Manuscript accepted on :05-12-2022
Published online on: 23-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Ammar Almulathanon
Second Review by: Dr. Niharika Kondepud
Final Approval by: Dr. Ayush Dogra
Manisha Bhatti1 , Jitender Singh2 and Divya Dhawal Bhandari 3*
, Jitender Singh2 and Divya Dhawal Bhandari 3*
1University Institute of Pharma Sciences, Chandigarh University, Gharuan, Mohali (Punjab), India
2Institute of Pharmaceutical Sciences, IET Bhaddal Technical Campus, Bhaddal, District Rupnagar, Punjab India
Corresponding Author E-mail: nainagumber@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2671
Abstract
Background: Paspalum Scrobiculatum (Kodomillet) plant is found in various regions of India and possesses several pharmacological activities such as nutritive, anti-fungal, anti-diabetic, etc. The researchers are more focused these days on exploring other potential activities of this plant and the constituents present. Methods: Present study emphasized preliminary analysis such as macroscopic study, phytochemical analysis, Physicochemical investigation, determination of ash content, (Total flavonoid content), antioxidant property and characterization of extracted components with different spectral techniques and antiulcer potential. later, this study will be helpful in the determination of its medicinal importance in Pharmacy. Results: In macroscopic analysis, it was studied that paspalum scrobiculatum seeds are light brown to dark brown colored, round to oval shaped grain having a size approximately 4mm. Further, the seeds were analyzed for determination of the extractive values and found to be 2.82 % w/w, 0.79 %w/w, 4.26 % w/w 2.40 % w/w in pet. ether, chloroform, ethanol, and water extracts respectively, and extractive value was found highest in ethanol extract. Furthermore, phytochemical analysis of all the different extracts revealed the presence of phenolic components, flavonoids amino acids, protein, and carbohydrates in ethanolic extract, whereas in aqueous extract there was the presence of saponins and carbohydrates. Then, spectral characterization analysis was done with Infrared spectroscopy, Nuclear magnetic resonance, and Mass spectroscopy techniques and revealed the presence of different active metabolites in the ethanolic extract which may be considered for numerous pharmacological properties, thus further processed for anti-ulcer potential. The ethanolic extract has given promising results as an antiulcer agent against the standard drug omeprazole. Conclusion: The present research concluded from various parameters such as macroscopic evaluation, successive extraction, physicochemical investigation, phytochemical investigation, and spectral analysis that ethanolic extract of plant P. Scrobiculatum contains the maximum amount of secondary metabolites and was further investigated for anti-ulcer potential at a dose of 250 mg/kg and 500 mg/kg and the mean ulcer index was found to be 3.39±0.10 and 2.78±0.10 respectively against the standard drug omeprazole.
Keywords
Antioxidant Activity; Antiulcer potential; Paspalum Scrobiculatum; Physicochemical Properties; Phytochemical Profile; Spectral Analysis; TPC; TFC
Download this article as:| Copy the following to cite this article: Bhatti M, Singh J, Bhandari D. D. Compendium of P. Scrobiculatum: Phytochemical, Physicochemical, Antioxidant, Antiulcer, and Spectroscopic Analysis. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Bhatti M, Singh J, Bhandari D. D. Compendium of P. Scrobiculatum: Phytochemical, Physicochemical, Antioxidant, Antiulcer, and Spectroscopic Analysis. Biomed Pharmacol J 2023;16(2). |
Introduction
Peptic ulcer disease affects people globally, the estimated lifetime prevalence and the annual incidences of peptic ulcer disease in the general population are 5–10% and 1–3%, respectively. It is the most dangerous disorder associated with hyperacidity since it can lead to bleeding ulcers, a condition that can be fatal. The identification of Helicobacter pylori infection and the widespread use of non-steroidal anti-inflammatory drugs (NSAIDs) are known to be the major causes of peptic ulcers. Several anti-ulcer agents are available in the market but with profound side effects, some of them even being carcinogenic so the upcoming strategy is to target herbal products to explore their anti-ulcer potential.
Paspalum Scrobiculatum L. is described in “The Ayurvedic Pharmacopoeia of India” Part-I, Volume V. possessing the property to reduce burning sensation and inflammation (“shit and dahashaman”) and aids to wound healing (“Vranaropan”) which may be useful in healing ulcers. Paspalum scrobiculatum Linn. belongs to the family ‘Poaceae’ is and commonly known as ‘Kodo millet’. It is a feathered everlasting grass of 120-150 cm in height. It is an extremely drought-resistant crop. This plant flourishes even in very poor soils. Kodo millet is a minor grain crop grown throughout India, but to a greater extent in the Deccan and South India. Distributed in Madhya Pradesh, Chhattisgarh, and Karnataka. Kodo millet is reported to be rich in proteins when compared to other minor millets. 1 Flavonoids and phenolic acids are reported in Kodo millet.2,3 Kodo millets are enriched in phenolic acids, tannins, and phytates, that may be active as antioxidants and useful in protection against oxidative stress and maintaining blood glucose response. 4 Kodo millet extract (Methanolic) was found to be a potent drug in the treatment of skin wounds.2,5 The crude extracts and the pure isolates were reported to have nutritive, anti-fungal effects.6 The presence of several phytochemicals leads to the existence of various pharmacological activities. Furthermore, various spectral analytical techniques (UV, NMR, IR, and MS), etc help in the confirmation of the structure of particular phytoconstituents.
