Manuscript accepted on :20-04-2023
Published online on: 21-06-2023
Plagiarism Check: Yes
Reviewed by: Dr. Revathi Shenoy and Dr. Nataliya Kitsera
Second Review by: Dr. Kulvinder Kaur
Final Approval by: Dr. Patorn Piromchai
Seema Srivastava1* , Raksha Sharma1
, Raksha Sharma1 and Manish Kumar Sharma2
and Manish Kumar Sharma2
1Department of Zoology, Reproductive Physiology Lab, University of Rajasthan, Jaipur-302004, Rajasthan, India.
2Department of Zoology, Raj Rishi Autonomous College, Alwar, Rajasthan, India.
Corresponding Author E-mail: drseemaa07@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2657
Abstract
The p53 gene is a tumor suppressor gene and, thus, plays an important role in cell cycle, cell senescence, DNA repair, and cell death. Since testicular tissues go through a continuous and complex process of spermatogenesis, p53 is likely to play a significant role in the regulation of germ cell proliferation and spermiogenesis. In the present study, the specific localization of p53 in testicular tissues was determined by comparing it with BPA induced toxicity. Four groups containing 10 albino rats each were designated as Group I: Control, Group II: 10 mg/kg BPA, Group III: 50 mg/kg BPA, and Group IV: 100 mg/kg BPA. Daily administration of BPA was carried out through oral gavage for 6 weeks by dissolving the assigned weight of BPA in olive oil. Testicular tissues were investigated for expression of p53 by immunohistochemistry, and testicular sperms were examined under a scanning electron microscope. Results showed that p53 was exclusively expressed in the spermatogonia of animals exposed to 10 mg/kg BPA. The highest expression of p53 was present in animals exposed to 50 mg/kg BPA; besides spermatogonia, spermatocytes and spermatids also indicated positive expression. However, relatively lower expression was evident in animals exposed to 100 mg/kg BPA, as most cellular architecture was already distorted significantly, and germ cells appeared to have fallen into the lumen of seminiferous tubules. The ultrastructure of testicular sperm indicated specific damage to the perforatorium, plasma membrane, and connecting pieces around the neck, and tail. Damages occurring in the head cap segment of the perforatorium indicated an alteration during spermiogenesis. In conclusion, it is highly likely that a BPA induced alteration in the expression of p53 may have affected spermiogenesis through spermatogenesis.
Keywords
Bisphenol A; p53; Spermatogenesis; Spermiogenesis
Download this article as:| Copy the following to cite this article: Srivastava S, Sharma R, Sharma M. K, Immunohistochemical and Ultrastructural Evaluation of Spermatogenic Alteration by P53 under the Influence of Bisphenol-A. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Srivastava S, Sharma R, Sharma M. K, Immunohistochemical and Ultrastructural Evaluation of Spermatogenic Alteration by P53 under the Influence of Bisphenol-A. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/43NQPr3 |
Introduction
Cellular growth and proliferation are highly regulated processes. Among various factors, p53 superfamily proteins play a significant role in cellular proliferation. In cases of abnormal stress or cellular damage, p53 signals repair machinery to maintain genomic integrity. In cases where repair is not possible, it induces cell-cycle arrest and apoptosis.1 According to many studies, p53 is silenced in most cancerous cells.2,3 Apoptosis is an important phenomenon in cell growth and proliferation, in the absence of it, tumours may form in most cells. Hair follicles, skin cells, gastrointestinal cells, lymphocytes, germ cells, etc. require stringent control over proliferation and differentiation. Under slight variations in the cellular microenvironment, the role of p53 becomes extremely valuable in the regulation of damage destined to occur.
