Manuscript accepted on :21-12-2022
Published online on: 21-07-2023
Plagiarism Check: Yes
Reviewed by: Dr. Nishu Raina
Second Review by: Dr. Ahmed Albarbary
Final Approval by: Dr. Anton R Kiselev
Vartika Jain1 , Bhavika Kunwar1
, Bhavika Kunwar1 and S. K. Verma2*
and S. K. Verma2*
1Department of Botany, Government Meera Girls’ College, Udaipur, Rajasthan, India,
2Department of Medicine, Pacific Medical College and Hospitals, Udaipur-313001, Rajasthan, india.
Corresponding Author E-mail: skvermaster@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2709
Abstract
Impaired thrombolysis is one of the causes of the development of cardiovascular diseases (CVD). The synthetic thrombolytic agents such as streptokinase, urokinase and antistreplase have their own side effects. Plants are always considered as safe and cost-effective therapeutic agents. Dietary therapeutics is an emerging branch for the prevention and treatment of several ailments. The present article compiles 43 edible plants which have shown in vitro thrombolytic potential and are also employed in the diets of several ethnic communities in India. Among these, Bauhinia purpurea and Baccaurea ramiflora are two plants having more than 70% in vitro clot lysis potential; Coccinia grandis, Curcuma longa, Cyperus rotundus, and Typha domingensis have 50-70% thrombolytic activity; and the rest of the plants have 11-49% thrombolytic activity. These 43 plants also include spices and condiments such as Turmeric, Black pepper, Indian Bayleaf, Coriander and Ginger, which affirms the traditional saying of using food as medicine. Besides, these edible plants also possess various phyto-constituents and health-beneficial pharmacological activities. If these plants could be incorporated into a routine diet, it might be possible to prevent or delay the onset of CVD. However, detailed studies are required to evaluate the pattern of CVD in ethnic communities consuming such plants, as well as systematic clinical trials are warranted to investigate the thrombolytic efficacy of these plants.
Keywords
Bauhinia purpurea; Cardiovascular Disease; Food; Streptokinase; Traditional Medicine; Turmeric
Download this article as:| Copy the following to cite this article: Jain V, Kunwar B, Verma S. K. A Review on Thrombolysis Enhancing Indian Edible Plants. Biomed Pharmacol J 2023;16(3). |
| Copy the following to cite this URL: Jain V, Kunwar B, Verma S. K. A Review on Thrombolysis Enhancing Indian Edible Plants. Biomed Pharmacol J 2023;16(3). Available from: https://bit.ly/3O53QWu |
Introduction
Plants are nature’s wonderful gift to mankind, not only for providing oxygen as one of the most essential requirements of human existence but also for providing food, fodder, fiber, fuel, medicine, dye, timber, etc. Primitive people lived in forests and used natural resources for survival. They realized the importance of various plant species available in the surroundings and started using those species to fulfil the needs of daily life. They also noticed that plants can also act as medicine for prevention and cure of many diseases and ailments and therefore, included several plants in diets. The study of such man-plant relationships forms the discipline of Ethnobotany1.
Several edible plants play a very important role in the lives of ethnic communities as they not only provide fresh food but also useful sources of nutrients, medicines, firewood, dyes, building materials, and help in generating income. Moreover, cultivation of edible plants also helps in the conservation of many wild plants which are under pressure due to various biodiversity threats. Therefore, a number of studies have been carried out in various parts of the world to document the ethnobotanical knowledge associated with wild edible plant species2-7. Interestingly, many such plant species have demonstrated significant pharmacological activities in animal and human studies, such as antioxidant, anti-inflammatory, analgesic, antimicrobial, hypoglycemic, hepatoprotective, anticonvulsant, anti-platelet aggregation, adaptogenic, immunomodulatory, hypotensive, hypolipidemic, cytotoxic, anti-proliferative, diuretic, and nootropic8-10. Furthermore, these plants are rich in various phytochemicals such as flavonoids, phenols, tannins, alkaloids, saponins, steroids, etc. The presence of bioactive molecules and pharmacological activities scientifically validate many of the folk medicinal claims for the edible plant species and serve as good evidence to recommend them as functional foods.
Thrombus is the main culprit behind cardio-vascular diseases (CVD), leading towards the development of diseases like stroke, embolism, ischemia, deep vein thrombosis etc. Lysis of thrombus is an important event naturally going on in the body with the help of processes such as fibrinolysis. The mechanism behind thrombolytic action is by activating plasminogen, which forms plasmin. Plasmin thus formed cleaves the fibrin and the clot is finally dissolved. If the body’s natural thrombolysis is reduced or impaired for several reasons, it may have serious consequences. In that case, modern medicine takes the help of synthetic thrombolytic agents such as streptokinase, urokinase, tissue plasminogen activator and/or anistreplase, reteplase, tenecteplase. These agents mainly activate plasminogen to start a cascade of events related to thrombolysis. However, some serious side effects are also associated with these agents, necessitating the need for the development of comparatively safer as well as cost-effective thrombolytic molecules11-13.
The diet-disease relationship has been explored in many scientific studies, and changes in dietary behaviour have been shown to reduce the risk of cardio-metabolic disorders. Dietary modifications to improve various health conditions are now a preferred method of treatment as well as prevention of upcoming diseases14. Plants have played an important role in various therapeutic diets, such as, Mediterranean diet and Dietary Approaches to Stop Hypertension (DASH) diet15. It is recommended that intake of plants which are easily digestible and have high fiber content could be less stressful for heart during acute stages of heart disease16. In view of this, plants have been explored to provide safe, effective, and cheaper thrombolytic molecules. Many plant species have exhibited in vitro thrombolytic potential in scientific studies carried out in different parts of the world17. Moreover, some of those studied plants are also used as edibles among Indian ethnic communities. The present paper is to provide an overview of current knowledge on edible plant species that have demonstrated in vitro thrombolytic potential and to provide scope for future research.
Materials and Methods
For this purpose, first, a listing of plants which have shown in vitro thrombolytic activity was done by screening online databases such as Pubmed, Google Scholar, Research Gate, Science Direct, Taylor and Francis, and Springer Link as well as books and non-impact and non-indexed journals using keywords such as ‘in vitro thrombolysis, clot lysis, plants, herbs’ up to July 2022. Further short-listing of those plants was carried out by using ‘Compendium of Indian Folk Medicine and Ethnobotany’6 as a reference book to find out whether those plant parts are consumed in diets of Indian ethnic communities. The resultant plants were categorised into four, i.e., having more than 70%, between 50 and 70%, between 30 and 50%, and less than 30% thrombolytic potential and details are given in Tables 1-4 respectively in which the plants are listed alphabetically by botanical names, along with their families, common names in English, habit, plant part used, percent clot lysis, and the corresponding reference. Updated botanical nomenclature for all the plants was used as available on the website 151.
Results and Discussion
The present paper provides the botanical names and families of 43 edible plant species along with their in vitro percent clot lysis activity. These 43 plants have been distributed in 33 Angiosperm families and the most dominant family was Asteraceae with four plants, followed by Fabaceae with three plants and two each in the Cucurbitaceae, Solanaceae, Rutaceae, Zingiberaceae, and Araceae families. Rest 26 families represent a single plant species. Seven plants belong to the monocotyledon group and 36 plants belong to the dicotyledon group (Tables 1-4). The highest number of plants is represented as herbs (58.13%) followed by trees (23.25%), shrubs, and climbers (9.3% each) as depicted in Figure 1.
Table 1: Edible plants with in vitro thrombolytic potential > 70%
|
Botanical name & Family |
Common name |
Habit |
Plant part |
Percent clot lysis |
References |
|
Baccaurea ramiflora Lour. (Euphorbiaceae) |
Burmese grape |
Tree |
Seed |
88.21 |
18 |
|
Bauhinia purpurea L. (Fabaceae) |
Purple butterfly tree |
Tree |
Leaves |
91.02 |
19 |
The studies included in this paper have a range of 41 to 86% thrombolytic activity for the positive control, streptokinase, and a two-to-ten percent range of thrombolytic activity of distilled water as a negative control.
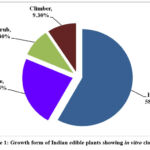 |
Figure 1: Growth form of Indian edible plants showing in vitro clot lysis |
Table 2: Edible plants with in vitro thrombolytic potential between 50-70%
|
Botanical name & Family |
Common name |
Habit |
Plant part |
Percent clot lysis |
References |
|
Coccinia grandis (L.) Voigt. syn. Coccinia indica Wight. & Arn. (Cucurbitaceae) |
Scarlet-fruited Ivy gourd |
Climber |
Leaf |
57.94 |
20 |
|
Curcuma longa L. (Zingiberaceae) |
Turmeric |
Herb |
Rhizome |
53.32 |
21 |
|
Cyperus rotundus L. (Cyperaceae) Typha domingensis Pers. (Typhaceae) |
Common Nut Sedge Southern Cattail |
Herb
Herb |
Rhizome
Whole plant |
60.00
67.16 |
22
23 |
Notably, two plants have shown more than 70% thrombolytic potential (Table 1) and four have shown 50-70% thrombolytic potential (Table 2). Twenty-six plants have shown between 30 and 50% in vitro thrombolytic potential (Table 3) and twelve plants have shown less than 30% thrombolytic potential (Table 4).