Thus, the present work is directed towards discovering the antiulcer efficacy of plant Paspalum Scrobiculatum L. isolating and investigating their phytoconstituents responsible for ulcer healing potency
Methodology
Plant collection
P. scrobiculatum grains were procured in bulk quantity from Rookhraj podhshala, Rajasthan in the month of August after confirming authenticity. The grains of Kodo millet were collected, cleaned, shade dried, coarsely powdered using a grinder, weighed, and passed through sieve no. 40 and then allow keeping in an air-closed container.
Authentication
The collected P. scrobiculatum grains were then allowed for the identification of morphological features first and later subjected to authentication by Taxonomist Dr. Sunita Garg, Head RHMD, Delhi, (CSIR-NISCAIR) New Delhi. An herbarium sample has also been conserved in the Pharmacy branch, UIPS, CU, Gharuan (Mohali) for the future reference UIPS/HB/MB/01/2020.
Macroscopic Identification
The shape of grains is round to oval, plano-convex, and approx. length is about 4 mm; brown colored pericarp, closely adherent to grains, can be seen using a hand lens, caryopsis is present on the convex side, plane surface there is one central line, 3 lines; shiny brown colored seed inside pericarp; three prominent ridges are there on seed upon convex side and in between these ridges, fine striations are there; whereas, seed’s plane side shows very finely striated oval central depression, pointed apical side [Figure 1]. 7
 |
Figure 1: P. Scrobiculatum (A) Plant [8] (B) Seeds and (C) powdered seeds. |
Physicochemical Investigation
Chemicals and Instruments
The chemicals used were Methanol (CH3OH), Petroleum ether, Chloroform (CHCl3), Ethanol (C2H5OH), H2O, DPPH, TCA, Potassium ferricyanide, Picric acid, Wagner’s reagent, Dragendroff’s reagent, Nitric acid, Fehling’solution, GAA, 1M sodium hydroxide, CuSO4 AgNO3, and HCL all these chemicals were procured from sigma chemicals. All standard drugs such as Gallic acid, quercetin, ascorbic acid, Folin‑ Ciocalteu reagent, and ferric chloride were obtained from Merck. UV‑graph was studied in Shimadzu 1800 UV‑Visible spectrophotometer, Bruker Avance II 400 MHz spectrometer, Perkin Elmer Spectrum Version 10.03.08
Ash values Determination
Total Ash Value Determination
For total ash value investigation, 2 g of ground grains were put in a tarred silica crucible and then completely ignited. During Incineration the heating temperature of the muffle should not exceed 450 0C, and allow heating to continue till it becomes carbon-free. Finally, allowed to cool, and weighing was performed. The same process is repeated to obtain successive weighing. The difference shouldn’t be >1 milligram. The total ash %age was calculated. 9,10
Acid Insoluble Ash Determination
Acid insoluble ash was analyzed by the ash so obtained from the total ash value procedure and then boiled with 2M 25ml of HCl for approx. 5mins. The matter which is insoluble in nature first accumulates in a crucible or on a piece of filter paper which should be ashless, further allowing hot water washing and then incineration. Later, the residue was allowed to cool and subjected to the determination of weight. 9,10
Water Soluble Ash Determination
The water-soluble ash value was studied, by boil of the total ash content separately for 5 mins using approx. 20-25milliliter of water. The non-soluble matter is taken, then allowed for hot water washing, and later, incinerated for 10-15 minutes at a temperature that should not exceed 450 0C. Further, by subtracting the weight of non-soluble matter from ash weight, the weight of water-soluble ash was calculated. 9,10
Fluorescence Analysis
The powdered drug was subjected the fluorescence analysis using different chemicals such as picric acid, Wagner’s reagent, Dragendroff’s reagent, HNO3, Fehling’s solution, GAA, 1M sodium Hydroxide, 5% CuSO4 and 5% AgNO3 and further observed under 254 nm, 365 nm, and normal day-light. A further study done under fluorescence light shows specific fluorescent colors which further help in drug identification as well as the determination of fluorescent impurities. It is one of the important tools for the identification of adulteration of the drug. 11,12
Phytochemical Investigation
Extraction by Successive Solvents
For Soxhlet extraction, 100gm of the powdered drug was taken in a thimble and then allowed for successive extraction using various solvents such as petroleum ether, chloroform, ethanol, and water from non-polar to polar nature for 4-6 hrs. Before treatment with the successive solvent, the powdered mass was completely dried in the open environment and then in a hot air oven at a temperature not exceeding 50 0C. The extraction completion was confirmed by taking each extract in a portion of 0.5-1 ml from the thimble on a watch glass and allowed to evaporate to ensure that no residue should leave behind after complete evaporation of the solvent 9.