Spermatogenesis is an ideal function to understand the importance of p53. Mature sperm develop from the complex differentiation of spermatogonial cells, spermatocytes, and spermatids. However, before spermatogonia turn into spermatocytes, they follow a series of five subsequent divisions, such as; A1, A2, A3, A4, and B 4. A study by Beumer et al.5 showed that differentiation of A2-B spermatogonia was relatively more radioresistant than that of their parallel controls in p53 knockout mice. Similarly, other studies also revealed a significant role for p53 during the prophase of meiosis (Beumer et al.6 leading to higher expression of p53 in spermatocytes.7
Bisphenol A is a polymerizing agent used in plastics and plastic products. BPA is an endocrine disruptor that negatively interferes with the regulation of spermatogenesis.8 There is ample information on BPA that it causes apoptotic damage in Sertoli cells 9-12 and germ cells.13 A study by Lloyd et al.14 reported that BPA has a stimulatory effect on p53. BPA and p53 are also associated with each other by means of oxidative stress; previous studies have associated BPA with targeted oxidative stress in testicular tissue.15,16 Interestingly, p53 regulates antioxidant activities to ensure cell survival during low oxidative stress while promoting cell death during high oxidative stress.17 Oxidative damages in sperms follow a typical pattern such as head tail separation, axonemal damage, and multiple morphological defects.18 Based on earlier studies, the present study attempted to evaluate the role of p53 at various stages of spermatogenesis in animals administered with BPA. This study also investigated patterns in types of deformities associated with oxidative stress in testicular sperm and related theoretically with p53 expression.
Materials and methods
Test material
Bisphenol A or 2,2-bis(4-hydroxyphenyl) propane (≥99%) was made commercially available from Sigma Aldrich, MO, USA.
Test animals
Male albino rats (Rattus norvegicus) were used in the present study. These rats were selected based on their age (3 months) and weight (150–200 g). All animals were maintained under the strict observation of a veterinary expert. The university’s departmental facility provided polypropylene cages (43×27×15 cm) and housing conditions where 12:12-h of light: dark was ensured. Experiments carried out under the guidelines of the Committee for the Purpose of Control and Supervision of Experiments and Animals.19 Institutional Animal Ethics Committee (IAEC)-approved experimental protocols were used and performed under the procedures of the Indian National Science Academy (INSA), New Delhi, for the care and use of animals.
Experimental design
Animals were randomly divided into four groups, consisting of 10 animals each. Group I: vehicle-treated control; Group II: administered with 10 mg BPA/kg b.w. dissolved in olive oil; similarly, Group III: 50 mg BPA/kg b.w. dissolved in olive oil; and Group IV: 100 mg BPA/kg b.w. BPA was administered through oral gavage by dissolving it in olive oil in a 1:1 ratio. Accordingly, BPA was administered daily for 6 weeks.
Detection and localization of Apoptotic marker
The immunohistochemistry of testis tissue was done by the Avidin-Biotin Complex (ABC) immunostaining method as described in the kit. Formalin-fixed, paraffin-embedded tissues were heated to expose antigenic residue. A concentration of 2.5 μg/ml (1:1000) of primary antibody was used for staining, and the solution was incubated overnight at room temperature. After incubation with the primary antibody, slides were incubated with biotinylated secondary antibodies, followed by horseradish peroxidase-streptavidin and chromogen.20 Apoptotic profiles were calculated based on set criteria in the focal plane.
Scanning Electron Microscopy
Sperm collected from the cauda epididymis were washed twice with phosphate buffer (pH 7.0) and centrifuged at 1500 rpm for 15 minutes. Sperm pellets were fixed in 2.5% glutaraldehyde for 30 minutes and washed three times in phosphate buffer followed by distilled water. A thin film of spermatozoa was smeared on a clean glass slide, air dried, and mounted on an SEM stub with silver paint. A coated sputter at 350 Åwas observed under ascanning electron microscope.
