then embedded in paraffin. Sample tissue was sectioned at 5 µm and stained with Hematoxylin and Eosin (H&E). Sections were examined with a light microscope at magnifications of 100× and 400× 26-30.
Data analysis
MDA levels, StAR gene expression, testosterone hormone, and Leydig cell count were statistically analyzed using SPSS 22 for Windows software (IBM, USA). Independent T-Test and sequential path analysis were used to determine the effect of treatment between the control and BBLE groups as well as the direct and major effect of tissue MDA levels and StAR mRNA expression on Leydig cell number and testosterone secretion. Graph views were processed using GraphPad Prism 8.0 (GraphPad Software, Inc., San Diego, CA) 22.
Results and Discussion
MDA levels
Testicular MDA levels of hypercholesterolemic rats differed significantly (p<0.05) in the two groups (Table 1). The MDA level of the testicular tissue of the control rats was 8.61 ± 0.61 μm and significantly higher than the BBLE group which was 5.27 ± 0.82 μm (Figure 1). Our results confirm that BBLE apart from having an anti-hypercholesterolemic effect had antioxidant properties.
Currently, many studies prove a decrease in male fertility due to high cholesterol foods. The development of herbal medicines to treat hypercholesterolemia continues to be carried out, including research on sembung (B. balsamifera) plants with leaves that are traditionally used in many areas, especially tropical areas such as Indonesia. The positive effect of BBLE on hypercholesterolemia subjects had not been comprehensively studied, especially as indicated by markers of oxidative stress in the reproductive system. The chemical composition and several parameters of BBLE reproductive performance in rats induced by high cholesterol feed had previously been studied and this raw material with the potential to be developed in biomedical industries22.
Table 1: MDA tissue levels, StAR mRNA expression, Leydig cell counts, and testosterone levels in the control and treatment groups.
|
Groups |
Mean±SD |
|||
|
Testicular tissue MDA levels (μmol/mL) |
StAR mRNA expression (fold change) |
Leydig cells counts |
Testosterone levels (ng/L) |
|
|
Control |
8. 61 ± 0. 61 |
1 |
9. 07 ± 0. 95 |
310. 98 ± 4. 94 |
|
BBLE |
5. 27 ± 0. 82 |
3. 09 |
13. 07 ± 1. 47 |
426. 02 ± 9. 37 |
|
P value |
0. 00 |
0. 03 |
0. 00 |
0. 00 |
Note: The value of p<0.05 showed significantly different interpretations between the control and BBLE groups.
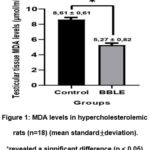 |
Figure 1: MDA levels in hypercholesterolemic rats (n=18) (mean standard±deviation).*revealed a significant difference (p < 0.05). |
The low levels of MDA in the BBLE group can be caused by the secondary metabolites contained therein that were dominated by the flavonoid group 21,22. Based on the results, the flavonoids in BBLE restored the antioxidant defense system and reduced lipid peroxidation by binding to free radicals and turning them into more stable compounds 30. In contrast, in the control group, hypercholesterolemia can trigger lipid peroxidation31. BBLE has also been shown in other research to reduce blood sugar levels and enhance the Langerhans cell in on pancreas of hyperglycemia mice32. The presence of flavonoids is also thought to affect the increase in glycogenic and glycolytic pathways that work outside the pancreas 33.
The number of Leydig cells
The number of Leydig cells in the control group was 9.07 ± 0.95, which tended to be lower than the BBLE group, that was 13.07 ± 1.47 (Figure 2). Interestingly, the effect of a high-cholesterol diet affected the proliferation of Leydig cells in the testes. The histological appearance of Leydig cells in both groups can be seen in Figure 3.