Extractive Values Findings
In extractive value determination, the different solvent extracts were collected separately. Further, extracts were allowed to concentrate till a thick residue is obtained and then allowed to evaporate at low temperature till dryness. The dried matter was weighed and the w/w % age of the dried extract was calculated.
Qualitative Analysis of Extract
The qualitative analysis was conducted for all different extracts of grains of P. scrobiculatum for determination of the phytoconstituents such as alkaloidal molecules, glycosidic components, tannins, phytosterol compounds, phenolic acids, flavonoids, etc. 13,14
Estimation of Phytoconstituents Quantitatively
TPC determination
The % w/w TPC determination was done by the Folin‑Ciocalteu method where gallic acid was taken as standard. 0.005 g weighed dried extract was dissolved in a 10 ml mixture of (4:6 v/v ratio) methanol‑water. Further, 0.2 mL quantity of solution was pipetted out in a test tube and 1 ml of Folin‑Ciocalteu reagent and 0.8 ml of sodium carbonate (7.5%) were added. The mixture was then allowed to stand at room temperature for about 30 min and the absorbance was recorded at 765 nm.15
Estimation of TFC
The %w/w TFC content was measured by the Dowd method using quercetin as standard. For experimentation, 5 mL of 2% aluminium trichloride (AlCl3) was prepared in methanol and then mixed with the same proportion of the plant extract solution (0.5 mg/mL). Further, at 415 nm absorption was recorded.16
Spectroscopic Analysis
FTIR Analysis
The FT-IR spectral results were obtained from Perkin Elmer Spectrum Version 10.03.08, potassium bromide disc method was considered (νmax in cm-1) for the sample preparation. The sample extract was mixed with potassium bromide (KBr) in a ratio of 1:10 and further hydraulic press/IR press was used for pellet preparation. Then the Pellet was inserted in the sample slit of the instrument for determination of transmittance.17,18
(1H NMR) Proton Nuclear Magnetic Resonance
The Bruker Avance II 400 MHz spectrometer was used for NMR spectra recording with deuterated chloroform (CDCl3) or deuterated dimethyl sulfoxide (DMSO-d6) as the solvents, and the chemical shifts (δ) were measured in (ppm) lying downfield from the internal standard (tetramethyl silane (Me4Si)). The spin multiplicities were mentioned as singlet (s), doublet (d), double doublet (dd), triplet (t), quintet (quint), etc.19,20,21
Radical scavenging Property
DPPH Radical Scavenging Method
According to the Blois method, in the presence of a stable DPPH radical, the scavenging property of DPPH or donation of hydrogen capacity was quantified. [22] Accordingly, in a DPPH methanolic solution of 100 mM, 2.95 ml, and 0.05 ml of sample extracts in methanol were added at various concentrations of 20 µg/ml,40 µg/ml, 60 µg/ml, 80 µg/ml, and 100 µg/ml. After mixing thoroughly absorbance was determined at 517nm. Ascorbic acid was taken as standard. The scavenging efficacy of the extracts is determined by the degree of discoloration. In triplicate, the readings were expressed and %age scavenging activity was measured by the given equation:
% DDPH Scavenging Activity = (Control Solution−Test Solution) × 100/ Control
Determination of Antiulcer Activity
Aspirin-induced ulcer model
Experimental Animals
Wistar Rats of both sexes of weight 200-250g were used for experimental study as per approval under Protocol no. (CU/2021/IAEC/3/06) by the animal ethical committee of UIPS, Chandigarh University, Gharuan, Mohali. Under standard laboratory conditions, rats were given food and water.
Animals fasted for 24 h before treatment as per the groups mentioned in the aspirin-induced ulcer model [Table 1]. All treatments were administered 1h before the administration of aspirin. Ulcers were induced by the administration of aspirin orally at the dose of 200 mg/kg. 23 Animals were sacrificed after 6h of administration of aspirin along with greater curvature and ulcer score was determined.
Table 1: Antiulcer Experimental Animal Design
| Sr. No | Groups | Experimental Design |
| 1. | Group I | Control Wistar Rat received vehicle (Normal Saline 10 ml/kg/day, p.o.) |
| 2. | Group II | Positive Control Wistar Rat received aspirin (NSAIDs, 200mg/kg, p.o) |
| 3. | Group III | Wistar Rat received Omeprazole (standard drug 20 mg/kg, p.o) |
| 4. | Group IV | Wistar Rat received an ethanolic extract of P. scrobiculatum at a dose of 250 mg/kg |
| 5. | Group V | Wistar Rat received an ethanolic extract of P.scrobiculatum at a dose of 500 mg/kg |
Calculation of Gastric Ulcer Score
Once the gastric ulceration induction is done, the stomach of the animal is allowed to open with large curvature of the stomach and then, allowed to wash with tap water first and later with a normal salt solution. Stomach mucosal layers where the stomach was opened along with greater curvature are thoroughly washed with normal saline. Gastric mucosal lesions were stated in the ulcer index (U.I.) form 24. Calculations were done using an ulcer scoring index from 0-3 based on the severity of ulceration on the mucous membrane of the stomach. The severity index is defined based on the measurement of the ulceration lesions.