Results
Detection and localization of an apoptotic marker
Results indicated limited cellular expression of p53 in control (Group I) animals. The most stained areas were in and around Leydig cells. In Group II, the degree of p53 expression was higher, and these expressions were mostly localised in and around Leydig cells. Expression of p53 was also evident in seminiferous tubules, more specifically, in the lumen, but staining was not very strong. Group III’s histological slide indicated higher expression of p53 compared to control and Group II. Besides Leydig cells, the basal lamina also indicated high expression of p53. Group IV, notably, indicated lower expression of p53 in areas proximal to the basal lamina, compared to Group II. However, there were clear indications of higher expression in germ cells, which appeared to have fallen into the lumen. The lower expression at spermatogonial location was only due to a low cell count and high disorientation at cellular architecture (Figure 1A–1D).
 |
Figure 1: Immunostaining of testicular tissues against p53 showed minimum number of positive spermatogonia in control (Group I) (A), likewise, slightly higher but limited to spermatogonial cells were found positive for p53 in Group II (B), |
Scanning Electron Microscopy
SEM analysis of cauda epididymis spermatozoa was carried out to assess surface morphology. Control animals indicated normal morphology; sperm contained a perfect hook-shaped head with an uninterrupted surface and an intact acrosome (Figure 2A). Intact homogeneous plasma membrane throughout the head, middle piece, neck, and tail. Sperm midpiece width and sperm head width at the neck and head joints were normal. Treatment groups evidently showed various types of deformities in sperms (Figures 2B–H). These include a flattened apical perforatorium, an irregular head cap segment, and a perifossal zone indicating abnormal appearance and damage to the connecting piece (Figure 2B). Dissolution of the dorsal acrosomal system with limited leakage of cellular fluid (Figure 2C). A combination of head and tail separation, an abnormal head, shrinkage in curvature, and a flattened perforatorium were apparent in BPA treated animals (Figure 2D). Abnormal rough surface on the head; cytoplasmic leakage at the acrosomal sheath; dysplasia of the fibrous sheath; and a damaged mid-piece were evident (Figures 2E–F). Most BPA treated animals showed spermatozoons having coiled and looped tails (Figures 2G–H).
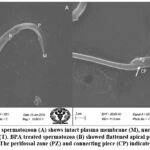 |
Figure 2.(A-B): Control spermatozoon (A) shows intact plasma membrane (M), nucleus (N), acrosome (R), perforatorium (P) and tail (T). BPA treated spermatozoa (B) showed flattened apical perforatorium and distorted head cap segment (HCS). |
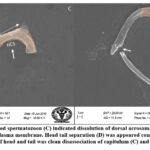 |
Figure 2.(C-D): BPA treated spermatozoon (C) indicated dissolution of dorsal acrosomal system (AS) with partial leakage through dorsal plasma membrane. |
 |
Figure 2.(E-F): Treated animals indicated spermatozoa with damaged plasma membrane and formation of cytoplasmic droplets (Cd) in mid-piece (MP) and head. |
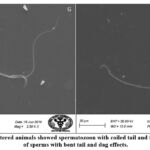 |
Figure 2.G-H: BPA administered animals showed spermatozoon with coiled tail and free tail. There were number of sperms with bent tail and dag effects. |
Discussion
The tumour suppressor molecule p53 is also referred to as the guardian of cell cycle21 has specific roles in cellular development and proliferation. According to various studies, checkpoints such as; G1/S and G2/M are stringently controlled by p53.22-24 Genetic instability through internal or external interferences can upregulate expression of p53, leading to cell cycle arrest and eventually apoptosis.25-26 Expression of p53 could be dose-dependent, as an earlier study by Lloyd et al.14 reported that an increase in the concentration of low-level BPA can inversely affect p53 expression. Similarly, another study by Dairkee et al.46 reported that BPA induces aberrant expression of crucial p53 checkpoints. In response to high oxidative stress, prooxidative genes are upregulated by the p53, causing an increased ROS level that promotes apoptosis in targeted cells17. BPA is well researched toxicant that has targeted induction of oxidative stress in testicular tissues.15, 27-28 In the present study, modulation of p53 expression in testicular tissues of BPA treated animals was investigated.
