Manuscript accepted on :11-11-2022
Published online on: 12-05-2023
Plagiarism Check: Yes
Reviewed by: Dr. Francisco Solano
Second Review by: Dr. Hind Shakir
Final Approval by: Dr. Ayush Dogra
Arsh Singh1 , Amit Gupta1*
, Amit Gupta1* , Simran Srivastava1
, Simran Srivastava1 , Bhavya Choudhury1
, Bhavya Choudhury1 , Sidharth Jain1
, Sidharth Jain1 and AB Bajpai2
and AB Bajpai2
1Department of Microbiology, Graphic Era (Deemed to be) University, Dehradun, Uttarakhand, India
2Department of Botany, D.B.S. PG. College, Dehradun, Uttarakhand, India.
Corresponding Author E-mail: dr.amitgupta.bt@geu.ac.in
DOI : https://dx.doi.org/10.13005/bpj/2672
Abstract
Natural products are believed to be one of the richest sources of prophylactic and therapeutic-based compounds which show a wide range of applications in the food and pharmaceutical industries. An aqueous extract of Momordica charantia was evaluated systematically for antioxidant (2,2-diphenyl-1-picrylhydrazyl, DPPH free radical assay), antimicrobial (bacterial strains), and anti-inflammatory (heat-induced hemolysis, proteinase inhibitory activity, protein denaturation, i.e., bovine serum albumin, BSA, and typhoid vaccine) agents, as well as its phenolic content. The results showed its higher concentration of phenolic content, as reported in this study, which is directly correlated with the antioxidant activity of Momordica charantia against DPPH. Similar results were obtained in the antimicrobial assay, where the diameter of the inhibition zone against bacterial strains is measured in millimetres (mm), which is wider in the case of the aqueous extract. In addition, anti-inflammatory studies were also taken into consideration, and the results suggest that higher doses of Momordica charantia may enhanced the percentage of protein denaturation as compared to BSA or typhoid vaccine alone and also induced heat induce hemolysis and proteinase inhibitory activity, In short, Momordica charantia may have shown several immunopharmacological properties, and this study may have been used further have used for isolation of desired prophylactic or therapeutic based compounds and to develop better nutraceutical or pharmaceutical agents.
Keywords
Antimicrobial; Antioxidant; Anti-Inflammatory; Momordica Charantia
Download this article as:| Copy the following to cite this article: Singh A, Gupta A, Srivastava S, Choudhury B, Jain S, Bajpai A. Assessment its Antioxidant, Antimicrobial and Anti-Inflammatory Potential of Momordica Charantia. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Singh A, Gupta A, Srivastava S, Choudhury B, Jain S, Bajpai A. Assessment its Antioxidant, Antimicrobial and Anti-Inflammatory Potential of Momordica Charantia. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3BPol41 |
Introduction
The use of these natural products has been demonstrated to be effective against a variety of infectious diseases. These activities could be due to the presence of inorganic and organic compounds that are present in these natural products [1]. So, pharmacists are interested in looking at these types of compounds because of their therapeutic performance and low toxicity. In addition, consumption of fruits and vegetables is directly or indirectly correlated with improved mental wellbeing and is also traditionally used as a therapeutic agent [2, 3]. In addition to the fruits and vegetables, twigs, sap, stems, leaves, roots, fruit, and bark are used as ingredients for conventional medicine. These parts are widely used by locals as a folk remedy to treat a variety of illnesses such as, asthma, fever, cough, diarrhoea, digestive disorders, and skin disorders [4]. Eating a good diet with the involvement of different fruits and vegetables can significantly lessen the chances of stroke, heart disease, and certain types of cancer, reduce the risk of eye damage and digestive problems and have a positive effect on blood sugar maintenance, which can help control appetite. Even weight loss can be achieved by consuming non-starchy fruits and vegetables, namely apples, pears, and green leafy vegetables [5, 6]. Their low glycemic load prevents spikes in blood sugar levels that can increase hunger. There are essentially nine different families of vegetables and fruits, all containing indefinite medicinal properties due to the plant compounds present. Eat a spread of colours and types of produce so as to offer our body the combination of nutrients it requires. This ensures a major range of advantageous plant chemicals, as well as impressive meals [7, 8]. In modern medicine, extracts from different parts of the plant, including the fruit, have been used more for their medicinal advantages as anti-microbial, anti-leukemic, anti-clastogenic, anti-hypercholesterolemic, anti-fungal, anti-atherosclerotic, and anti-proliferative agents [9, 10].