Severeness of ulcer occurrence is 0 = when there is no layer of the ulcer; the score is 1 = if ulceration< 1 mm in length; the score is 2 = ulceration 2–4 mm in length and the score is 3 = ulceration> 4 mm in length. The lesions or ulceration scoring for every animal was determined. The mean ulcer index (UI) was calculated 23,24,25.
U.I.= Area exposed to ulceration/whole area of animal Stomach ×100
Percentage of inhibition of ulcer= [U.I. in Control Group- U.I in Test Group] ×100/Ulceration in Control Group
Thus:
The gastric ulcerated part was separated and then allowed to place and fix over a cork plate. Further, the number count of and severity of ulceration were recorded by a stereo-microscope.
0 = color of stomach normal
0.5 = Reddish color
1 = ulceration Spotted
1.5 = Hemorrhagic streaks
2 = Ulceration found ≥ 3 but ≤5
Statistical analysis
A statistical evaluation was done by using one-way Analysis of Variance (ANOVA). The results were presented in terms of the mean and standard errors of the mean (SEM) and a P value of less than 0.05 was regarded as statistically significant after a post-hoc test using Student-Newman-Keuls.
Results and Discussion
Physicochemical Evaluation
Ash Values
Different types of Ash Values TAV, AIAV, and WSAV values (Total Ash Value, Acid Insoluble Ash Value and Water-Soluble Ash Value) were analyzed. The TAV of grains was 2.41±0.14% while water-soluble ash was 1.82±0.16 and acid-insoluble ash was 1.45±0.15 [Table 2]. The presence of the high ash value showed the presence of a high amount of inorganic matter because heating results in the loss of the organic materials in the form of CO2 gas whereas, only inorganic matter is left behind.
Table 2: Determination of Total Ash value of Paspalum Scrobiculatum Grains
| Type of Ash | % Age (w/w) Ash |
| Total Ash Value (%w/w) | 2.41±0.14 |
| Water Soluble(%w/w) | 1.82±0.16 |
| Acid Insoluble(%w/w) | 1.45±0.15 |
Fluorescence Analysis
The fluorescence analysis of several samples was carried out at various light wavelengths, which aids in the identification of fluorescent contaminants with certain luminous hues as well as the detection of drug adulteration, as shown in Table 3. Polarity is one of the most crucial physical factors in the fluorescence process. The process of absorbing the excitation energy from UV radiation is impacted by this situation. This has an impact on the way Paspalum Scrobiculatum seeds are de-excited, which alters the way that the medicinal plant extract produces fluorescence with varying intensities and spectral features. Different chemicals, including acids (conc. HNO3, conc. HCl, Glacial acetic acid, and H2SO4), alkalis (NaOH), picric acid, Wagner’s reagents, silver nitrate, Dragondorff’s reagent, and Fehling’s solution were used in this investigation (Table 3). The amount of fluorescence intensity determined with different sorts of solvents with various levels of polarity used in the maceration process to ascertain how solvent polarity affects the outcomes of extraction.
Table 3: Analysis of Powdered Seeds of P. scrobiculatum under fluorescent light
| S. no. | Test | Seeds | ||
| Observations in normal day Light | Observations at
254nm |
Observations at
365nm |
||
| 1 | Dry Powdered plant part | Brownish | Brownish | Dark
Brownish |
| 2 | Powdered plant part + Conc.
HNO3 |
Yellowish
Brown |
Brownish | DarkBrownish |
| 3 | Powdered plant part + Conc. HCl | Light
Brownish |
Dark Brownish | Black
Brownish |
| 4 | Powdered plant part + Glacial
Acetic acid |
Light yellow | Light yellow | Green |
| 5 | Powdered plant part + Aq.
NaOH |
Darkbrown | Blackish
Brown |
Black |
| 6 | Powdered plant part + Alc.
NaOH |
Pale brown | Greenish Brown | Green Brown |
| 7 | Powdered plant part + 5%
AgNO3 |
Cream | Colorless | Greenish
White |
| 8 | Powdered plant part + Wagner
Reagent |
Darkish red | Dark Black | Brown
Blackish brown |
| 9 | Powdered plant part+ 10%
Picric acid |
Yellowish | Yellow
brownish |
Greenish brown |
| 10 | Powdered plant part+
Dragondorff reagent |
Red-dark brown | Blackish | Blackish red |
| 11 | Powdered plant part + Fehling
Solution A |
Blue color | Blue color | Blue color |
| 12 | Powdered plant part+ 50%
Conc.H2SO4 |
Red
brownish |
Blackish | Blackish |
Phytochemical evaluation
Extraction by successive solvents
In the successive extraction process, the phytoconstituents present in the plant have been separated based on their solubility in various solvents such as petroleum ether, chloroform, ethanol, and water from non-polar to polar for 4-6 hrs using a soxhlation process. Extract of each solvent was collected separately, concentrated, and further proceed for extractive value determination.