The present study noted positive expression of p53 in the interstitial spaces between seminiferous tubules in those animals treated with BPA. The p53 expression in Leydig cells was common in all dose groups and relatively higher than control. A study by Inoue and Wada29 examined the nuclear accumulation of p53 in testicular tumours of dogs and discovered that Leydig cells were associated with a high level of p53 accumulation, whereas, weak accumulation was evident in primary spermatocytes. There are many studies that corroborate damages in Leydig cells under testicular oxidative stress 30. It appeared that under the influence of BPA, Leydig cells showed a dose dependent increase in p53 expression. Similarly, BPA-administered animals indicated higher p53 positive spermatogonia than control animals. It was apparent that spermatogonia in animals administered with 50 mg/kg b.w. of BPA were highly upregulated compared to animals administered with 10 mg/kg b.w. and 100 mg/kg b.w. BPA doses. Since most germ cells were severely disoriented in the 100 mg/kg group and appeared to have fallen into the lumen, comparing the spermatogonial expression of p53 was not parallel. Although a significant increase in the expression of p53 was noted in the 50 mg/kg groups compared to the 10 mg/kg group, It emerged that spermatogonia were more tolerant to oxidative stress compared to Leydig cells. A study by Huang et al.31 revealed that spermatogonia were more tolerant to toxicants (Pb) than Leydig cells. Other studies also reported that spermatogonia are highly tolerant to oxidative stress.32-33 It could be speculated that spermatogonia may have a higher tolerance for BPA-induced oxidative stress. However, at higher doses, expression of p53 was localised in various cells, including Leydig cells, spermatogonia, and spermatocytes. Interestingly, spermatocytes showed maximum tolerance against BPA-induced oxidative stress and respective expression of p53. Nonetheless, animals administered 100 mg/kg BPA indicated relatively higher numbers of p53-positive spermatocytes. Although there is limited information on spermatocytes potential tolerability against reactive oxygen species (ROS), nevertheless, Agarwal et al.34 reported that spermatogonia were more tolerant to ROS-generated oxidative stress compared to primary spermatocytes. In contrast, the present study indicated greater toleration in spermatocytes; a possible explanation for this could be alternative action induced by oxidative stress (other than p53 activation) such as autophagy, which inhibits the progression of apoptosis.35
A SEM micrograph showed specific damages in the testicular spermatozoon, such as; flattened perforatorium area, indicating an acrosomal defect. It is a part of the perinuclear theca, consisting of the post-acrosomal sheath, subacrosomal layer, and perforatorium.36-37 The perforatorium is formed early during spermiogenesis, more specifically around elongation phase.38 This particular part is not distinctly present in human sperm. Thus, damages found in this region of sperm indicate a significant alteration in morphosis during the elongation phase of spermiogenesis. Spermiogenesis occurs quickly after meiotic divisions. An alteration in the activity of p53 can impact spermiogenesis, leading to spermatids undergoing apoptosis.39 A study by Sharma et al.40 reviewed how oxidative insult can affect the epigenetic mechanisms of spermatogenesis. Perforatorium forms during the elongation phase of spermatogenesis, therefore, it is highly likely that defects occurred during this stage, which is promoted by BPA-induced oxidative stress.47 Although there is no clear