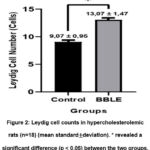 |
Figure 2: Leydig cell counts in hypercholesterolemic rats (n=18) (mean standard±deviation). * revealed a significant difference (p < 0.05) between the two groups. |
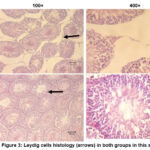 |
Figure 3: Leydig cells histology (arrows) in both groups in this study. |
The repair effect on the Leydig cells given BBLE in this study was through three main mechanisms. I) maintaining the working of the HPT axis as evidenced by the increased expression of the StAR gene in Leydig cells due to optimal stimulation of the Luteinizing hormone (LH). II) protecting the testicular organs from exposure to free radicals as evidenced by the MDA levels of the testicular tissue that was given low BBLE, and III) the mechanism of action of the flavonoids found in BBLE had a high probability of binding to androgen receptors in the testicular Leydig cells34.
Testosterone Levels
Testosterone levels in the control group showed a significant difference (p<0.05) compared to the BBLE group (Table 1). Testosterone levels in the BBLE group were 426.02 ± 9.37 ng/L, it was higher than the control group which was 310.98 ± 4.94 ng/L (Figure 4). Our findings reveal that there was a positive relationship between a high-cholesterol diet given for 50 days and a decrease in testosterone hormone secretion shown in the control group. Likewise, the ability of BBLE is important to highlight because it can maintain the function of Leydig cells for testosterone biosynthesis.
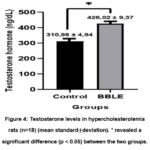 |
Figure 4: Testosterone levels in hypercholesterolemia rats (n=18) (mean standard±deviation). * revealed a significant difference (p < 0.05) between the two groups. |
Testosterone is a hormone produced by testicular interstitial cells or Leydig cells through steroidogenesis processes. The administration of BBLE can effectively maintain the function of Leydig cells which correlates with the increase in the amount of testosterone in this study. It has been confirmed by our previous studies, which observed that providing Wistar rats with a high-fat diet for 50 days increased the number of spermatogenic cells and the diameter of the seminiferous tubules. The testosterone hormone testosterone, released by the testicular Leydig cells, promoted spermatogenesis in male animals35. The important role of testosterone hormone especially in spermatogenesis was to initiate, maintain and restore spermatogenesis36. Cholesterol is the main ingredient in the biosynthesis of the testosterone hormone37. Interestingly, the accumulation of cholesterol in mice with hypercholesterolemia had inhibition of testosterone synthesis due to decreased activity of steroidogenesis enzymes, as well as increased levels of stress biomarkers in the endoplasmic reticulum and activation of ATF-638.
StAR mRNA Expression
The results showed that administration of BBLE for 30 days was able to up-regulate StAR gene expression by threefold (fold change) when compared to the control group (Figure 5). We suspected that the phytochemical compounds in BBLE that were dominated by flavonoids from our previous researchcan maintain StAR gene expression that in turn affects the performance of steroidogenesis thereby maintaining testosterone production22.
 |
Figure 5: The StAR mRNA expression in hypercholesterolemic rats in the control and BBLE groups. The * sign showed that |
StAR triggers the movement of cholesterol, the major component of steroidogenesis, from the mitochondrial outer membrane to the inner membrane in male animals. In this study, StAR gene expression was up-regulated threefold (fold change) in the BBLE group compared to the control group. Flavonoid compounds in BBLE were thought to play an important role in increasing StAR activity by suppressing the protein performance of DAX-1, an AHC critical region on the X-chromosome gene, that was a protein repressor for StAR gene transcription 39. The effectiveness of flavonoid compounds in increasing StAR activity was also revealed by Martin and Touaibia through the inhibition of COX-2 (cyclooxygenase-2) expression. Likewise, inhibition of COX-2 expression had been found to inhibit the action of the StAR protein in aging mouse Leydig cells39.
Path analysis
The results of pathway analysis showed that MDA levels, and StAR mRNA expression had a significant direct effect (p<0.05) on the Leydig cell numbers. The contribution of MDA levels and StAR gene expression to the number of Leydig cells was 89.4%, while the remaining 10.6% was contributed by other not examined variables. The direct effect of testicular tissue MDA levels, StAR mRNA expression, and the number of Leydig cells was significant on serum testosterone hormone secretion (p<0.05). MDA levels, StAR mRNA expression values, and the number of Leydig cells contributed as much as 94.2% to testosterone secretion. The indirect effect of tissue MDA levels on testosterone secretion through the number of Leydig cells was significant from the direct effect. However, the indirect effect of StAR mRNA expression values on testosterone secretion was not significant compared to the direct effect (Table 2).