Extractive Values Findings
Extraction values were determined and weighed for various extracts of seeds of P. scrobiculaum as shown in Table 4 & Figure 2. These results revealed that the extractive values of seeds extract of Pet. ether, chloroform, ethanol, and aqueous were found to be 2.82, 0.79, 4.26, and 2.40 (%w/w) respectively. Out of all the four extracts the extractive findings of the ethanolic seed extract of the plant were highest which is assuring the existence of high alcohol-soluble components in this extract, this was a valuable parameter for further studies on ethanolic seed extract of the plant.
Table 4: % age values of different extracts of seeds of Paspalum Scrobiculatum
| S. No | Plant Extract | Seeds(%w/w) |
| 1 | Pet. ether (60-800) | 2.82 |
| 2 | Chloroform | 0.79 |
| 3 | Ethanol | 4.26 |
| 4 | Water | 2.40 |
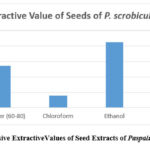 |
Figure 2: Successive ExtractiveValues of Seed Extracts of Paspalum Scrobiculatum |
Qualitative Analysis of Extract
The phytochemical screening of different extracts confirmed the presence of Phytoconstituents shown in Table 5. The pet ether extract of the plant showed the presence of only fixed oils and fats whereas, in chloroform plant extract, none of the phytoconstituents were observed. In contrast, the ethanolic extract of the plant showed the presence of phenolic components, tannins, flavonoids, amino acids, proteins, and carbohydrates. And the aqueous extract of the plant confirmed the presence of saponins and carbohydrates. These results revealed that the ethanolic plant extract possessed the maximum number of phytoconstituents which helped to further investigate ethanolic plant extract for further studies.
Table 5: Phytoconstituent testing of successive seed extract of P. scrobiculatum
| S. No. | Tests | Petroleum ether grain extract | Chloroform grain extract | Ethanolic grain extract | Aqueous grain extract |
| 1. | Phenolic constituents | -ve | -ve | +ve | -ve |
| 2. | Tannins | -ve | -ve | +ve | -ve |
| 3. | Alkaloids | -ve | -ve | -ve | -ve |
| 4. | Flavonoids | -ve | -ve | +ve | -ve |
| 5. | Glycosides | -ve | -ve | -ve | -ve |
| 6. | Amino acids | -ve | -ve | +ve | -ve |
| 7. | Fixed oils and fats | +ve | -ve | -ve | -ve |
| 8. | Saponins | -ve | -ve | -ve | +ve |
| 9. | Proteins | -ve | -ve | +ve | -ve |
| 10. | Carbohydrates | -ve | -ve | +ve | +ve |
(+ve) -Presence of constituent (-ve) – Absence of constituents
Estimation of Phytoconstituents Quantitatively
Determination of TPC and TFC
The folin-Ciocalteu procedure was used in TPC determination, considering gallic acid as the standard and standard curve (y=0.008×0.001, r2=0.998). Whereas, TFC, was reported as quercetin equivalent by reference in terms of %w/w (y=0.006x+0.018, r2=0.994). TPC of P. scrobiculatum seeds extracts of pet ether, chloroform, ethanol, and water was found to be 0.05, 0.08, 0.19, and 0.12 %w/w respectively whereas, TFC in pet ether, chloroform, ethanol, and water was 0.03, 0.05, 2.14 and 1.79%w/w respectively. These results revealed that the value of TPC and TFC was highest in ethanolic plant seed extract. Generally, a great content of phenols and flavonoids confirms the presence of polyphenolic, phenolic, and flavonoid moieties in plant parts which further signifies antioxidant properties. Table 6 shows the total content of phenols and flavonoids in the different P. scrobiculatum seed extracts.
Table 6: TPC and TFC of seeds in various extracts of P. scrobiculatum
| Sr. no | Extract | TPC (%w/w) | TFC (%w/w) |
| 1 | Pet ether | 0.05±0.21 mg GAE/g | 0.03±0.01 |
| 2 | Chloroform | 0.08±0.12 mg GAE/g | 0.05±0.02 |
| 3 | Ethanol | 0.19 ± 0.87 mg GAE/g | 2.14±0.01 |
| 4 | Water | 0.12 ± 0.92 mg GAE/g | 1.79±0.21 |
All the results figures are represented as mean ± std of minimum three replications(n=3)
Spectral Analysis
FT-IR Analysis
From the spectral studies viz. FT-IR, NMR, and LC-MS studies it can be found that the extract shows the presence of primary and secondary metabolites including esters of fatty acids, and polyphenols. Phytochemical screening showed a better response to the presence of 9-octadecanoic acid methyl ester. FT-IR spectra listed in Table 7 and Figure 4, were recorded to determine functional moieties present in molecule. The spectra of the plant showed absorption peaks in the region of 3783 cm -1 and 3297 cm-1 relates to hydroxyl moiety in carbonic acid. Further, the peaks at 3009, 2923, 2854, and 2676 cm-1 can corresponding to -CH of alkene & alkane. Bands at 1742 cm-1and 1713 cm-1 correspond to C = O and C = O ester groups. The 1459 cm-1 and 1373 cm-1 bands assign to CH and O-H bends. The 1164 cm-1 band confirmed the C-O stretching of the ester group.