evidence that oxidative stress may cause flattened perforatorium in spermatozoa, oxidative damage in sperm may lead to other head deformities. A review by Lohiya et al.41 reported that reactive oxygen species can variably affect spermatozoa, leading to eventual loss of fertility. Authors claimed that the plasma membrane of spermatozoa is highly responsive to oxidative stress, leading to damage in the acrosome and absence of plasma membrane in the mid-piece and tail. The present study also observed the dissolution of the plasma membrane and dorsal acrosomal system. Besides, loss of segmented columns and numeric aberration of the centriole in the neck are also effects of oxidative stress.42 This study indicated a similar pattern in the head and tail separation. It was noticeable that both a flattened perforatorium and head-tail separation existed simultaneously. Irregular expression of proteins leads to changes in the post-acrosomal sheath38. It is important to note that these alterations may have been carried out during spermiogenesis, which indicates interference by BPA in spermatogenesis. Previous studies have indicated impairment of spermatogenesis in BPA-exposed animals.13,43 There is no direct impact of p53 on spermiogenesis, but it appears to have an impact on overall spermatogenesis. The role of Sertoli cells during spermiogenesis is suspected to have played an important role in deformities, however, the present study did not find a significant role for the p53 response in these cells. This study further suggests that apoptosis in Leydig cells may have been associated with undernourished germ cells and subsequent deformities in sperm. This study also observed typical coiling, bent tail, and dag effects indicative of oxidative stress. Lipid peroxidation of the plasma membrane and cytoplasmic droplets have a direct association.44 Appearances of cytoplasmic droplets are indicative of failure to resist oxidative stress. There is another alternative for the presence of cytoplasmic residue: during spermiogenesis, a small amount of cytoplasmic residue normally remains in sperm. These sperm could be defective even after maturation and may impact fertility.45
Conclusion
Localization of p53 revealed differential expression in germ cells. It appeared that spermatocytes and spermatogonial cells were more tolerant to low-dose BPA-induced toxicity compared to other testicular cells. In conclusion, p53 positively impacted spermatogenesis under the influence of BPA, and in addition, it also impacted spermiogenesis. However, the present study was based on qualitative immunohistochemical observations thus further quantitative study on each phase of spermatogenesis would reveal more details on activities of p53 following BPA exposure. It appeared that p53 may have contributed to sperm deformities through specific metamorphic transitions from spermatocytes to spermatids. For further investigation of the role of Sertoli cells during spermiogenesis under targeted effects, BPA must be investigated in association with p53 and other apoptotic factors to understand specific sperm deformities.
Conflict of Interest
There are no conflict of interest.
Funding Sources
The authors are thankful to the Department of Science and
Technology (DST), New Delhi, for financial assistance provided by the DST
INSPIRE FELLOWSHIP. The grant number is IF160160 and the Department of Zoology,
University of Rajasthan, for providing necessary facilities.
References
- Finlay, C. A., Hinds, P. W., & Levine, A. J. (1989). The p53 proto-oncogene can act as a suppressor of transformation. Cell, 57(7), 1083–1093. doi:10.1016/0092-8674(89)90045-7