Table 2: Effect of testicular tissue MDA levels, StAR gene expression, and the number of Leydig cells on testosterone levels in hypercholesterolemic Wistar rats
|
Variable |
Type of Effect |
R-square value |
The amount of Effect (β) |
p-value |
|
MDA Leydig cell |
Direct |
0,894 |
-0. 712 |
0. 00 |
|
Ct-StAR Leydig cell |
Direct |
-0. 331 |
0. 00 |
|
|
MDA testosterone |
Direct |
0,942 |
-0. 540 |
0. 00 |
|
Ct-StAR testosterone |
Direct |
0. 287 |
0. 01 |
|
|
sel Leydig testosterone |
Direct |
0. 634 |
0. 00 |
|
|
MDA testosterone |
Indirect |
|
-0. 451 |
– |
|
Ct-StAR testosterone |
Indirect |
|
-0. 209 |
– |
The results showed that the administration of BBLE had a protective effect against oxidative stress directly in the testes as evidenced by lower MDA levels. Reducing oxidative stress had the effect of regenerating Leydig cells that produce testosterone22. On the other hand, administration of BBLE also resulted in up-regulation of StAR gene expression with a direct influence on testosterone biosynthesis by Leydig cells.
Conclusion
The results show a protective effect against oxidative stress by decreasing MDA levels in testicular tissue in hypercholesterolemia Wistar rats. The results of this study also prove the effectiveness of the BBLE antioxidant that acts directly on the testes and improves Leydig cell regeneration and stimulates the regulation of StAR gene expression thereby increasing testosterone secretion. Further, research is still needed to examine several steroidogenesis markers that can complete information about BBLE’s ability to increase testosterone secretion.
Acknowledgment
The author would like to thank Mr. I Gede Wiranata, M.Si from the Laboratory of Pharmacology, Faculty of Medicine, Udayana University (UNUD) who has assisted in this research through the maintenance and intervention of BBLE in experimental animals. The author also thanks the Laboratory of Biochemistry and Molecular Biology of the Faculty of Medicine, Udayana University (UNUD), Histopathology Laboratory, Denpasar Veterinary Center (BBVET), and Laboratory of Molecular Biology of Institute of Tropical Disease Center (ITD) of Universitas Airlangga (UNAIR) who has assisted in the provision of reagents and analysis of research samples.
Conflict of Interest
The authors reported no declarations of interest.
Funding Source
This research was funded by the Ministry of Research, Technology and Higher Education of the Republic of Indonesia (KEMENRISTEKDIKTI) through the Domestic Postgraduate Education Scholarship Program (BPPDN) with Contract Number: B/276/D3.2/KD. 02.00/2019.
References
- Pizzino G, Irrera N, Cucinotta M, et al. Oxidative Stress: Harms and Benefits for Human Health. Oxid Med Cell Longev. 2017;2017:1-13. doi:10.1155/2017/8416763
- Andersson KE. Oxidative stress and its possible relation to lower urinary tract functional pathology. BJU Int. 2018;121(4):527-533. doi:10.1111/bju.14063
- Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC. Impact of Oxidative Stress on the Heart and Vasculature. J Am Coll Cardiol. 2017;70(2):212- 229. doi:10.1016/j.jacc.2017.05.035
- Asadi N. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J Clin DIAGNOSTIC Res. Published online 2017. doi:10.7860/JCDR/2017/23927.9886
- Ghosh S, Mukherjee S. Testicular germ cell apoptosis and sperm defects in mice upon long‐term high fat diet feeding. J Cell Physiol. 2018;233(10):6896-6909. doi:10.1002/jcp.26581
- Pushpendra A, GC J. Hyper-Lipidemia and Male Fertility: A Critical Review of Literature. Andrology-Open Access. 2015;04(02). doi:10.4172/2167-0250.1000141
- Cui L, Guan QB. Regulation of lipid metabolism in rat leydig cells testosterone synthesis and proliferation. Int J Clin Exp Med. 2016;9(5):8224-8229.