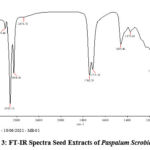 |
Figure 3: FT-IR Spectra Seed Extracts of Paspalum Scrobiculatum. |
Table 7: Components recovered from Fourier-transform infrared spectroscopy spectrum
| S. No. | Peak (cm-1) | Functional Moiety |
| 1 | 3783 | OH (carboxylic acid) |
| 2 | 3297 | OH (carboxylic acid) |
| 3 | 3009 | -CH stretching (Alkene) |
| 4 | 2923,2854,2676 | -CH stretching (Alkane) |
| 5 | 1742, 1713 | C=O, C-O (Ester) |
| 6 | 1459 | -CH bending (Alkane) |
| 7 | 1373 | -OH bending (carboxylic acid) |
| 8 | 1164 | C-O stretching (Ester) |
(1H-NMR) Proton Nuclear Magnetic Resonance
The proton NMR showed multiplet at δ 0.879-1.694 for hydrogens of alkyl group and the other alkyl hydrogens can be seen in a range of δ 2.069-2.004. The alkenes protons showed in range of δ 2.342-2.293 and 2.780-2.754. The carboxylic acid protons hydroxyl group protonsshowed in range of δ 5.401-5.257, whereas a singlet at δ 7.278 representing aryl protons. (Table 8 and Figure 4).
Table 8: Components recovered from 1H-NMR
| S. No. | Parts per million | Components |
| 1 | 7.278 | Aryl Hydrogen |
| 2 | 5.401-5.257 | Alcohol/Acid/Ester |
| 3 | 4.316-4.294 | Methoxy |
| 4 | 4.173-4.127 | Methoxy |
| 5 | 2.780-2.754 | Alkene |
| 6 | 2.342-2.293 | Alkene |
| 7 | 2.069-2.004 | Alkyl |
| 8 | 1.624-0.879 | Alkyl |
 |
Figure 4: 1H-NMR Spectra Seed Extracts of Paspalum Scrobiculatum. |
From the LC-MS data, it can be seen that the M+ peak at the value of 296, depicts presence of 9-octadecanoic methyl ester. Another molecule which can be probably present are phenolic acids depicted by M+ peak at 205.
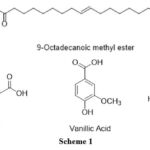 |
Scheme 1 |
Radical scavenging Property
Antioxidant Activity Analysis by DPPH Scavenging Method
The principle behind the antioxidant activity is that the DPPH molecule is a nitrogen-centred molecule and a stable free radical. Due to the reduction reaction, there is a change in color from pinkish-violet to yellow to pale yellow. So, the molecules showing this reaction are known as antioxidants. Moreover, the antioxidant potential of all the various extracts (Pet ether, chloroform, ethanol, and water) showed a rise with a rise in concentration. Furthermore, higher values of TPC and TFC demonstrated good DPPH scavenging power. The ethanolic seed extract which contained high phenolic and flavonoid content possessed the best antioxidant profile also. The antioxidant activity of the ethanolic extract of P. scrobiculatum was found to be maximum i.e. from 8.9% to 51.8% with an increase in concentration. Also, there was a rise in the antioxidant profile of other different extracts with an increase in concentration from 20-100 µg/ml i.e. 2.6%-26.1% in pet ether extract, 3.9%-29.1% in chloroform extract and 8.4%-44.1% in the water extract. Furthermore, the IC50 values for pet ether, chloroform, ethanol, and water extracts were reported as 198, 144, 82, and 95 µg/ml and it has shown that ethanolic extract being least IC50 value possessed the highest antioxidant potential which has been expressed in . Later %age radical-scavenging ability was determined by using the following formula:
Scavenging radical power in % = {(absorption of control –absorption of test sample)/absorption of control sample} × 100
The absorbance of control is the absorption of antioxidant radical ion i.e DPPH in methanolic solution, And test sample absorbance is the absorbance of antioxidant radical ion i.e DPPH and extract of the test sample.