Table 3: Edible plants with in vitro thrombolytic potential between 30-50%
|
Botanical name & Family |
Common name |
Habit |
Plant part |
Percent clot lysis |
References |
|
Acmella paniculata (Wall. ex DC.) R.K. Jansen syn. Spilanthes paniculata Wall. ex DC. (Asteraceae) |
Panicled spot flower |
Herb |
Leaves |
42.77 |
24 |
|
Anacardium occidentale L. (Anacardiaceae) |
Cashew nut |
Tree |
Nut |
33.79 |
25 |
|
Bacopa monnieri Wettst. (Plantaginaceae) |
Water hyssop |
Herb |
Leaf |
47.39 |
26 |
|
Boerhavia diffusa L. (Nyctaginaceae) |
Horse Purslane |
Herb |
Leaf |
38.42 |
27 |
|
Brassica oleracea L. (Brassicaceae) Capparis decidua Edgew. (Capparaceae) |
Cabbage
Bare Caper |
Herb
Shrub |
Flower Leaves Fruit |
42.75 30.24 32.39 |
28 29 30 |
|
Capsicum frutescens L. (Solanaceae) |
Capsicum |
Herb |
Fruit |
36.87 |
29 |
|
Coriandrum sativum L. (Apiaceae) |
Coriander |
Herb |
Fruit |
43.25 |
21 |
|
Cuscuta reflexa Roxb. (Cuscutaceae) |
Devils hair |
Climber |
Whole plant |
44.63 |
31 |
|
Ficus racemosa L. syn. Ficus glomerata Roxb. (Moraceae) |
Cluster fig |
Tree |
Fruits |
47.23 |
32 |
|
Homalomena aromatica (Spreng.) Schott (Araceae) |
Gandh kochu |
Herb |
Leaf |
33.31 |
33 |
|
Leea indica (Burm.f.) Merr. (Leeaceae) Luffa cylindrica L. (Cucurbitaceae) |
Bandicoot berry Sponge gourd |
Shrub
Climber |
Leaf
Fruit |
39.30
45 |
34
35 |
|
Merremia vitifolia (Burm.f.) Hallier. f. (Convolvulaceae) |
Grape-leaf wood rose |
Herb |
Leaf |
42.48 |
36 |
|
Moringa oleifera Lam. (Moringaceae) |
Horse Raddish Tree |
Tree |
Leaf |
41.40 |
37 |
|
Ocimum tenuiflorum L. syn. Ocimum sanctum L. (Lamiaceae) |
Holy Basil |
Shrub |
Leaves |
30.01 |
25 |
|
Piper nigrum L. (Piperaceae) Punica granatum L. (Punicaceae) |
Black Pepper Pomegranate |
Climber
Shrub |
Fruit
Fruit |
35.4
38 |
38
39 |
|
Sesamum indicum L. (Pedaliaceae) |
Sesame |
Herb |
Seed |
32.94 |
38 |
|
Spinacia oleracea L. (Amaranthaceae) |
Spinach |
Herb |
Leaves |
40.9 |
40 |
|
Solanum torvum Swartz. (Solanaceae) |
Turkey berry |
Herb |
Fruit |
31.51 |
34 |
|
Syzygium aromaticum Merr. & L.M.Perry. (Myrtaceae) Tribulus terrestris L.(Zygophyllaceae) Vigna mungo (L.) Hepper (Fabaceae) |
Clove
Puncture vine
Black Gram |
Tree
Herb
Herb |
Flower buds Seed
Seed |
32.18
33
31.52 |
21
41
42 |
|
Vigna unguiculata (L.) Walp. (Fabaceae) Zingiber officinale Roscoe (Zingiberaceae) |
Cowpea
Ginger |
Herb
Herb |
Seed
Rhizome |
40.33
30.13 |
43
44 |
Table 4: Edible plants with in vitro thrombolytic potential <30%
|
Botanical name & Family |
Common name |
Habit |
Plant part |
Percent clot lysis |
References |
|
Averrhoa bilimbi L. (Oxalidaceae) Camellia sinensis (L.) O. Kuntze (Theaceae) |
Bilimbi
Black Tea |
Tree
Herb |
Fruit
Leaves |
23.94
11.64 |
45
46 |
|
Cinnamomum tamala T. Nees & Eberm. (Lauraceae) |
Indian bay leaf |
Tree |
Leaves |
22.10 |
21 |
|
Eclipta prostrata (L.) L. (Asteraceae) |
False Daisy |
Herb |
Leaves |
15.19 |
24 |
|
Emilia sonchifolia (L.) DC.ex DC. (Asteraceae) |
Red tassel-flower |
Herb |
Leaf |
28.71 |
24 |
|
Launaea sarmentosa Schult. Bip. ex Kuntze (Asteraceae) |
Beach Launaea |
Herb |
Whole plant |
22.57 |
47 |
|
Moringa oleifera Lam. (Moringaceae) |
Horse Raddish Tree |
Tree |
Flower |
20.52 |
37 |
|
Murraya koenigii Sprin (Rutaceae) |
Curry leaf tree |
Tree |
Leaves |
22.14 |
40 |
|
Musa sp. var. Nanjangud rasa bale (Musaceae) |
Banana |
Herb |
Flower pseudostem |
18 13 |
48 |
|
Nigella sativa L. (Ranunculaceae) |
Black cumin |
Herb |
Seeds |
28.49 |
21 |
|
Pistia stratiotes L. (Araceae) |
Water lettuce |
Herb |
Leaves |
12.06 |
49 |
|
Zanthoxylum rhetsa DC. (Rutaceae) |
Indian prickly ash |
Tree |
Leaves |
25.23 |
50 |
One plant, namely, Moringa oleifera (Fig. 2), has been listed twice in Tables 3 and 4 with different plant parts (leaves and flowers) and counted only once. M. oleifera is considered as ‘The Miracle tree’ with its multifarious beneficial activities for human health. Though its leaves and flowers possess moderate thrombolytic potential (between 20 to 42%), but along with many nutritive and therapeutic phytochemicals and other pharmacological activities, this plant could be very well recommended for dietary therapeutics in the prevention of cardiovascular diseases52.
Plants having >70% thrombolytic activity
Interestingly, ethanolic extract of Bauhinia purpurea (Fig. 3) leaves have shown 91.02% clot lysis activity, which was more than the standard drug streptokinase, having 72.83% clot lysis activity (Table 1). Leaves of B. purpurea are used as vegetables in Bengal, Bihar, Odisha, Jharkhand, Madhya Pradesh and some North-East states of India6. Leaves have shown presence of flavonoids, quercetin, rutin, apigenin and apigenin 7-O-glucoside along with lupeol (34.48%), stigmasterol (15.63 %), lanosterol (4.15 %), ergosterol (2.82 %), hexadeconic acid, hexadeconic acid methyl esters, octadecadienoic acids and octadecatrienoic acid, beta-tocopherol, phytol, and vitamin E acetate53,54 as well as hypoglycemic, antidiarrhoeal and antimicrobial55 activities. Similarly, aqueous extract of Baccaurea ramiflora seeds have shown 88.21% clot lysis, which was more than the standard drug streptokinase, which had 78.98% clot lysis (Table 1). B. ramiflora is a wild edible plant native to north-eastern states such as Meghalaya, Manipur, and Arunachal Pradesh6. It is also used during the holy rituals in the‘Rathyatra’ procession of Lord Jagannath in Odisha56. The fruits of B. ramiflora are rich in vitamin C, protein, and iron54. Seeds have shown presence of Sapidolide A and cytotoxic, analgesic, anti-inflammatory, CNS depressant and antidiarrheal57-58, antioxidant59, hypolipidemic60 and antimicrobial activities61. Seed oil contains palmitic acid (33.67%), stearic acid (19.38%), arachidic acid (9.38%), oleic acid (24.48%), 11-transeicosenoic acid (12.75%) and a high iodine value of 80.3262. In view of this, seeds of B. ramiflora could be included in the diet for nutritional as well as therapeutic benefits.
Plants having 50-70% thrombolytic activity
Table 2 shows that there are four plants that have shown more than 50% but less than 70% thrombolytic potential. These are Coccinia grandis (Fig. 4), Curcuma longa, Cyperus rotundus and Typha domingensis. Leaves of C. grandis have been shown to possess anti-inflammatory, antioxidant, hypoglycemic, hypolipidemic, analgesic, and antipyretic activities with beta-sitosterol as an important phyto-constituent along with phenolic and flavonoid compounds63. These are added advantage to its in vitro thrombolytic effect, which was 57.94% as observed in a study by Sultana et al.20. Methanolic extract of rhizome of C. longa has shown 53.32% in vitro clot lysis potential. In view of this and its other activities such as platelet aggregation inhibition, anti-inflammatory, antioxidant activities, it is one of the most useful spices used in Indian cuisines64,65. The active ingredient of Turmeric is Curcumin, which possesses anti-viral and immunomodulatory activities along with other pharmacological activities66. Tuberous roots of Cyperus rotundusL. are popularly known as ‘Nagarmotha’ in Western India and are used to treat various ailments by native communities of India. It is also used as an edible inNorth Bihar, Rajasthan and the Attapadi hills situated in the Western Ghats of India6. Ethanolic extract of its rhizome (200µg/ml) has shown 60% in vitro thrombolytic potential22 which validate the traditional knowledge on its use to treat heart stroke by Taungya community in Terai Arc Landscape of India67. Antioxidant, anti-inflammatory, cardioprotective, hypolipidemic, antidiabetic, antiobesity, antiplatelet etc. are some of its other health-beneficial activities, recommending it for dietary intake68. Leaves and shoots of Typha domingensis are used for food purpose by Konda Reddis of Rampa Agency, East Godavari District, Andhra Pradesh, and the inflorescence is consumed in North-East states of Assam and Arunachal Pradesh6. It has been shown to possess antioxidant, vasodilator, hypolipidemic, bronchodilator, cytotoxic, antimicrobial activities and is rich in vitamin E, nonacosane, piperine, triacontane, n-hexadecanoic acid, decanoic acid, tetracosane, oleic acid, phytol, sitosterol, naringenin, n-acetoacetyl-deacetylcolchicine and many other phyto-therapeutic compounds23,69.