- Widhiantara IG, Permatasari AAAP, Siswanto FM, Dewi NPES. Ekstrak Daun Sembung (Blumea balsamifera) Memperbaiki Histologi Testis Tikus Wistar Yang Diinduksi Pakan Tinggi Lemak. J Bioteknol Biosains Indones. 2018;5(2):111. doi:10.29122/jbbi.v5i2.2868
- Widhiantara IG, Permatasari AAAP, Rosiana IW, Sutirtayasa IWP, Siswanto FM. Role of HIF-1, Siah-1 and SKN-1 in inducing adiposity for caenorhabditis elegans under hypoxic conditions. Indones Biomed J. 2020;12(1):51-56. doi:10.18585/INABJ.V12I1.1007
- Ayala A, Muñoz MF, Argüelles S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid Med Cell Longev. 2014;2014:1-31. doi:10.1155/2014/360438
- Djordjević A, Kotnik P, Horvat D, Knez Ž, Antonič M. Pharmacodynamics of malondialdehyde as indirect oxidative stress marker after arrested-heart cardiopulmonary bypass surgery. Biomed Pharmacother. 2020;132:110877. doi:10.1016/j.biopha.2020.110877
- Manna PR, Stetson CL, Slominski AT, Pruitt K. Role of the steroidogenic acute regulatory protein in health and disease. Endocrine. 2016;51(1):7-21. doi:10.1007/s12020-015-0715-6
- Larsen MC, Lee J, Jorgensen JS, Jefcoate CR. STARD1 Functions in Mitochondrial Cholesterol Metabolism and Nascent HDL Formation. Gene Expression and Molecular mRNA Imaging Show Novel Splicing and a 1:1 Mitochondrial Association. Front Endocrinol (Lausanne). 2020;11. doi:10.3389/fendo.2020.559674
- Widhiantara IG, Putri Permatasari AAA, Sutirta Yasa IWP. Spermatogenic and Leydig Cells Induced Hyperlipidemia: A Review. Res J Pharm Technol. Published online October 31, 2021:5573-5578. doi:10.52711/0974-360X.2021.00971
- Qu X, Yan L, Guo R, Li H, Shi Z. ROS-induced GATA4 and GATA6 downregulation inhibits StAR expression in LPS-treated porcine granulosa-lutein cells. Oxid Med Cell Longev. 2019;2019. doi:10.1155/2019/5432792
- Nteeba J, Ganesan S, Keating AF. Progressive Obesity Alters Ovarian Folliculogenesis with Impacts on Pro-Inflammatory and Steroidogenic Signaling in Female Mice1. Biol Reprod. 2014;91(4). doi:10.1095/biolreprod.114.121343
- Lin PH, Kuo TH, Chen CC, et al. Downregulation of testosterone production through luteinizing hormone receptor regulation in male rats exposed to 17α-ethynylestradiol. Sci Rep. 2020;10(1):1576. doi:10.1038/s41598-020-58125-0
- Santillo A, Giacco A, Falvo S, et al. Mild Exercise Rescues Steroidogenesis and Spermatogenesis in Rats Submitted to Food Withdrawal. Front Endocrinol (Lausanne). 2020;11. doi:10.3389/fendo.2020.00302
- Widhiantara IG, Permatasari AAAP, Wiradana PA. The Effect of Sembung Leaf Extract (Blumea balsamifera) On The Number and Diameter of Rats Leydig Cells Induced By High-Fat Diet. Plant Arch. 2021;21(April):356-361. doi:10.51470/PLANTARCHIVES.2021.v21.no1.049
- Li FS, Weng JK. Demystifying traditional herbal medicine with modern approach. Nat Plants. 2017;3(8):17109. doi:10.1038/nplants.2017.109
- Widhiantara IG, Jawi IM. Phytochemical composition and health properties of Sembung plant (Blumea balsamifera): A review. Vet World. 2021;14(5):1185-1196. doi:10.14202/vetworld.2021.1185-1196
- Widhiantara IG, Permatasari AAAP, Rosiana IW, Wiradana PA, Widiastini LP, Jawi IM. Antihypercholesterolemic and Antioxidant Effects of Blumea balsamifera L. Leaf Extracts to Maintain Luteinizing Hormone Secretion in Rats Induced by High-Cholesterol Diets. Indones Biomed J. 2021;13(4):396-402. doi:10.18585/inabj.v13i4.1694
- Panche AN, Diwan AD, Chandra SR. Flavonoids: An overview. J Nutr Sci. 2016;5:1-15. doi:10.1017/jns.2016.41
- Widhiantara IG, Putri Permatasari AAA, Rosiana IW, et al. The role of biopolymers as candidates for promoting health agents: A review. J Appl Pharm Sci. 2022;13(1):042-055. doi:10.7324/JAPS.2023.130104-1
- Radhika S, Smila K, Muthezhilan R. Antidiabetic and hypolipidemic activity of Punica granatum linn on alloxan induced rats. World J Med Sci. 2011;6(4):178-182.