Table 9: % Antioxidant power of different seeds extracts of P. scrobiculatum at different concentrations
| % Scavenging Activity (mean±standard deviation, n=3) | ||||
| Conc (µg/ml) | Pet Ether(60-80oC) | Chloroform | Ethanol | Water |
| 20 | 2.6±0.11 | 3.9±0.16 | 8.9±0.10 | 8.4±0.12 |
| 40 | 6.9±0.12 | 10±0.18 | 23.5±0.12 | 14.7±0.24 |
| 60 | 15.2±0.19 | 18.2±0.24 | 30.3±0.18 | 22.2±0.16 |
| 80 | 22.8±0.16 | 26.1±0.28 | 42.4±0.21 | 32.3±0.19 |
| 100 | 26.1±0.14 | 29.1±0.19 | 51.8±0.14 | 44.1±0.23 |
| IC50 | 198 | 144 | 82 | 95 |
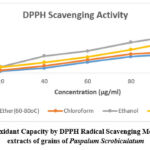 |
Figure 5: Antioxidant Capacity by DPPH Radical Scavenging Method of various extracts of grains of Paspalum Scrobiculatum. |
Determination of Antiulcer Activity
Preliminary anti-ulcer activity of extracts of P. scrobiculatum was carried out using an Aspirin-induced ulcer model. The standard agent, omeprazole in 20 mg/kg, and samples of plant extract at different doses (250 & 500 mg/kg) resulted in a remarkable reduction in ulceration (𝑃< 0.05) as depicted in Table 10 & Figure 6. The mean ulcer index (MUI) score was calculated. The MUI of P. scrobiculatum at 250 mg/kg & 500 mg/kg was found to be 3.39±0.10 & 2.78±0.10 (p<0.05) respectively and these results have significantly varied with control and standard as 4.08±0.09 and 0.76±0.02 respectively.
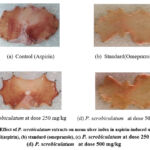 |
Figure 6: Effect of P. scrobiculatum extracts on mean ulcer index in aspirin-induced ulcer model (a) control(aspirin), (b) standard (omeprazole), (c) P. scrobiculatum at dose 250 mg/kg, (d) P. scrobiculatum at dose 500 mg/kg. |
Table 10: Effect of P. scrobiculatum extracts on mean ulcer index in aspirin-induced ulcer model
|
Treatment Group |
Dose | Mean Ulcer Index |
| Control | 200mg/kg |
4.08±0.09 |
|
Standard |
20mg/kg | 0.76±0.02* |
| P. scrobiculatum | 250mg/kg |
3.39±0.10* |
|
P. scrobiculatum |
500mg/kg |
2.78±0.10* |
All values are expressed as a mean ± SEM, n=5, *p<0.05 as compared to the control group (One Way Analysis of Variance (ANOVA) followed by multiple comparisons Dunnet’s test).
Conclusions
P. scrobiculatum grains were extracted successively using solvents of varying polarity such as petroleum ether, ethanol, chloroform, and water. P. scorbiculatum has been screened macroscopically, physicochemically, and phytochemically and the ethanolic extract has been found to contain several secondary metabolites such as flavonoids, phenols, tannins, amino acids, proteins, and carbohydrates which may be responsible for antioxidant as well as anti-ulcer potential. Furthermore, ulcer index and % ulcer protection parameters were studied using an aspirin-induced ulcer model and it was found that plant extract at 500mg/kg dose showed effective results with mean ulcer index score 1.34±0.21 and % ulcer protection was found to be 49.24% as compared with standard and control. The anti-ulcer potential of the plant has shown promising results in comparison with standard drug. Further investigations can be done on the plant extract to separate more secondary metabolites which can be tested further to explore antiulcer potential thus opening a new platform for researchers to overcome the side effects associated with allopathic anti-ulcer drugs. Thus, this plant possesses properties that can be utilized commercially by research institutes as well as pharmaceutical companies either alone or in combination with other plants or allopathic drugs for the synthesis of new anti-ulcer drugs.
Acknowledgment
All the authors are sincerely thankful to Chandigarh University for providing necessary facilities for the completion of research.
Conflicts of Interest
The authors declare no conflict of interest.
Funding sources
This research received no external funding.
References
- Dey, S., Saxena, A., Kumar, Y., Maity, T., & Tarafdar, A. (2022). Understanding the Antinutritional Factors and Bioactive Compounds of Kodo Millet (Paspalum scrobiculatum) and Little Millet (Panicum sumatrense). Journal of Food Quality, 2022.
- Kiran, P., Denni, M., & Daniel, M. (2014). Antidiabetic principles, phospholipids, and fixed oil of Kodo millet (Paspalum scrobiculatum). Indian J Appl Res, 4, 13-5.
CrossRef - Chandrasekara, A., & Shahidi, F. (2010). Content of insoluble bound phenolics in millets and their contribution to antioxidant capacity. Journal of agricultural and food chemistry, 58(11), 6706-6714.
CrossRef - Jain, S., Bhatia, G., Barik, R., Kumar, P., Jain, A., & Dixit, V. K. (2010). Antidiabetic activity of Paspalum scrobiculatum in alloxan-induced diabetic rats. Journal of ethnopharmacology, 127(2), 325-328.