 |
Figure 2: Moringa oleifera |
 |
Figure 3: Bauhinia purpurea |
 |
Figure 4: Coccinia grandis |
Spices having thrombolytic activity
Spices have shown moderate thrombolytic potential such as Clove (Syzygium aromaticum),Black pepper (Piper nigrum), Ginger (Zingiber officinale), Red chilly (Capsicum frutescens), Coriander (Coriandrum sativum), Indian Bayleaf (Cinnamomum tamala) as depicted in Table 3 and 4. Some of these spices have also demonstrated anti-platelet potential, which is an important mechanism of clot lysis17. Figure 5 depicts 20 common edible plants having in vitro thrombolytic activity out of the 43 scrutinized plant species. These species also possess several therapeutic bioactive compounds. For example, capsaicinoids, flavonoids, carotenoids, steroids, saponins in Capsicum; monoterpene, sesquiterpene, geraniol, linolol, bornyl acetate, phytosterols, caryophylene oxide, pcoumaric acid, vanillic acid etc. in Cinnamomum tamala; flavonoids, gallic acid, ferulic acid, coumarins, salicylic acid, tartaric acid, maleic acid, arbutin etc. in Coriandrum sativum; murrayazolidine, murrayazoline, murrayacine, koenimbine, koenine, mahanimbine,
 |
Figure 5: Some common edible plants demonstrating in vitro thrombolytic activity |
girinimbine, mukoeic acid etc. in Murraya koenigii (Fig. 6); p-cymene, carvacrol, thymoquinone, thymohydroquinone, dithymoquinone, 4-terpineol, tanethol, sesquiterpene longifolene α-pinene etc. in Nigella sativa; eugenol, carvacrol, linalool, β-caryophyllene, rosmarinic acid, oleanolic acid, ursolic acid etc. in Ocimum tenuiflorum (Fig. 7); gingerol, shoagaol, paradol, quercetin, zingerone, gingerenone-A in Zingiber officinale (Fig. 8); Piperine as major constituent in Piper nigrum besides volatile oil, oleoresins, and alkaloids; sesamin, sesamol, sesamolin, pinoresinol etc. in Sesamum indicum (Fig. 9) and eugenol, eugenyl acetate, β-caryophyllene etc. in Syzygium aromaticum70. Besides, many pharmacological activities such as antioxidant, anticancer, antidiabetic, anti-inflammatory, immuno-modulatory, hepatoprotective, platelet aggregation inhibition, neuro-protective, nephroprotective, and cardio-protective have also been reported from these plants8,70-72. This further emphasizes that use of these plants in the diet could be beneficial for many purposes.
 |
Figure 6: Murraya koenigii |
 |
Figure 7: Ocimum tenuiflorum |
 |
Figure 8: Zingiber officinale |
 |
Figure 9: Sesamum indicum |
Thrombolytic activity of leaves of edible plants
Plant based diets including leafy green vegetables provide strong evidence for benefits in cardiovascular risk factors such as obesity, diabetes, hypertension, high lipid levels etc.73. In the present analysis, leaves of 42% of the plants were consumed and reported to possess thrombolytic activity, followed by fruits (22%), seeds (13.33%), flowers (8.88%), and rhizome and whole plant (6.66% each), which is depicted in Figure 10.
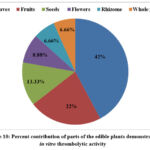 |
Figure 10: Percent contribution of parts of the edible plants demonstrated in vitro thrombolytic activity. |
Spinacia oleracea is a well-known leafy vegetable that has shown 40.9% thrombolytic potential (Table 3). It is considered a functional food as it is rich in various vitamins and minerals as well as many therapeutic phytochemicals such as flavones, flavanols, glucuronides, methylenedioxyflavonol glucuronides, and carotenoids. Spinach helps in scavenging reactive oxygen species and thereby prevents macromolecular oxidative damage. It has also been shown to modulate genes which are involved in metabolism, proliferation, inflammation, and antioxidant defense and therefore, demonstrates various pharmacological activities such as antioxidant, hypolipidemic, hypoglycemic, anti-hypertriglyceridemia, anti-obesity, anticancer, anti-inflammatory, anti-α-amylase, and bileacid binding capacity74,75. In view of this, intake of spinach can help in thrombolysis and also provide protection against several other ailments.
 |
Figure 11: Anacardium occidentale |
Leaves of Murraya koenigii; known as Curry leaf tree is used for flavor in various Indian cuisines6 and exhibited 22.14% in vitro thrombolytic potential (Table 4). The cardioprotective potential of Murraya koenigii has been demonstrated in doxorubicin-induced cardiotoxicity in Wistar albino rats. Lyophilized leaf extract of M. koenigii was administered orally in a concentration of 2 g/kg to animals for 14 days, which significantly reduced the levels of cardiac troponin I, NT-pro BNP, aspartate aminotransferase, lactate dehydrogenase, myeloperoxidase along with improvement in reduced glutathione, glutathione reductase, glutathione peroxidase, total antioxidant capacity, superoxide dismutase, and catalase activity, along with a reduction in lipid peroxidation levels76. This clearly indicates the cardio-protection ability of M. koenigii along with antioxidant and anti-inflammatory activities, and therefore, its daily consumption could be beneficial for the heart. Leaves of Bacopa monnieri; a well-known memory-improving plant, have shown 47.39% thrombolytic activity (Table 3). This small herb is rich in several phytochemicals for example, luteolin, quercetin, apigenin, ursolic acid, bacopasides, β-sitosterol, stigmasterol, ascorbic acid, bacopasaponins, bacoside, cucurbitacins, wogonin and exhibited various health-beneficial activities such as adaptogenic, antioxidant, antihypertensive, antilipidemia, anti-inflammatory, analgesic, antidiabetic, antiarthritic, anticancer, smooth muscle relaxant antipyretic, neuroprotective, and hepatoprotective77. Thus, clot lysis activity is an additional benefit gained by people after consuming its leaves. The leaves of sacred Holy Basil (Ocimum tenuiflorum) have also demonstrated anticoagulant properties by prolonging prothrombin time and activated partial thromboplastin time with linolenic acid as a major constituent78. The cardioprotective potential of hydroalcoholic extract of O. tenuiflorum has also been observed in isoproterenol induced myocardial infarction in rats at a dose of 50 mg/kg further indicating the importance of this herb in CVD79.
Leaves of Emilia sonchifolia are used to prepare vegetable in western Maharashtra and Nagaland states of India. It is also recommended to treat chest pain by tribal communities in India6. Notably, it has demonstrated 28.71% clot lysis activity (Table 4). This plant is rich in flavonoids, flavones glycosides, beta-sitosterol, stigmasterol, ursolic acid, quercetin, quercitrin, rutin, kaempferol 3-β-D-galactoside, senkirkine, doronine, n-hexacosanol, and triacontane and has been shown to possess antioxidant, antidiabetic, anti-cataract, anti-inflammatory, antiviral, analgesic and anti-cancer activities80. Leaves of Acmella paniculata are used for making vegetables in the north-eastern states of India and are mostly used for the treatment of toothache6. It is rich in phyto-constituents such as spilanthol, β-sitosterol, stigmasterol, α-and β-amyrin, vanillic acid, limonene, β-caryophyllene, (z)-β-ocimene, germacrene-D, scopoletin and trans-ferulic acid and has also demonstrated anti-inflammatory, antioxidant, vasorelaxant, immunomodulatory, and analgesic activities in animal studies81.
Brassica oleracea is another popular vegetable in India. Its flowers and leaves contain phenolics, polyphenols, saponins, tannins, steroids, flavonoids, alkaloids, glucosinolates, reducing sugars, and vitamin C. It has been shown to possess strong antioxidant activity in different antioxidant assays. Moreover, inhibition of DNA methylation, prevention of DNA damage and threats to cancer and cardiovascular diseases are its other benefits. All these bioactive molecules and pharmacological activities make it a good candidate for recommending as a nutraceutical in daily diet82. Whole plant of Launaea sarmentosa is used as vegetable in Lakshadweep Island6 and also in Vietnam as nutritious vegetable83. It has also shown anti-inflammatory, antioxidant, antidiabetic, and hepatoprotective properties along with 22.57% clot lysis potential84.