CrossRef - Vousden, K. H., & Lane, D. P. (2007). p53 in health and disease. Nature Reviews. Molecular Cell Biology, 8(4), 275–283. doi:10.1038/nrm2147
CrossRef - Brady, C. A., & Attardi, L. D. (2010). p53 at a glance. Journal of Cell Science, 123(15), 2527–2532. doi:10.1242/jcs.064501
CrossRef - Griswold, M. D. (2016). Spermatogenesis: The commitment to meiosis. Physiological Reviews, 96(1), 1–17. doi:10.1152/physrev.00013.2015
CrossRef - Beumer, T. L., Roepers-Gajadien, H. L., Gademan, I. S., van Buul, P. P., Gil-Gomez, G., Rutgers, D. H., & de Rooij, D. G. (1998). The role of the tumor suppressor p53 in spermatogenesis. Cell Death and Differentiation, 5(8), 669–677. doi:10.1038/sj.cdd.4400396
CrossRef - Beumer, T. L., Roepers-Gajadien, H. L., Gademan, I. S., Rutgers, D. H., & de Rooij, D. G. (1997). P2 (Cip1/WAF1) expression in the mouse testis before and after X-irradiation. Molecular Reproduction and Development, 47(3), 240–247. doi:10.1002/(SICI)1098-2795(199707)47:3<240::AID-MRD2>3.0.CO;2-L
CrossRef - Iida, H., Maehara, K., Doiguchi, M., Mōri, T., & Yamada, F. (2003). Bisphenol A-induced apoptosis of cultured rat Sertoli cells. Reproductive Toxicology, 17(4), 457–464. doi:10.1016/s0890-6238(03)00034-0
CrossRef - Qi, S., Fu, W., Wang, C., Liu, C., Quan, C., Kourouma, A., . . . Yang, K. (2014). BPA-induced apoptosis of rat Sertoli cells through Fas/FASL and JNKs/p38 MAPK pathways. Reproductive Toxicology, 50, 108–116. doi:10.1016/j.reprotox.2014.10.013
CrossRef - Qian, W., Zhu, J., Mao, C., Liu, J., Wang, Y., Wang, Q., . . . Wang, J. (2014). Involvement of CaMCaMKII- ERK in bisphenol A-induced Sertoli cell apoptosis. Toxicology, 324, 27–34. doi:10.1016/j.tox.2014.06.001
CrossRef - Wang, C., Fu, W., Quan, C., Yan, M., Liu, C., Qi, S., & Yang, K. (2015). The role of Pten/Akt signaling pathway involved in BPA-induced apoptosis of rat Sertoli cells. Environmental Toxicology, 30(7), 793–802. doi:10.1002/tox.21958
CrossRef - Jin, P., Wang, X., Chang, F., Bai, Y., Li, Y., Zhou, R., & Chen, L. (2013). Low dose bisphenol A impairs spermatogenesis by suppressing reproductive hormone production and promoting germ cell apoptosis in adult rats. Journal of Biomedical Research, 27(2), 135–144. doi:10.7555/JBR.27.20120076
CrossRef - Lloyd, V., Morse, M., Purakal, B., Parker, J., Benard, P., Crone, M., . . . Dinda, S. (2019). Hormone-like effects of bisphenol A on p53 and estrogen receptor alpha in breast cancer cells. BioResearch Open Access, 8(1), 169–184. doi:10.1089/biores.2018.0048
CrossRef - Meli, R., Monnolo, A., Annunziata, C., Pirozzi, C., & Ferrante, M. C. (2020). Oxidative stress and BPA toxicity: An antioxidant approach for male and female reproductive dysfunction. Antioxidants, 9(5), 405. doi:10.3390/antiox9050405
CrossRef - Pallotti, F., Pelloni, M., Gianfrilli, D., Lenzi, A., Lombardo, F., & Paoli, D. (2020). Mechanisms of testicular disruption from exposure to bisphenol A and phtalates. Journal of Clinical Medicine, 9(2), 471. doi:10.3390/jcm9020471
CrossRef - Liu, D., & Xu, Y. (2011): p53. p53, oxidative stress, and aging. Antioxidants and Redox Signaling, 15(6), 1669–1678. doi:10.1089/ars.2010.3644
CrossRef - Kurkowska, W., Bogacz, A., Janiszewska, M., Gabryś, E., Tiszler, M., Bellanti, F., . . . Kasperczyk, A. (2020). Oxidative stress is associated with reduced sperm motility in normal semen. American Journal of Men’s Health, 14(5), 1557988320939731. doi:10.1177/1557988320939731
CrossRef - CPCSEA. (2010). Guidelines on the regulation of scientific experiments on animals. New Delhi: Ministry of Environment and Forests, CPCSEA standard operating procedures for institutional animals Ethics Committee (IAEC).