CrossRef - Hegde, P. S., Anitha, B., & Chandra, T. S. (2005). In vivo effect of whole grain flour of finger millet (Eleusine coracana) and Kodo millet (Paspalum scrobiculatum) on rat dermal wound healing. Indian journal of experimental biology, 43(3), 254-258.
- Parameswaran, K. P., & Thayumanavan, B. (1995). Homologies between prolamins of different minor millets. Plant Foods for Human Nutrition, 48(2), 119-126.
CrossRef - Bhatti, M., Singh, J., & Bhandari, D. D. (2020). Review Article on Phytoconstituents and Pharmacological Profile of Portulaca quadrifida and Paspalum scrobiculatum Linn. Plant Cell Biotechnology and Molecular Biology, 234-239.
- https://www.shutterstock.com/search/kodo-millet.
- Kokate C.K, Practical Pharmacognosy, Vallabh Prakashan, Delhi. 125 (2001) 108-111.
- Kokate C.K, Purohit A.P and Gokhle S.B, Textbook of Pharmacognosy, Nirali prakashan, pune. (1996) 533-537.
CrossRef - Adediwura, F. J., & Ayotunde, A. (2012). Phytochemical and pharmacognostic studies of telosma africanum (NE Br) Colville leaf and stem. JPSR, 3, 1860-2.
- Selvam, A. B. D., & Bandyopadhyay, S. (2005). Fluorescence analysis on the roots of Rauvolfia serpentina (L.) Benth. Ex kurz under UV radiation. Ancient science of life, 24(4), 164.
- Trease G, Evans SM. Pharmacognosy. 15th ed. London: Bailer Tindal; 2002, p. 23-67.
- Herborne, J. B. (1973). Phytochemical methods. A guide to modern techniques of plant analysis, 2, 5-11.
- Siddhuraju, P., & Becker, K. (2003). Antioxidant properties of various solvent extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera) leaves. Journal of agricultural and food chemistry, 51(8), 2144-2155.
CrossRef - Zhishen, J., Mengcheng, T., & Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64(4), 555-559.
CrossRef - Chouhan, N., Ameta, R., & Meena, R. K. (2017). Biogenic silver nanoparticles from Trachyspermum Ammi (Ajwain) seeds extract for catalytic reduction of p-nitrophenol to p-aminophenol in excess of NaBH4. Journal of Molecular Liquids, 230, 74-84.
CrossRef - Rajvanshi, S. K., & Dwivedi, D. H. (2017). Screening of secondary phytometabolite of hydro-distilled essential oil from fresh flower and leaves of African marigold (Tagetes erecta). International Journal of Minor Fruits, Medicinal and Aromatic Plants, 3(2), 1-7.
CrossRef - Dhaiwal, K., Chahal, K. K., Kataria, D., & Kumar, A. (2017). Gas chromatography‐mass spectrometry analysis and in vitro antioxidant potential of ajwain seed (Trachyspermum Ammi) essential oil and its extracts. Journal of Food Biochemistry, 41(3), e12364.
CrossRef - Nazeer, M., Waheed, H., Saeed, M., Ali, S. Y., Choudhary, M. I., Ul-Haq, Z., & Ahmed, A. (2019). Purification and characterization of a nonspecific lipid transfer protein 1 (nsLTP1) from Ajwain (Trachyspermum ammi) seeds. Scientific reports, 9(1), 1-13.
CrossRef - Chauhan, N. S., Sharma, V., Thakur, M., & Dixit, V. K. (2010). Curculigo orchioides: the black gold with numerous health benefits. Zhong xi yi jie he xue bao= Journal of Chinese integrative medicine, 8(7), 613-623.
CrossRef - Blois, M. S. (1958). Antioxidant determinations by the use of a stable free radical. Nature, 181(4617), 1199-1200.
CrossRef - Kumar, D., Hegde, H. V., Patil, P. A., Roy, S., & Kholkute, S. D. (2013). Antiulcer activity of water-soaked Glycine max grains in aspirin-induced model of gastric ulcer in Wistar rats. Journal of Ayurveda and Integrative Medicine, 4(3), 134.
CrossRef - Adefisayo, M. A., Akomolafe, R. O., Akinsomisoye, S. O., Alabi, Q. K., Ogundipe, O. L., Omole, J. G., & Olamilosoye, K. P. (2017). Gastro-protective effect of methanol extract of Vernonia amygdalina (del.) leaf on aspirin-induced gastric ulcer in Wistar rats. Toxicology reports, 4, 625-633.
CrossRef - Ali Khan, M. S., Mat Jais, A. M., & Afreen, A. (2013). Prostaglandin analogous and antioxidant activity mediated gastroprotective action of Tabernaemontana divaricata (L.) R. Br. flower methanolic extract against chemically induced gastric ulcers in rats. BioMed Research International, 2013.
CrossRef - The novelty of your study is unclear! You need to state the objective. In addition, the problem (the research question to be addressed). You have to provide a concise background: why the work was done.