Thrombolytic activity of fruits of edible plants
Out of these 43, fruits from 10 plants are used in the diet (Fig. 10). For example, Anacardium occidentale (Fig. 11) is one of the famous edible tree nuts. It is consumed as raw, roasted form or also used to prepare sweets in the winter season85. Its kernels are rich in protein, vitamin E, K, B6, riboflavin, minerals like potassium, calcium, magnesium, phosphorous, iron, copper, zinc, manganese, selenium etc., glutamic acid, arginine, cholesterol-lowering phytosterols, phosphatidylcholine, beta-sitosterol, lutein, zeaxanthin, epicatechin, catechin, polyphenols, flavanol etc. Cashewnuts have been shown to possess antioxidant, hypoglycemic and hypotensive potential86. Thus, the thrombolytic action of A. occidentale is an additional weapon for protection from several diseases, including cardiovascular diseases and metabolic syndrome. Pomegranate is another well-known fruit plant which is rich in ellagitannins, polyphenols, luteolin, kaempferol, quercetin, gallic acid, ellagic acid, punicalagin, gallagic acid, delphinidin, cyanidin, pelargonidin, catechin, punicalin, and minerals, such as sodium, potassium, calcium, magnesium, phosphorus, and nitrogen. Fruit and peel of Punica granatum have shown anti-thrombotic potential besides anti-inflammatory, anti-diabetic, hypolipidemic, anti-platelet, anticoagulant, cardio-protective and anticancer properties87-89.
Fruits of Ficus racemosa, known as ‘Gular’ in India, are rich in nutritive value and full of phytochemicals such as hentriacontane, β sitosterol, tiglic acid, esters of taraxasterol, lupeol acetate, phytosterol, euphol, euphorbinol, isoeuphorbol, tannins, steroids, tinyatoxin, tri-methyl ellagic acid, flavonoids, alkaloids and have demonstrated hypolipidemic, anti-diabetic, anti-carcinogenic, antioxidant, gastroprotective and analgesic activities in various scientific studies90. Fruits of Capparis decidua (Fig. 12) are used to prepare the famous traditional Rajasthani cuisine ‘Panchkuta’ and are also used to make pickle. Interestingly, a methanolic extract of its fruits has shown a 32.39% clot lysis potential (Table 3). This is an important addition to its pharmacological profile besides anti-atherosclerotic, hypolipidemic, antioxidant, and anti-inflammatory properties91. Therefore, it could be used in dietary modification for therapeutic benefits.
Fruits of Solanum torvum are used as vegetable and also to prepare chutney in eastern India. Ethnic communities of the Mayurbhanj district of Odisha utilize its fruits for the treatment of heart disease6. Fruits are rich in steroidal glycosides, phenolic compounds, isoflavonoids, etc. Interestingly, its fruits have shown a 31.51% clot lysis potential (Table 3). Besides, thrombolytic potential, fruits of S. torvum have also demonstrated anti-inflammatory, antidiabetic, anti-ulcerogenic, anti-hypertensive and anticancer activities in scientific studies92. Fruits of Luffa cylindrica have exhibited 45% clot lysis potential (Table 3) and used as vegetable in Rajasthan, Kerala and Sikkim6. Its fruits possess vitamin A, B5, B6, C, and dietary fibers as well as phyto-constituents such as gallic acid, caffeic acid, cinnamic acid, ferulic acid, ellagic acid, rutin, quercetin, luteolin, bobin, vitexin, myrecetin, catechin, noctacosane, n-heptacosan, n-hexacosane, n-tetracosane, n-tricosane, etc. Many pharmacological activities have also been demonstrated by fruits of L. cylindrica such as antioxidant, anti-inflammatory, hypoglycemic, antimicrobial and sedative which potentiate its use in daily diet93.
Fruits of Averrhoa bilimbi are rich in ascorbic and oxalic acids and possess carbohydrates, proteins, amino acids, coumarin, flavonoids, tannins, essential oils, terpenes, and valepotriates. Besides, many phytochemicals such as hexadecanoic acid, butyl nicotinate, (Z)-9-octadecenoic acid, nonanoic acid, nonanal, (Z)-9-pentacosene, (Z)-3-hexenol, (Z)-9-tricosene, octane, tricosane, (E)-2-decenal, 2-furfural, 2,4-dihydroxy-6-((4-methylpentyloxy) methyl) benzaldehyde etc. have been isolated from its fruits94. Interestingly, anticoagulant activity of ethanol extracts of leaves and fruitsof A. bilimbi has been demonstrated in normal and alloxan-induced diabetic rats after oral administration at a dose of 250 mg/kg for 14 days, which has significantly increased prothrombin time95. This further validates the results obtained through in vitro clot lysis activity (Table 4). Seeds of Tribulus terrestris (Fig. 13) are consumed as famine food in Rajasthan6 and have shown to possess 33% in vitro thrombolytic activity. T. terrestris possesses various phytochemicals such as quercetin, kaempferol, isorhamnetin, rutin, tribuloside, tribulusamide C, tribulusterine, tribulusin A, harmine, benzoic acid, vanillic acid, 2-methyl benzoic acid, and ferulic acid. Moreover, it has also demonstrated antioxidant, hypoglycemic, antimicrobial, anti-inflammatory, cardio-protective, anticancer, anti-ageing and hepatoprotective properties41,96.
 |
Figure 12: Capparis decidua |
 |
Figure 13: Tribulus terrestris |
Edible plants having thrombolytic and platelet aggregation inhibition activities
Inhibition of platelet aggregation is another important mechanism for maintaining the patency of blood vessels. Many of the phyto-constituents such as caffeic acid, epigallocatechin, catechin, quercetin, kaempferol, apigenin, gallic acid present in the plants listed in this paper have also shown platelet aggregation inhibition activity97. Likewise, some other compounds, such as Piperin, isolated from Piper nigrum have shown inhibition of platelet aggregation through attenuation of cytosolic phospholipase A2 and thromboxane A2 synthase involving arachidonic acid (AA) metabolism98. Curcumin; a polyphenol isolated from Curcuma longa, also demonstrated platelet aggregation inhibition by activating adenosine A2A receptor-stimulated protein kinase A activation and phosphorylation of vasodilator-stimulated phosphoprotein99. Gingerol, shogaol, paradol, and gingerol analogues isolated from Zingiber officinale have also demonstrated anti-platelet function100. Similarly, (+)-nootkatone isolated from Cyperus rotundus has also shown significant in vitro collagen-, thrombin-, and AA-induced platelet aggregation inhibition in a dose-dependent manner as well as ex vivo platelet aggregation inhibition in mice blood by increasing tail bleeding time101. Sesamum indicum also possesses many bioactive molecules such as sesamin, epi-sesamin, sesamol, γ-tocopherol, and sesamolin. Among these, epi-sesamin has shown a strong anti-thrombotic effect by preventing thrombin and activated blood coagulation factor X production, prolonging activated partial thromboplastin time and prothrombin time, reducing thrombin-catalyzed platelet aggregation in mice as well as inhibiting TNFα-induced secretion of plasminogen activator inhibitor type 1 in human umbilical vein endothelial cells102.
Cuscutaroside A and its acetyl derivative, as well as scrophenoside B; isolated from whole plants of Cuscuta reflexa (Fig. 14), have demonstrated a weak platelet aggregation inhibitory activity induced by collagen with IC50 values of 291.4 ± 47.9 μg/ml, 63.8 ± 4.4 μg/ml, and 180.5 ± 6.7 μg/ml, respectively. However, an acetylated derivative of Cuscutaroside A has shown strong platelet aggregation inhibition induced by AA with an IC50 value of 72.6 ± 10.5 μg/ml103. A dose-dependent adenosine diphosphate induced platelet aggregation inhibition has been demonstrated by 70% ethanolic extract of Eclipta prostrata leaves with 74.55%; 65.60%; 48.00% and 39.08% inhibition at the concentration of 100, 80, 60 and 40 mg/ml respectively with an IC50 value of 59.02 mg/ml104. The mechanism behind the anti-platelet property of E. prostrata and the corresponding bioactive molecules needs to be researched. However, all these studies indirectly support the anti-thrombotic potential of these edible plant species and provide motivation for initiating further research in this direction.
 |
Figure 14: Cuscuta reflexa |
 |
Figure 15: Boerhavia diffusa |
COVID-19 treatment potential of edible thrombolytic plants
Recent pandemic of COVID-19 has hugely affected the human population and thrombotic complications are one of the major causes of morbidity and mortality associated with this infectious viral disease105. COVID-19-related coagulation disorders were associated with an increase in D-Dimer, fibrin degradation products, prothrombin time, activated partial thromboplastin time, and a decrease in antithrombin106. Interestingly, herbal medicine was also employed to combat this virus and several in vitro, in vivo, and clinical trials have been carried out and are currently underway107. Plants like Curcuma longa, Nigella sativa, Zingiber officinale, Piper nigrum, Ocimum tenuiflorum, Cuscuta reflexa, Moringa oleifera, etc. were used in herbal treatment and prevention of COVID-19. Some of the isolated phytochemicals such as quercetin, piperin, curcumin, apigenin, kaempferol, luteolin, thymol, rutin, eugenol, ursolic acid, caffeic acid, and oleanolic acid have also shown their potential against the SARS-CoV-2 virus and exhibited anticoagulant activity106. In view of their thrombolytic potential, the addition of these plants to the diet for treatment or for prevention purposes seems quite relevant because this has been proven in scientific research related to the efficacy of these plants in COVID-1965,107.