- Kementerian Kesehatan Republik Indonesia (KEMENKES). Profil Kesehatan Indonesia Tahun 2019. Hardhana B, Sibuea F, Widiantini W, editors. KEMENKES RI; 2019.
- Min TS, Lee KH. Effects of nandrolone decanoate on expression of steroidogenic enzymes in the rat testis. Asian-Australasian J Anim Sci. 2018;31(5):658-671. doi:10.5713/ajas.17.0899
- Livak KJ, Schmittgen TD. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25(4):402-408. doi:10.1006/meth.2001.1262
- Hichem N, May M El, Laadhari N, Mrabet A, Gharbi R. Effect of Chronic Administration of Aluminum Trichloride on Testis among Adult Albino Wistar Rats. J Cytol Histol. 2013;04(05):4-5. doi:10.4172/2157-7099.1000195
- Lodhi P, Tandan N, Singh N, Kumar D, Kumar M. Camellia sinensis (L.) Kuntze Extract Ameliorates Chronic Ethanol-Induced Hepatotoxicity in Albino Rats. Evidence-Based Complement Altern Med. 2014;2014:1-7.
- Wahjuni S, Hafsia N, Bogoriani NW. Antihyperglycemic test of ethanol extract of sembung (Blumea balsamifera L.) leaves on male wistar rats (Rattus norvegicus). Intisari Sains Medis. 2020;11(2):582. doi:10.15562/ism.v11i2.670
- AL-Ishaq, Abotaleb, Kubatka, Kajo, Büsselberg. Flavonoids and Their Anti-Diabetic Effects: Cellular Mechanisms and Effects to Improve Blood Sugar Levels. Biomolecules. 2019;9(9):430. doi:10.3390/biom9090430
- Vyas N, Raval M. Aphrodisiac and spermatogenic potential of alkaloidal fraction of Argyreia nervosa (Burm. f.) Bojer roots in male rats. Nat Prod Res. 2022;36(5):1346-1351. doi:10.1080/14786419.2020.1869231
- Smith LB, Walker WH. The regulation of spermatogenesis by androgens. Semin Cell Dev Biol. 2014;30:2-13. doi:10.1016/j.semcdb.2014.02.012
- Ramaswamy S, Weinbauer GF. Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis. 2014;4(2):e996025. doi:10.1080/21565562.2014.996025
- Luo J, Yang H, Song BL. Mechanisms and regulation of cholesterol homeostasis. Nat Rev Mol Cell Biol. 2020;21(4):225-245. doi:10.1038/s41580-019-0190-7
- Yu C, Jiang F, Zhang M, et al. HC diet inhibited testosterone synthesis by activating endoplasmic reticulum stress in testicular Leydig cells. J Cell Mol Med. 2019;23(5):3140-3150. doi:10.1111/jcmm.14143
- Sablin EP, Woods A, Krylova IN, Hwang P, Ingraham HA, Fletterick RJ. The structure of corepressor Dax-1 bound to its target nuclear receptor LRH-1. 2008;2008:3-8.
- Wang X, Shen CL, Dyson MT, et al. Cyclooxygenase-2 Regulation of the Age-Related Decline in Testosterone Biosynthesis. Endocrinology. 2005;146(10):4202-4208. doi:10.1210/en.2005-0298