One such vegetable is Momordica charantia (bitter gourd), otherwise called resin pear, harsh melon, bitter gourd, or karela, this famous plant is used especially for the treatment of diabetes-related complications among the native people of India and its subcontinents [11, 12]. In literature, the organic product of this plant has a distinctively unpleasant taste, which is enhanced as it ages, hence the name “bitter gourd or bitter melon”. Biochemical and creature model investigations have come up with some useful information regarding the counter diabetic impacts of M. charantia [11-14]. In this study, we determined the antimicrobial and antioxidant activity of Momordica charantia.
Materials and Methods
Sample collection
The project work had been carried out in Graphic Era Deemed to be University (GEU), Dehradun. Momordica charantia was procured from the local market in Dehradun. The vegetable was washed and cleaned before being cut into pieces. For one week, the pieces were dried at room temperature in a shaded area. After that, dried pieces were ground and the powder was collected as a sample.
Phytochemical analysis
Preliminary phytochemical screening of Momordica charantia was carried out to determine the existence of plant secondary metabolites (qualitatively) using the standard methods [9].
Assessment of flavonoids and terpenoids [9]. – About 1 ml of the sample was dissolved in 1 M of HCl (5 ml each) and diluted NaOH. The existence of flavonoids was expressed when a yellow solution became colourless. Similarly, sample (1 ml) was thoroughly mixed with chloroform (2 ml) and concentrated sulfuric acid (3 ml) to form a layer. Appearance of reddish brown, which indicates the presence of terpenoids.
Assessment of Alkaloids [9]. – 200 mg of the sample were warmed up in a boiling water bath with 5 mL of 2N HCl. The chilled mixture was then purified and divided into two equal parts. One part was treated with a few drops of Mayer’s reagent and the other with Dragendroff’s reagent. The existence of alkaloids was expressed by the turbidity of the resulting precipitates.
Assessment of Saponins [9]. – About 200 mg of sample was shaken with 5ml of distilled water in a test tube and heated on a water bath to boil. The existence of saponins was expressed by the formation of strong and stable foam.
Estimation of total phenolic content
In this study, we evaluated the total phenolic content in an aqueous extract of Momordica charantia (5 g/50 ml) using a spectrophotometric method [15]. Briefly, Folin-Ciocalteu’s reagent (0.5 ml) and aqueous extract (1 ml with 10 mL of distilled water) were mixed together and incubated for 3 min. Following that, 1 mL of saturated Na2CO3 was added to the solution, increasing its volume to 25 mL. Finally, samples of Momordica charantia were laid down in a dark place for 1 h, and the absorbance was measured at 750 nm. Aqueous extract samples of Momordica charantia were analysed in triplicates, and its mean value of absorbance was taken. Gallic acid (5, 10, 20, 40, 60, 80, 100, and 150 mM) was used as the standard in this study, and it was taken at various concentrations to generate a calibration curve, after which the results were expressed in mM.
Evaluation of Antioxidant Activity
The antioxidant activity of Momordica charantia against the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical was evaluated through UV spectrophotometry at 518 nm [16]. In this experiment, variable concentrations of Momordica charantia (1-100 μg/ml) were prepared, whereas Vitamin C was used as an antioxidant standard. In this assay, M. charantia aqueous extract (1 ml) and methanol (3 ml) were mixed together, and then DPPH (1.0 ml) was added to the methanol. Finally, samples were incubated in the dark at room temperature for 15-20 minutes. Interchangeable proportions of solvent (methanol) and radical (DPPH) was assorted to make ready the blank solution. In this experiment, we analysed Momordica charantia samples in triplicate and obtained their absorbance (mean value).