The present article has compiled the Indian edible plants having thrombolytic potential which are commonly used in the diets of various ethnic groups without knowing their multifarious beneficial effects on human health. Theoretically, it is possible to get benefit from these plants in the situation of altered thrombolytic states. In this context, the work of Sarkar16 is worth quoting, who has advised that few of the plants, for example, Boerhavia diffusa (Fig. 15), Chenopodium album, etc., should be used during the acute stage of heart disease such as acute myocardial infarction. He has stressed that during the acute stage, the diet should preferably include these vegetables. The rationale behind it could be their thrombolytic potential, antioxidant property, anti-platelet and hypolipidemic effect. Moreover, these vegetables are easily digestible without posing undue stress on the heart, which requires rest during the recovery period, and the gut favorable because of high fiber content, which does not allow constipation. Because straining on defecation during this crucial time is also hazardous and can cause arrhythmia and sudden death. Scientific studies have also shown that the intake of dietary fibers can reduce the risk of CVD through various means108. For example, a recent cohort study in South Korea has also indicated the role of quality plant food in the prevention of metabolic syndrome109. Therefore, dietary selection of plants with the above-mentioned properties could be helpful for management of CVD.
Safety issues
Though the edible plants mentioned in this article have thrombolytic activity, they should be consumed with caution. Over-consumption of the plants may cause adverse effects. For example, studies have shown that intake of curcumin in doses of 500-12000 mg produced symptoms like diarrhea, yellow stool, headache, and rashes110. Similarly, heartburn, nausea, abdominal pain, gas, bloating, etc. are some of the reported side effects of consumption of Z. officinale (750-2000mg)111. Fruits of A. bilimbi can cause nephro- and neurotoxicity112 and consumption of 80% fresh plants of T. terrestris has shown harmful effects on cardiac muscle, liver and kidney of goats and sheep113. In various animal studies, after consumption of M. oleifera, genotoxicity, kidney and liver damage, necrosis of splenic blood vessels, and neuronal glial cells have been reported114. Hepatotoxicity and gastrointestinal disorders after consumption of C. sinensis on empty stomach have been reported115. S. oleracea grown in heavy metal polluted sites can create risk for cancer116. Likewise, nitrate poisoning has been observed after ingestion of Brassica oleracea var. capitata117. Some of these plants such as T. domingensis118, B. purpurea119, O. tenuiflorum120, S. torvum121, P. granatum122, B. monnieri123 and M. koenigii124 have yet to be tested for significant toxicity. However, plants having higher thrombolytic activity should be recommended cautiously to patients already taking anti-platelet and fibrinolysis enhancing drugs and supplements because there is a possibility of bleeding.
Conclusion
The present article provides an overview of 43 plants which have demonstrated thrombolytic potential and are also being utilized by ethnic communities for edible purposes. If these species could be added as a supplement to the diets of people who are predisposed / susceptible to cardio-vascular diseases, they may serve the role of preventive agents. Addition of these plant species to the diet, both before and after the development of disease, could be beneficial in myriad ways due to the multifarious therapeutic potential of plants, which mostly act in a synergistic manner. However, large scale clinical studies could be executed for scientific evaluation of the effect of the addition of these plant species in the diet on the prevention or betterment of CVD. In this regard, the two edible plants with the highest clot lysis potential, namely, Baccaurea ramiflora and Bauhinia purpurea, should be screened for their in vivo thrombolytic potential on a priority basis.
Conflict of Interest
Authors declare that there is no conflict of interest.
Funding sources
References
- Jain S. K and Jain V. Methods and Approaches in Ethnobotany (Concepts, Practices and Prospects). Deep Publications, New Delhi. 2017.
- Abera M. Ethnobotanical study of wild edible plants and their indigenous knowledge in Sedie Muja district, South Gondar Zone, Northwestern Ethiopia. Am. J. Plant Sci., 2022; 13: 241-264. DOI: 10.4236/ajps.2022.132015.
- Ashagre M, Asfaw Z and Kelbessa E. Ethnobotanical study of wild edible plants in Burji District, Segan Area Zone of Southern Nations, Nationalities and Peoples Region (SNNPR), Ethiopia. J. Ethnobiol. Ethnomed., 2016; 12: 32. DOI: 10.1186/s13002-016-0103-1
- Cao Y, Li R, Zhou S, Song L, Quan R and Hu H. Ethnobotanical study on wild edible plants used by three trans-boundary ethnic groups in Jiangcheng County, Pu’er, Southwest China. J Ethnobiol Ethnomed., 2020; 16: 66. DOI: 10.1186/s13002-020-00420-1
- Mallick S. N, Sahoo T, Naik S. K, Panda P. C. Ethnobotanical study of wild edible food plants used by the tribals and rural populations of Odisha, India for food and livelihood security. Plant Arch., 2020; 20: 661-669.
- Jain V and Jain S. K. Compendium of Indian Folk Medicine and Ethnobotany (1991-2015). Deep Publications, New Delhi, 2016.
- León-Lobos P, Díaz-Forestier J, Díaz R, Celis-Diez JL, Diazgranados M and Ulian T. Patterns of Traditional and Modern Uses of Wild Edible Native Plants of Chile: Challenges and Future Perspectives. Plants, 2022; 11: 744. DOI: 10.3390/ plants11060744
- Ray S and Saini M. K. Cure and prevention of cardiovascular diseases: Herbs for heart. Clin Phytosci., 2021; 7: 64. DOI: 10.1186/s40816-021-00294-0
- Al-Snafi A. E. Blood lipids lowering effect of medicinal plants. GSC Biol. Pharm. Sci., 2022; 19(03): 015–043. DOI: 10.30574/gscbps.2022.19.3.0213
- Mohd Nor N. H, Othman F, Mohd Tohit E. R and Md Noor S. Medicinal herbals with antiplatelet properties benefit in coronary atherothrombotic diseases. Thrombosis., 2016; DOI: 10.1155/2016/5952910.
- Baig M. U and Bodle J. Thrombolytic Therapy. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2022: Available from: https://www.ncbi.nlm.nih.gov/books/NBK557411/ PMID: 32491343
- Dunn P. S and Macaulay E. T. Drug-drug interactions associated with antiplatelet therapy. Cardiovasc. Hematol. Agents Med. Chem., 2011; 9: 231-240. DOI: 10.2174/187152511798120912
- Jilani T. N and Siddiqui A. H. Tissue Plasminogen Activator. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing., 2022; Available from: https://www.ncbi.nlm.nih.gov/books/NBK507917/
- Tapsell L. C. Dietary behaviour changes to improve nutritional quality and health outcomes. Chronic. Dis. Transl. Med., 2017; 3(3): 154-158. DOI: 10.1016/j.cdtm.2017.06.005.
- Estruch R, Ros E, Salas-Salvadó J, Covas M. I, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J and Lamuela-Raventos R. M. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med., 2018; 378(25): 2441-2442. DOI: 10.1056/NEJMc1806491.
- Sarkar P. R. Yogic treatments and natural remedies. 2nd ed. AMPS Publications, 1986; Tiljala, Calcutta.
- Subramani B and Sathiyarajeswaran P. Current update on herbal sources of antithrombotic activity-a comprehensive review. Egyptian J. Intern. Med., 2022; 26:1-12. DOI: 10.1186/s43162-021-00090-9
- Al-Masud KN, Runa MM, Hasan R, Khan M. N. M, Ahmed N, Chowdhury A. F and Keya S. I. Study of cytotoxic and thrombolytic activity of Baccaurea ramiflora in different extracts. Pharma. Innov., 2018; 7(10): 271-274.
- Kiranmayi G. V. N. Preliminary phytochemical screening and in vitro evaluation of antiinflammatory, antiarthritic, and thrombolytic activities of ethanolic leaf extract of Bauhinia purpurea. Int. J. Green Pharm., 2018; 12(1): 241-247.
- Sultana R, Nahar K, Bachar S. C. In-vitro membrane stabilizing, thrombolytic, antioxidant and antimicrobial activities of Bangladeshi origin Coccinia indica (Cucurbitaceae). African J. Pharm. Pharmacol., 2018; 12(16): 188-192. DOI: 10.5897/AJPP2018.4913
- Al-Mamun M. R, Amrin N, Begum J and Mazid M. A. Thrombolytic activity of some spices and plants available in Bangladesh. Thai J. Pharm. Sci., 2012; 36: 72-77.
- Prabhu N, Kiruthiga R, Kowsalya R, Jeevitha S, Singh M. V. P, Archana A and Gajendran T. Evaluation of phytochemicals and histochemicals of Cyperus rotandus and its thrombolytic activity. J. Pharma. Res. Int., 2022; 34(8B): 18-30.
- Dilshad R, Khan KUR, Ahmad S, Aati, HY, Al-qahtani JH, Sherif, AE, Hussain M, Ghalloo BA, Tahir H, Basit A and Ahmed M. Phytochemical profiling, in vitro biological activities, and in-silico molecular docking studies of Typha domingensis. Arabian J. Chem. 2022; 15(10):104133. https://doi.org/10.1016/j.arabjc.2022.104133
- Tabassum F, Chandi S. H, Mou K. N, Hasif K. I, Ahamed T and Akter M. In vitro thrombolytic activity and phytochemical evaluation of leaf extracts of four medicinal plants of Asteraceae family. J. Pharmacogn. Phytochem., 2017; 6(4): 1166-1169.
- Khan I. N, Habib M. R, Rahman M. M, Mannan A, Sarker MMI, Hawlader S. Thrombolytic potential of Ocimum sanctum L., Curcuma longa L., Azadirachta indica L. and Anacardium occidentale L. J. Basic Clin. Pharm., 2011; 2(3):125.
- Sai S. Y, Panigrahi M, Divya G. C, Beena D. B. Evaluation of in vitro thrombolytic activity of phytochemicals in Bacopa monnieri Linn. J. Pharm. Res., 2012; 5(1): 100-101.
- Kunwar B, Jain V and Verma S. K. In vitro clot lysis activity of Boerhavia diffusa L. leaves. Pacific J. Med. Health Sci., 2021; 3(3):1-7.