- Hsu, S. M., Raine, L., & Fanger, H. (1981). The use of antiavidin antibody and avidin-biotin-peroxidase complex in immunoperoxidase techniques. Journal of Histochemistry and Cytochemistry, 29(4), 577–580. doi:10.1177/29.4.6166661
CrossRef - Lane, D. P. (1992). Cancer. P53, guardian of the genome. Nature, 358(6381), 15–16. doi:10.1038/358015a0
CrossRef - Agarwal, M. L., Agarwal, A., Taylor, W. R., & Stark, G. R. (1995): p53 controls both the G2/M and the G1 cell cycle checkpoints and mediates reversible growth arrest in human fibroblasts. Acad. Sci. USA. Proceedings of the NAD, 92, 8493–8497.
CrossRef - Pellegata, N. S., Antoniono, R. J., Redpath, J. L., & Stanbridge, E. J. (1996). DNA damage and p53-mediated cell cycle arrest: A reevaluation. Proceedings of the National Academy of Sciences of the United States of America, 93(26), 15209–15214. doi:10.1073/pnas.93.26.15209
CrossRef - Ko, L. J., & Prives, C. (1996): p53. p53: Puzzle and paradigm. Genes and Development, 10(9), 1054–1072. doi:10.1101/gad.10.9.1054
CrossRef - Chernova, O. B., Chernov, M. V., & Agarwal, M. L. (1995), TaylorWRand StarkGR: The role of p53 in regulating genomic stability when DNA and RNA synthesis are inhibited. TIBS, 20, 431–434.
CrossRef - Guillouf, C., Rosselli, F., Krishnaraju, K., Moustacchi, E., Hoffman, B., & Liebermann, D. A. (1995). p53 involvement in control of G2 exit of the cell cycle: Role in DNA damage-induced apoptosis. Oncogene, 10(11), 2263–2270.
CrossRef - Olukole, S. G., Ola-Davies, E. O., Lanipekun, D. O., & Oke, B. O. (2020). Chronic exposure of adult male Wistar rats to bisphenol A causes testicular oxidative stress: Role of gallic acid. Endocrine Regulations, 54(1), 14–21. doi:10.2478/enr-2020-0003
CrossRef - Rezaee-Tazangi, F., Zeidooni, L., Rafiee, Z., Fakhredini, F., Kalantari, H., Alidadi, H., & Khorsandi, L. (2020). Taurine effects on bisphenol A-induced oxidative stress in the mouse testicular mitochondria and sperm motility. JBRA Assisted Reproduction, 24(4), 428–435. doi:10.5935/1518-0557.20200017
CrossRef - Inoue, M., & Wada, N. (2000). Immunohistochemical detection of p53 and p21 proteins in canine testicular tumours. Veterinary Record, 146(13), 370–372. doi:10.1136/vr.146.13.370
CrossRef - Aitken, R. J., & Roman, S. D. (2008). Antioxidant systems and oxidative stress in the testes. Oxidative Medicine and Cellular Longevity, 1(1), 15–24. doi:10.4161/oxim.1.1.6843
CrossRef - Huang, H., Wang, M., Hou, L., Lin, X., Pan, S., Zheng, P., & Zhao, Q. (2021). A potential mechanism associated with lead-induced spermatogonia and Leydig cell toxicity and mitigative effect of selenium in chicken. Ecotoxicology and Environmental Safety, 209, 111671. doi:10.1016/j.ecoenv.2020.111671
CrossRef - Celino, F. T., Yamaguchi, S., Miura, C., Ohta, T., Tozawa, Y., Iwai, T., & Miura, T. (2011). Tolerance of spermatogonia to oxidative stress is due to high levels of Zn and Cu/Zn superoxide dismutase. PLOS ONE, 6(2), e16938. doi:10.1371/journal.pone.0016938
CrossRef - Wu, P. Y., Scarlata, E., & O’Flaherty, C. (2020). Long-term adverse effects of oxidative stress on rat epididymis and spermatozoa. Antioxidants, 9(2), 170. doi:10.3390/antiox9020170