Evaluation of antimicrobial activity
The crude aqueous extract of M. charantia was subjected to antimicrobial evaluation against bacterial strains. The susceptibility of the bacterial strains to the aqueous extract of Momordica charantia was securitized using the disc diffusion method [17]. In this study, sterile blank discs (6.0 mm diameter), were saturated with different concentrations of Momordica charantia ranging from 2 mg/disc to 0.0312 mg/disc. So, these cultures were incubated overnight using a turbidometer to yield approximately 1 X 108 CFU/ml. Aqueous extract Momordica charantia-saturated discs (20 μl) were maintained on agar plates and incubated (37°C, 24 h). DMSO (20 µl) and vancomycin discs (30 µl) were used as negative and positive controls, respectively, in these studies. The antibacterial activities of Momordica charantia were then worked out by evaluating the zone of inhibition in millimetres (mm) [17].
In vitro anti-inflammatory activity
In this study, pathology lab collected whole human anti-coagulant EDTA blood samples (with consent letter) to determine anti-inflammatory activity in vitro. Whole blood samples were washed three times (centrifuged at 2500 rpm; 6 min) with an equal amount of 0.9% normal saline (NaCl). After centrifugation, the pellets containing blood cells were measured and then reconstituted in phosphate buffered saline (PBS, pH 7.4).
Heat-Induced Hemolysis
In this experiment, whole blood cell suspension (50 µl) containing a similar volume of different concentrations of Momordica charantia was dissolved in PBS (2.9 ml). Incubate the samples for 20-30 minutes at 54 oC in a shaking water bath. After incubation, centrifuged these samples at high speed, collected the supernatant, and measured its OD value at 570 nm using a UV-visible spectrophotometer. In this experiment, PBS served as the negative control for these studies. The level of hemolysis in whole blood cell suspension using Momordica charantia was calculated on the basis of this equation, as mentioned below:
% inhibition of hemolysis = 100 × (1 − A2/A1), where A1 = control absorbance, and A2 = Momordica charantia absorbance value.
Proteinase Inhibitory Activity
In this experiment, we prepared a reaction mixture (2 ml) consisting of trypsin (0.06 mg), Tris-HCl buffer (20 mM, pH 7.4) and added different concentrations of Momordica charantia. Incubate the samples at 37ºC for 10 min and then add casein (1 ml, 0.8 %). Again, incubate the samples for 30 min and then add perchloric acid (70 %; 2 ml) was added to stop its reaction rate. Centrifuging these sample mixtures at high speed and determined their absorbance value in the supernatant and measured them at 210 nm. In this experiment, PBS served as the control for these studies. In this assay, we determined its proteinase inhibitory activity on the basis of this equation as mentioned below-
% proteinase inhibitory activity = 100 × (1 − A2/A1),
where A1 = control absorbance, and A2 = Momordica charantia absorbance value.
Protein denaturation assay
To evaluate its denaturation of protein assay, an aqueous extract of Momordica charantia was applied against a specific protein antigen [18]. Briefly, an aqueous extract of Momordica charantia using a variable concentration (0.5-500 μg/ml) was homogenised separately with typhoid vaccine (25 µg/ml; 1 ml; Bharat Biotech company) and bovine serum albumin (BSA, 5g/100 ml; 1 ml). Incubate these extracts of Momordica charantia along with BSA or typhoid vaccine at 37°C for 30 minutes whereas the control tube had a combination of distilled water and BSA or typhoid vaccine. For this experiment, denaturation of the proteins with or without extracts was caused by placing the samples of an aqueous extract of Momordica charantia in a water bath for 10 minutes at 70°C. The mixture was cooling inside the ambient room temperature, and the activity of each mixture was measured at 660 nm. Each test was done three times. The following formula was used to calculate the inhibition percentage:
Statistical analysis
The overall results were expressed as mean standard error. The difference between the control and variable concentrations of aqueous extract of Momordica charantia is determined through a one-way ANOVA test.