- Kamal A. M, Chowdhury K. A. A, Shill L. K, Hossain M. R, Islam N, Anaytulla I. A and Hassan M. F. Phytochemical screening, cytotoxic and thrombolytic activity of extract of Brassica oleracea flower (cauliflower). Glob. J. Pharmacol., 2015; 9(1): 115-120.
- Emran T. B, Rahman M. A, Uddin M. M. N, Rahman, M. M, Uddin M. Z, Dash, R and Layzu C. Effects of organic extracts and their different fractions of five Bangladeshi plants on in vitro thrombolysis. BMC Complement. Altern. Med., 2015; 15(1):1-8. DOI: 10.1186/s12906-015-0643-2
- Kunwar B, Jain V and Verma S. K. Qualitative phytochemical screening and in vitro thrombolytic activity of Capparis decidua Edgew. Fruit. GSC Bio. Pharm. Sci., 2022; 19(3):160–167. DOI: 10.30574/gscbps.2022.19.3.0232
- Azad A. K, Laboni F. R, Rashid H, Ferdosh S, Rashid S. S, Kamal N, Labu Z. K, Islam M. S and Islam Sarker Z. In vitro evaluation of Cuscuta reflexa Roxb. for thrombolytic, antioxidant, membrane stabilizing and antimicrobial activities. Nat. Prod. Res., 2018; 18(3): 1-4. DOI: 10.1080/14786419.2018.1538216
- Shivasharanappa K and Londonkar R. Clot lysis and antimitotic study of Ficus glomerata Roxb. fruit extracts. ISRN Pharmacol., 2014; 2014: Article ID 975303. DOI: 10.1155/2014/975303
- Ali M, Sayem S. A. J, Quah Y, Lee E. B, Birhanu B. T, Suk, K and Park S. C. Investigation of potential antioxidants, thrombolytic and neuropharmacological activities of Homalomena aromatica leaves using experimental and in Silico Approaches. Molecules., 2021; 26(4): 975. DOI: 10.3390/molecules26040975
- Rahman M. A, Sultana R, Emran T. B, Islam M. S, Rahman M. A, Chakma J. S and Hasan C. M. M. Effects of organic extracts of six Bangladeshi plants on in vitro thrombolysis and cytotoxicity. BMC Complement. Altern. Med., 2013; 13(1):25. DOI: 10.1186/1472-6882-13-25
- Gandhamlla P, Buddola S. G, Rachakonda P, Manga R and Boggula N. Preliminary phytochemical analysis and thrombolytic screening of Luffa cylindrica Linn. fruits an in vitro study. Int. J. Innov., 2018; 6(1): 61-74.
- Akter S, Jahan I, Khatun M. R, Khan M. F, Arshad L, Jakaria M and Haque M. A. Pharmacological insights into Merremia vitifolia (Burm. f.) Hallier f. leaf for its antioxidant, thrombolytic, anti-arthritic and anti-nociceptive potential. Biosci. Rep., 2021; 41(1): DOI: 10.1042/BSR20203022
- Kunwar B, Jain V and Verma S. K. In vitro thrombolytic activity of Moringa oleifera. Nusantara Biosci., 2022; 14(1): 63-69.
- Emon N. U, Kaiser M, Islam M, Kabir M. F. I, Jamir M, Uddin M. A. J and Islam, M. N. Anxiolytic and thrombolytic investigation of methanol extract of Piper nigrum L. fruits and Sesamum indicum L. seeds. J. Adv. Biotechnol. Exp. Ther., 2020; 3(3): 158-164.
- Sampath R, Saravanan R, Pemiah B and Ramalingam S. Thrombolytic activity of Punica granatum fruit and peel extract. Asian J. Pharma. Clin. Res., 2016; 9(1): 268–271.
- Ramakrishnan PA and Amrithalingam P. Evaluation of thrombolytic activity of Murraya koenigii and Spinacia oleracea, in vivo and in vitro. Helix., 2014; 6: 622-630.
- Siddique MH, Andleeb R, Ashraf A, Zubair M, Fakhar-e-Alam M, Hayat S, Muzammil S, Atif M, Shafeeq S, Afzal M. Integration of in silico and in vitro approaches to evaluate antioxidant and anticancer properties of Tribulus terrestris extracts. Arabian J. Chem 2022; 15(8): 103984. https://doi.org/10.1016/j.arabjc.2022.103984
- Mowla T. E, Zahan S, Sami S. A, Uddin S. N and Rahman M. Potential effects and relevant lead compounds of Vigna mungo (L.) Hepper seeds against bacterial infection, helminthiasis, thrombosis and neuropharmacological disorders. Saudi J. Bio. Sci., 2022; 29(5): 3791-3805.
- Hussain M. S, Hossain M. S, Amin M. T and Millat M. S. In vitro thrombolytic potentials of methanolic extract of Vigna unguiculata Linn. (seed). J. Pharmacogn. Phytochem., 2016; 5(3):129.
- Manju P and Pushpa D. A. A. Study on thrombolytic and cytotoxic activity of methanolic extract of Zingiber officinale. Int. J. Life Sci. Pharma Res., 2020; 10(5): 1-5. 10(5):1-5. DOI 10.22376/ijpbs/lpr.2020.10.5.L1-5
- Ramjan A, Hossain M, Runa J. F, Md H and Mahmodul I. Evaluation of thrombolytic potential of three medicinal plants available in Bangladesh, as a potent source of thrombolytic compounds. Avicenna J. Phytomed., 2014; 4(6): 430.
- Ratnasooriya W. D, Fernando T. S. P and Madhubashini P. P. In vitro thrombolytic activity of Sri Lankan black tea (Camellia sinensis L.). J. Nat. Sci. Found. Sri Lanka. 2008; 36 (2): 179-181.
- Moghal M. M. R, Millat M. S, Hussain M. S, Islam M. R. Thrombolytic and membrane stabilizing activities of Launaea sarmentosa. Int J Pharmacogn., 2016; 3(8): 354-358. DOI:10.13040/IJPSR.0975-8232.IJP.3(8).354-58
- Ramu R, Shirahatti P, Zameer F, Lakkapa D. B and Prasad N. M. N. Evaluation of Banana (Musa sp. var. Nanjangud Rasa bale) flower and pseudostem extracts on antimicrobial, cytotoxicity and thrombolytic activities. Int. J. Pharm. Pharmaceut. Sci. 2015; 7(1): 136-40. https://innovareacademics.in/journals/index.php/ijpps/article/view/3531.
- Hossen S. M, Sarkar M. M. I and Jahid M. A. Assessment of thrombolytic activity of five Bangladeshi medicinal plants: Potential source for thrombolytic compounds. Int. Blood Res. Rev., 2014; 2(6): 262-269. DOI: 10.9734/IBRR/2014/9623
- Azad A. K, Islam O, Rima E, Islam M, Sultana C, Nesa, J. U andAhmed, F. Phytochemical Screenining and In-Vitro Thrombolytic Activity of Methanolic Leaf Extract of Zanthoxylum rhetsa. J. Pharm. Sci. Res., 2015; 7(6):302-304. DOI: 10.4236/am.2017.82012
- Website 1. https://powo.science.kew.org/
- Prajapati C, Ankola M, Upadhyay T. K, Sharangi A. B, Alabdallah N. M, Al-Saeed F. A, Muzammil K and Saeed M. Moringa oleifera: Miracle plant with a plethora of medicinal, therapeutic, and economic importance. Horticulturae., 2022: 8: 492. DOI: 10.3390/horticulturae8060492
- The Wealth of India – Raw materials, First Supplement Series. 2004. 1: First reprint, NISCAIR, New Delhi.
- Gupta A. K, Sharma M and Tandon N. 2004. Reviews on Indian Medicinal Plants. Vol. 4: ICMR, New Delhi.
- Negi B. S, Dave B. P, Agarwal Y. K. Evaluation of antimicrobial activity of Bauhinia purpurea leaves under in vitro conditions. Indian J. Microbiol., 2012; 52(3): 360–365. DOI: 10.1007/s12088-012-0264-0
- Goyal A. K, Middha S. K and Usha T. Baccaurea ramiflora Lour.: a comprehensive review from traditional usage to pharmacological evidence. Adv. Tradit. Med. 2020. DOI: 10.1007/s13596-020-00489-9
- Bordoloi M, Barua N. C, Mohan S, Dutta S. C, Mathur R. K, Ghosh A. C and Rychlewska U. Sapidolide A: An unprecedented spherical carbocyclic lactone from Baccaurea sapida seed kernels: Is it a meroisoprenoid? Tetrahedron Lett., 1996; 7(37): 6791-6792. DOI: 10.1016/s0040-4039(96)01480-3
- Nesa M. L, Karim S, Api K, Sarker M, Islam M. M, Kabir A, Sarker M, Nahar K, Asadujjaman M and Munir M. S.. Screening of Baccaurea ramiflora (Lour.) extracts for cytotoxic, analgesic, anti-inflammatory, neuropharmacological and antidiarrheal activities. BMC Complement. Altern Med., 2018; 18(1): 35. DOI: 10.1186/s12906-018-2100-5
- Uddin M, Sahab Md, Al Mamun A, Tewari D, Asaduzzaman M, Islam M. S and Abdel-Daim M. M.. Phytochemical analysis and antioxidant profile of methanolic extract of seed, pulp and peel of Baccaurea ramiflora Lour. Asian Pac. J. Trop. Med. 2018; 11: 443-450. DOI: 10.4103/1995-7645.237189
- Alam Y, Hossain S, Fakir S, Das A, Afia I. J and Podder, P. S. Hypolipidemic effect of ethanolic seeds extract of Baccaurea ramiflora in wister albino rats. Inter. Res. J. Pharm. Med. Sci., 2019; 3(1): 25-27.