CrossRef - Agarwal, A., Virk, G., Ong, C., & du Plessis, S. S. (2014). Effect of oxidative stress on male reproduction. World Journal of Men’s Health, 32(1), 1–17. doi:10.5534/wjmh.2014.32.1.1
CrossRef - Yin, J., Ni, B., Yang, Y. D., Tang, Z. W., Gao, Z. Q., Feng, L., . . . Gao, Y. Q. (2018). Elevation of autophagy rescues spermatogenesis by inhibiting apoptosis of mouse spermatocytes. Reproduction, 156(6), 545–558. doi:10.1530/REP-18-0243
CrossRef - Oko, R., & Maravei, D. (1994). Protein composition of the perinuclear theca of bull spermatozoa. Biology of Reproduction, 50(5), 1000–1014. doi:10.1095/biolreprod50.5.1000
CrossRef - Oko, R. J. (1995). Developmental expression and possible role of perinuclear theca proteins in mammalian spermatozoa. Reproduction, Fertility, and Development, 7(4), 777–797. doi:10.1071/rd9950777
CrossRef - Protopapas, N., Hamilton, L. E., Warkentin, R., Xu, W., Sutovsky, P., & Oko, R. (2019). The perforatorium and postacrosomal sheath of rat spermatozoa share common developmental origins and protein constituents†. Biology of Reproduction, 100(6), 1461–1472. doi:10.1093/biolre/ioz052
CrossRef - Allemand, I., Anglo, A., Jeantet, A. Y., Cerutti, I., & May, E. (1999). Testicular wild-type p53 expression in transgenic mice induces spermiogenesis alterations ranging from differentiation defects to apoptosis. Oncogene, 18(47), 6521–6530. doi:10.1038/sj.onc.1203052
CrossRef - Sharma, P., Ghanghas, P., Kaushal, N., Kaur, J., & Kaur, P. (2019). Epigenetics and oxidative stress: A twin-edged sword in spermatogenesis. Andrologia, 51(11), e13432. doi:10.1111/and.13432
CrossRef - Lohiya, N. K., Alam, I., Hussain, M., Khan, S. R., & Ansari, A. S. (2014). RISUG: An intravasal injectable male contraceptive. Indian Journal of Medical Research, 140, Suppl.(Suppl 1): S63-72, S63–S72.
- Aitken, R. J., & Baker, M. A. (2006). Oxidative stress, sperm survival and fertility control. Molecular and Cellular Endocrinology, 250(1–2), 66–69. doi:10.1016/j.mce.2005.12.026
CrossRef - Chianese, R., Viggiano, A., Urbanek, K., Cappetta, D., Troisi, J., Scafuro, M., . . . Meccariello, R. (2018). Chronic exposure to low dose of bisphenol A impacts on the first round of spermatogenesis via SIRT1 modulation. Scientific Reports, 8(1), 2961. doi:10.1038/s41598-018-21076-8
CrossRef - Nichi, M., Goovaerts, I. G., Cortada, C. N., Barnabe, V. H., De Clercq, J. B., & Bols, P. E. (2007). Roles of lipid peroxidation and cytoplasmic droplets on in vitro fertilization capacity of sperm collected from bovine epididymis stored at 4 and 34 degrees C. Theriogenology, 67(2), 334–340. doi:10.1016/j.theriogenology.2006.08.002
CrossRef - Cooper, T. G. (2011). The epididymis, cytoplasmic droplets and male fertility. Asian Journal of Andrology, 13(1), 130–138. doi:10.1038/aja.2010.97
CrossRef - Dairkee, S. H., Luciani-Torres, M. G., Moore, D. H., & Goodson, W. H. 3rd. (2013). Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis, 34(3), 703–712. doi:10.1093/carcin/bgs379
CrossRef - Aitken, R. J., Smith, T. B., Jobling, M. S., Baker, M. A., & De Iuliis, G. N. (2014). Oxidative stress and male reproductive health. Asian Journal of Andrology, 16(1), 31–38. doi:10.4103/1008-682X.122203
CrossRef