Results
Phytochemical analysis and total phenolic content
Phytochemical analysis of M. charantia qualitatively revealed the existence of flavonoids, terpenoids, alkaloids, and saponins. In addition, the total phenolic content of Momordica charantia was also calculated and showed redox properties, and acted as an antioxidant. The results of these studies are shown in Table 1 where aqueous extract at higher doses showed the highest extraction yield as compared to standard and control. In this study, the total phenolic contents of Momordica charantia were determined using the Folin-Ciocalteu assay by constructing a standard curve with gallic acid (GA) taking into consideration the relationship between absorbance and concentration.
Table 1: Estimation of total phenolic content from M. charantia.
| S.No. | Momordica charantia (Aqueous extract, µg/ml) | Total phenolic content (mM) |
| 1 | 3.9 | 7.84 ± 0.22 |
| 2 | 7.8 | 11.2 ± 0.64 |
| 3 | 15.6 | 18.14 ± 0.78 |
| 4 | 31.25 | 26.6 ± 1.14 |
| 5 | 62.5 | 33.4 ± 1.98 |
| 6 | 125 | 43.6 ± 1.56 |
| 7 | 250 | 57.2 ± 2.12 |
| 8 | 500 | 66.4 ± 1.94 |
Readings were taken and calculated the phenolic content through calibration curve obtained from standard (gallic acid) was linear with y = 0.0343x + 0.091 6; R2 = 0.972.
Antioxidant activity (DPPH assay)
The effect of Momordica charantia using an aqueous extract against DPPH is shown in Table 2. The results showed that the aqueous extract at higher concentrations showed significant inhibition of DPPH as compared to the control. Vitamin C was used as a standard for these studies.
Table 2: Percentage inhibition of DPPH for Momordica charantia compared with Vitamin C
| Concentration (µg/ml) | Percentage inhibition by Vitamin C | Percentage inhibition (Momordica charantia) |
| 3.9 | 24.6 ± 0.88 | 14.8 ± 0.56 |
| 7.8 | 39.8 ± 1.46 | 19.2 ± 0.22 |
| 15.6 | 44.2 ± 1.34 | 26.4 ± 0.80 |
| 31.25 | 68.4 ± 1.78 | 44.2 ± 2.04 |
| 62.5 | 78.4 ± 1.78* | 60.4 ± 1.46 |
| 125 | 88.4 ± 1.06** | 76.4 ± 1.30 |
| 250 | 96.6 ± 1.22*** | 82.2 ± 0.98* |
| 500 | 100.2 ± 0.2*** | 88.4 ± 1.22** |
Values were expressed as Mean ± S.E.; one-way ANOVA test (*P<0.05, **P<0.01 and ***P<0.001).
Antimicrobial activity
The effect of Momordica charantia using an aqueous extract against two bacterial strains is shown in Table 3 and the results are expressed in millimetres (mm). The results showed that the aqueous extract at higher concentration showed significant antimicrobial activity as compared to the control. Gentamycin (0.125-1 mg/disc) used as a standard and showed significant inhibition.
Table 3: Inhibition zone (mm) of Momordica charantia against bacterial strains.
| Concentration of crude extracts of Momordica charantia against bacterial strains (mg/disc) | Antibiotic (Gentamycin) (inhibition zone in mm) | Staphylococcus aureus | Pseudomonas aeruginosa |
| 4 | – | 19.8 ± 1.24** | 18.6 ± 1.56** |
| 2 | – | 14.6 ± 1.74* | 14.2 ± 1.92* |
| 1 | 21.4 ± 1.84** | 13.2 ± 1.18* | 12.6 ± 1.62 |
| 0.5 | 18.8 ± 1.56** | 10.4 ± 0.88 | 9.2 ± 0.96 |
| 0.25 | 15.4 ± 1.24* | 6.4 ± 0.42 | 0 |
| 0.125 | 11.2 ± 0.78 | 0 | 0 |
Values were expressed as Mean ± S.E.; one-way ANOVA test (*P<0.05, **P<0.01 and ***P<0.001).