- Akter S and Sarker A. Antimicrobial activities of seeds of Diospyros blancoi and Baccuarea ramiflora. Int. J. Adv. Pharmacy Biol. Chem., 2015; 4(4): 789-793.
- Gogoi B. Baccaurea ramiflora Lour.: Biochemical and ethnobotanical value with scope for bio-prospection. Ann. Plant Sci., 2017; 6:1649-1652. DOI:10.21746/aps.2017.07.001
- Kumar M, Alok S, Chanchal D. K, Bijauliya R. K, Yadav R. D and Sabharwal M. An updated pharmacological activity of Coccinia indica (Wight & Arn.). Int. J. Pharm. Sci. Res., 2018; 9(2): 456-65. DOI: 10.13040/IJPSR.0975-8232.9(2).456-65
- Srivastava K. C, Bordia A and Verma S. K. Curcumin, – a major component of food spice turmeric (Curcuma longa) inhibits aggregation and alters eicosanoid metabolism in human blood platelets. Prost Leuk Essen Fatty acids., 1995; 52: 223-227. DOI: 10.1016/0952-3278(95)90040-3
- Jain A and Jain V. An overview of some potential traditional medicinal plant species against Covid-19: Harvesting details for optimal bioactive. In: Medicinal Plants Phytochemistry and Therapeutics (Ed.: Patni, V.) Agrobios Research , 2021. Jodhpur, India.
- Zahedipour F, Hosseini SA, Sathyapalan T, Majeed M, et al. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother. Res. 2020 ; 34(11):2911–2920. DOI: 10.1002/ptr.6738
- Kumari P and Singh G. S. Ethnobotanical study of medicinal plants used by the Taungya community in Terai Arc Landscape, India. J Ethnopharmacol., 2009; 123: 167-176. DOI: 10.1016/j.jep.2009.02.037
- Pirzada A. M, Ali H. H, Naeem M, Latif M, Bukhari A. H and Tanveer A. Cyperus rotundus L.: Traditional uses, phytochemistry, and pharmacological activities. J. Ethnopharmacol., 2015; 174: 540–560. DOI: 10.1016/j.jep.2015.08.012
- Akram A and Jabeen Q. Pharmacological evaluation of Typha domingensis for its potentials against diet-induced hyperlipidemia and associated complications. Trop. J. Pharma. Res., 2022; 21 (3): 563-569
- Khanal A, Devkota H. P, Kaundinnyayana S, Gyawali P, Ananda R and Adhikari R. Culinary herbs and spices in Nepal: A review of their traditional uses, chemical constituents, and pharmacological activities. Ethnobot. Res. Appl. 2021; 21: 40. DOI: 10.32859/era.21.40.1-18
- Masoodi M. H, Rehman M. U. Edible Plants in Health and Diseases. Volume II: Phytochemical and Pharmacological Properties. Springer, Singapore., 2022; DOI: 10.1007/978-981-16-4959-2
- Mao Q. Q, Xu X. Y, Cao S. Y, Gan R. Y, Corke H, Beta T and Li H. B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods., 2019; 8(6): 185. DOI: 10.3390/foods8060185. PMID: 31151279; PMCID: PMC6616534.
- Pallazola V. A, Davis D. M, Whelton S. P, Cardoso A, Latina J. M, Michos E. D, Sarkar S, Blumenthal R. S, Arnett D. K, Stone N. J and Welty F. K, A clinician’s guide to healthy eating for cardiovascular disease prevention. Mayo Clinic Proceedings: Innovations, Quality & Outcomes., 2019; 3(3): 251-267. DOI: 10.1016/j.mayocpiqo.2019.05.001
- Roberts J. L and Moreau R. Functional properties of spinach (Spinacia oleracea L.) phytochemicals and bioactives. Food Funct., 2016; 7(8): 3337-53. DOI: 10.1039/c6fo00051g.
- Gutierrez R. M. P, Velazquez E. G and Carrera S. P. P. Spinacia oleracea Linn. considered as one of the most perfect foods: A Pharmacological and Phytochemical Review. Mini. Rev. Med. Chem., 2019; 19(20): 1666-1680. DOI: 10.2174/1389557519666190603090347
- Jayasinghe A. N, Hewawasam S. R. P, Jayatilaka K. A. P. W, Mudduwa L. K. B. Cardioprotective potential of Murraya koenigii (L.) Spreng. Leaf Extract against Doxorubicin-Induced Cardiotoxicity in Rats. Evidence-Based Complement. Altern. Med., 2020, Article ID 6023737. DOI: 10.1155/2020/6023737
- Jeyasri R, Muthuramalingam P, Suba V, Ramesh M and Chen J-T. Bacopa monnieri and their bioactive compounds inferred multi-target treatment strategy for neurological diseases: A cheminformatics and system pharmacology approach. Biomolecules., 2020; 10(4): 536. DOI: 10.3390/biom10040536
- Gunendren M, Nordin S, Ramachandran M and Samad N. Effect of Ocimum sanctum (Tulsi) aqueous leaf extract on prothrombin time (PT), activated partial thromboplastin time (APTT) and thrombin time (TT) of human plasma. J Biomed Clin Sci., 2017; 2(1): 62-68. http://apps.amdi.usm.my/journal/index.php/jbcs/article/view/77
- Sharma M, Kishore K, Gupta S. K, Joshi S and Arya D. S. Cardioprotective potential of Ocimum sanctum in isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem., 2001; 225(1): 75-83. DOI: 10.1023/a:1012220908636. PMID: 11716367.
- Dash G. K, Syafiq A. M and Ruhaiyem Y. Traditional uses, phytochemical and pharmacological aspects of Emilia sonchifolia (L.) DC. Int. J. Res. Ayurveda Pharm., 2015; 6: 551-556. DOI: 10.7897/2277-4343.064103
- Dubey S, Maity S, Singh M, Saraf S. A and Saha S. Phytochemistry, pharmacology and toxicology of Spilanthes acmella: a review. Adv. pharmacol. sci., 2013; DOI: 10.1155/2013/423750
- Nawaz H, Shad M. A and Muzaffar S. Phytochemical Composition and Antioxidant Potential of Brassica. In: El-Esawi MA. editor. Brassica Germplasm – Characterization, Breeding and Utilization [Internet]. London: IntechOpen., 2018; DOI: 10.5772/intechopen.76120
- Them L.T., Tuong Nguyen Dung, P., Thi Nhat Trinh P., Tong Hung Q., Tuong L.N., Trong Tuan, N., Duc Lam T., Thuy Nguyen V., Dung L.T. Saponin, Polyphenol, Flavonoid content and α-glucosidase Inhibitory Activity, Antioxidant Potential of Launaea sarmentosa Leaves grown in Ben Tre province, Vietnam. IOP Conf. Ser. Mater. Sci. Eng. 2019 ; 542: 012036.
- Nguyen T. Q. C, Binh T. D, Kusunoki R, Pham T. L. A, Nguyen Y. D. H, Nguyen T. T, Kanaori K and Kamei K. Effects of Launaea sarmentosa extract on Lipopolysaccharide-Induced Inflammation via Suppression of NF-κB/MAPK Signaling and Nrf2 Activation. Nutrients., 2020; 12(9): 2586. DOI: 10.3390/nu12092586.
- Jain V. Sweets as traditional medicine in winter season: An ethnobotanical study in Udaipur city, India. Ethnobot Res Appl (Online)., 2020; 20: 1-17. DOI: 10.32859/era.20.31.1-17
- Perez J. Food as Medicine
Cashew (Anacardium occidentale, Anacardiaceae). Herbalgram. 2020; Available online at: https://www.herbalgram.org/resources/herbalegram/volumes/volume-17/number-10-october-2020/food-as-medicine-cashew/food-as-medicine-cashew/ - Prakash C.V.S and Prakash I. Bioactive chemical constituents from pomegranate (Punica granatum) juice, seed and peel-a re-view. Int. J. Res. Chem. Environ. Technol., 2011; 1–18.
- Riaz A and Khan R. A. Anticoagulant, antiplatelet and antianemic effects of Punica granatum (pomegranate) juice in rabbits. Blood Coagul. Fibrinolysis., 2016; 3:287-293.
- Moga M. A, Dimienescu O G, Bălan A, Dima L, Toma S. I, Bîgiu N. F and Blidaru A. Pharmacological and therapeutic properties of Punica granatum Phytochemicals: possible roles in breast cancer. Molecules, 2021; 26(4):1054. DOI: 10.3390/molecules26041054
- Chaware G. K, Kumar V, Kumar S and Kumar P. Bioactive compounds, pharmacological activity and food application of Ficus racemosa: A Critical Review, Int. J. Fruit Sci., 2020; 20(sup2): S969-S986. DOI: 10.1080/15538362.2020.1774467
- Nazar S, Hussain M. A, Khan A, Muhammad G and Tahir M. N. Capparis decidua Edgew (Forssk.): A comprehensive review of its traditional uses, phytochemistry, pharmacology and nutrapharmaceutical potential. Arabian J. Chem., 2020; 13(1): 1901-1916.
- Harley B. K, Neglo D, Tawiah P, Pipim M. A, Mireku-Gyimah N. A, Tettey CO, Amengor C. D, Fleischer T. C and Waikhom S. D. Bioactive triterpenoids from Solanum torvum fruits with antifungal, resistance modulatory and antibiofilm formation activities against fluconazoleresistant candida albicans strains. PLoS ONE., 2021; 16(12): e0260956. DOI: 10.1371/journal. pone.0260956
- Akinwumi K. A , Eleyowo O. O and Oladipo O. O. A Review on the ethnobotanical uses, phytochemistry and pharmacological effect of Luffa cylindrinca. In (Ed.), Natural Drugs from Plants. IntechOpen. in H. A. El-Shemy (ed.), Natural Drugs from Plants, IntechOpen, 2021; London. DOI: 10.5772/intechopen.98405
- Alhassan A. M and Ahmed Q. U. Averrhoa bilimbi Linn.: A review of its ethnomedicinal uses, phytochemistry, and pharmacology. J Pharm Bioallied Sci., 2016; 8(4): 265–271. DOI: 10.4103/0975-7406.199342
- Daud N, Hashim H and Samsulrizal N. Anticoagulant activity of Averrhoa bilimbi Linn. in normal and alloxan-induced diabetic rats. Open Conf. Proc. J., 2013; 4(Suppl 2, M6): 21–6. DOI: 10.2174/2210289201304020021
- ZhuW, Du Y, Meng H and Li L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem. Cent. J. 11., 2017; 60 (2017): DOI: 10.1186/s13065-017-0289-x
- Tamer F, Tullemans B. M. E, Kuijpers M. J. E, Claushuis T. A. M and Heemskerk J. W. M. Nutrition Phytochemicals Affecting Platelet Signaling and Responsiveness: Implications for Thrombosis and Hemostasis. Thrombo. Haemost., 2022; 122(06): 879-894. DOI: 10.1055/a-1683-5599.
- Son D. J, Akiba S, Hong J. T, Yun Y. P, Hwang S. Y, Park Y. H and Lee S. E. Piperine inhibits the activities of platelet cytosolic phospholipase A2 and thromboxane A2 synthase without affecting cyclooxygenase-1 activity: different mechanisms of action are involved in the inhibition of platelet aggregation and macrophage inflammatory response. Nutrients., 2014; 6(8): 3336-52. DOI: 10.3390/nu6083336.
- Rukoyatkina N, Shpakova V, Bogoutdinova A, Kharazova A, Mindukshev I and Gambaryan S. Curcumin by activation of adenosine A2A receptor stimulates protein kinase a and potentiates inhibitory effect of cangrelor on platelets. Biochem. Biophysic Res. Comm., 2022; 586: 20-26. DOI: 10.1016/j.bbrc.2021.11.006
- Nurtjahja-Tjendraputra E, Am.mit A. J, Roufogalis B. D, Tran V. H and Duke C. C. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb. Res., 2003; 111(4-5): 259-65. DOI: 10.1016/j.thromres.2003.09.009. PMID: 14693173.
- Seo E. J, Lee D. U, Kwak J. H, Lee S. M, Kim Y.S and Jung Y. S. Antiplatelet effects of Cyperus rotundus and its component (+)-nootkatone. J. Ethnopharmacol., 2011; 135(1): 48-54. DOI: 10.1016/j.jep.2011.02.025.
- Dalibalta S, Majdalawieh A. F and Manjikian H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm. J., 2020; 28(10): 1276-1289. DOI: 10.1016/j.jsps.2020.08.018.
- Aung T. T. T, Xia M. Y, Hein P. P, Tang R. Zhang D. D, Yang J, Yang X. F, Hu D. B and Wang Y. H. Chemical Constituents from the whole plant of Cuscuta reflexa. Nat. Prod. Bioprospect., 2020; 10: 337–344. DOI: 10.1007/s13659-020-00265-x
- Sandhiutami N. M. D and Desmiaty Y. Inhibitory Effect of Lantana camara L., Eclipta prostrata (L.) L. and Cosmos caudatus Kunth. Leaf extracts on ADP-Induced Platelet Aggregation. Pharmacog. J., 2018; 10(3): 581-585. DOI : 10.5530/pj.2018.3.95
- Avila J, Long B, Holladay D and Gottlieb M. Thrombotic complications of COVID-19. Am. J. Emerg. Med., 2021; 39: 213-218. DOI 10.1016/j.ajem.2020.09.065
- Lamponi S. Potential use of plants and their extracts in the treatment of coagulation disorders in COVID-19 disease: a narrative review. Longhua Chin. Med., 2021; 4: 26. DOI: 10.21037/lcm-21-23
- Alam S, Sarker M. M. R, Afrin S, Richi F. T, Zhao C, Zhou J. R and Mohamed I. N. Traditional herbal medicines, bioactive metabolites, and plant products against COVID-19: update on clinical trials and mechanism of actions. Front Pharmacol., 2021; 12: 671498. DOI: 10.3389/fphar.2021.671498
- Soliman GA. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients., 2019 ; 11(5):1155. doi: 10.3390/nu11051155
- Kim H, Lee K, Rebholz C. M and Kim J. Plant-based diets and incident metabolic syndrome: Results from a South Korean prospective cohort study. PLoS Med., 2020; 17(11): e1003371. DOI: 10.1371/journal.pmed.1003371.
- Hewlings S. J, Kalman D. S. Curcumin: A review of its effects on human health. Foods, 2017; 6(10): 92. DOI: 10.3390/foods6100092.
- Anh N. H, Kim S J, Long N. P, Min J. E, Yoon Y. C, Lee E. G, Kim M, Kim T. J, Yang Y. Y, Son E. Y, Yoon S. J, Diem N. C, Kim H. M and Kwon S. W. Ginger on human health: a comprehensive systematic review of 109 randomized controlled trials. Nutrients, 2020; 12(1):157. DOI: 10.3390/nu12010157.
- Wong K. W and Lansing M. G. Case of acute kidney injury due to A. bilimbi fruit ingestion. BMJ Case Rep., 2021;14(7):e242325. DOI: 10.1136/bcr-2021-242325.
- Pokrywka A, Obmiński Z, Malczewska-Lenczowska J, Fijałek Z, Turek-Lepa E and Grucza R. Insights into supplements with Tribulus terrestris used by athletes. J. Hum. Kinet., 2014; 41:99-105. DOI: 10.2478/hukin-2014-0037.
- Stohs S. J and Hartman M. J. Review of the safety and efficacy of Moringa oleifera. Phytother. Res., 2015; 29(6):796-804. DOI: 10.1002/ptr.5325.
- Bedrood Z, Rameshrad M and Hosseinzadeh H. Toxicological effects of Camellia sinensis (green tea): A review. Phytother. Res., 2018; 32(7):1163-1180. DOI: 10.1002/ptr.6063.
- Xiang G, Wu X and Long S. Evaluating the heavy metal risk in Spinacia oleracea L. and its surrounding soil with varied biochar levels: A pot experiment. Sustainability, 2021; 13(19):10843. DOI: 10.3390/su131910843.
- Nguta J. M. Nitrate Poisoning due to Ingestion of Cabbages (Brassica oleracea var. capitata L.) (Brassicaceae) in Kitui County, Kenya. Sci. World J., 2019; 8716518. DOI: 10.1155/2019/8716518.
- Dilshad R, Khan K. U, Saeed L, Sherif A. E, Ahmad S, Ovatlarnporn C, Nasim J, Hussain M, Ghalloo B. A, Basit A and Mukhtar I. Chemical composition and biological evaluation of Typha domingensis Pers. to ameliorate health pathologies: In Vitro and In Silico approaches. Biomed. Res. Int., 2022; 2022: 8010395. DOI: 10.1155/2022/8010395.
- Kumar S, Kumar R, Gupta Y. K and Singh S. In vivo anti-arthritic activity of Bauhinia purpurea Linn. bark extract. Indian J. Pharmacol., 2019; 51(1):25-30. DOI: 10.4103/ijp.IJP_107_16.
- Jamshidi N and Cohen M. M. The clinical efficacy and safety of tulsi in humans: a systematic review of the literature. Evid. Based Complement. Alternat. Med., 2017; 2017: 9217567. DOI: 10.1155/2017/9217567.
- Vadakkan K. Acute and sub-acute toxicity study of bacterial signaling inhibitor Solanum torvum root extract in Wister rats. Clin. Phytosci., 2019; 5(19). DOI: 10.1186/s40816-019-0113-3.
- Patel C, Dadhaniya P, Hingorani L and Soni M. G. Safety assessment of pomegranate fruit extract: acute and subchronic toxicity studies. Food. Chem. Toxicol., 2008; 46(8):2728-35. DOI: 10.1016/j.fct.2008.04.035.
- Sireeratawong S, Jaijoy K, Khonsung P, Lertprasertsuk N. and Ingkaninan K. Acute and chronic toxicities of Bacopa monnieri extract in Sprague-Dawley rats. BMC Complement. Altern. Med., 2016; 16:249. https://doi.org/10.1186/s12906-016-1236-4
- Balakrishnan R, Vijayraja D, Jo S. H, Ganesan P, Su-Kim I and Choi D. K. Medicinal profile, phytochemistry, and pharmacological activities of Murraya koenigii and its Primary bioactive compounds. Antioxidants (Basel), 2020; 9(2):101. DOI: 10.3390/antiox9020101.