In vitro anti-inflammatory activity
An aqueous extract of Momordica charantia in human whole blood cell suspension was shown to inhibit hemolysis in a concentration-dependent manner (Fig. 1) to determine its in vitro anti-inflammatory activity. Similarly, anti-inflammatory activity was also estimated against BSA and typhoid vaccines using a variable concentration of the aqueous extract of Momordica charantia shown in Fig.2. The results showed that the aqueous extract at higher concentrations showed significant declines in protein denaturation with reference to BSA and typhoid vaccine as compared to the control. In addition, the proteinase inhibitory activity of Momordica charantia is shown in Fig. 3, and the inhibition levels were within the range of 21.2–45.4 % as compared to control.
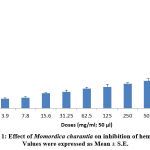 |
Figure 1: Effect of Momordica charantia on inhibition of hemolysis. |
Values were expressed as Mean ± S.E.
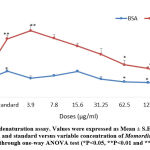 |
Figure 2: Protein denaturation assay. |
Values were expressed as Mean ± S.E. The difference between control and standard versus variable concentration of Momordica charantia is determined through one-way ANOVA test (*P<0.05, **P<0.01 and ***P<0.001)
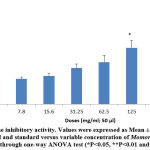 |
Figure 3: Proteinase inhibitory activity. |
Values were expressed as Mean ± S.E. The difference between control and standard versus variable concentration of Momordica charantia is determined through one-way ANOVA test (*P<0.05, **P<0.01 and ***P<0.001).
Discussion
In literature, scientific based studies related to medicinal plants were taken into consideration to understand their economic and medicinal importance. Some of the most familiar examples of medicinal plants are Azadirachta indica, Boswellia serrata, withania sominiferra, Picrohiza kurroa etc. [19, 20]. So, these medicinal plant based studies are well appreciated by authors all over the world, who understand their immunobiological activities. In view of this, our major objective is to evaluate the antimicrobial, antioxidant, and anti-inflammatory activity of Momordica charantia.
Preliminary studies were carried out on Momordica charantia to identify the existence of secondary metabolites that are present in the aqueous extract. So, these studies revealed the existence of flavonoids, terpenoids, alkaloids and saponins in Momordica charantia. In addition, we also estimate the phenolic content which is one of the most common antioxidants found in plants. In light of this, our primary focus is on phenolic content (an important indicator of antioxidant potential) and correlates phenolic content data with Momordica charantia antioxidant activity. Our results may found that higher phenolic content is reported at higher concentrations using gallic acid as the standard. In other words, Momordica charantia regarded them as one of the richest sources of phenolic compounds, and they were widely used in traditional medical systems. So, Momordica charantia using multiple doses which showed a large variation in antioxidant potential (using DPPH) as shown in Table 1 whereas Vitamin C was used as a standard for these studies. In most cases, aqueous extracts are non-toxic, and the isolation of antioxidant-based compounds is neither useful nor necessary. Hence, it may be concluded that the aqueous extract (Momordica charantia) has a higher antioxidant potential against the DPPH radical assay. In continuation of this, one of the most dominant or active compounds i.e. phenolics which showed significant antimicrobial potential against bacteria and fungi. In view of this, we focused on the phenolic content in an aqueous extract of Momordica charantia and showed its antimicrobial potential against bacterial strains. These antimicrobial-based studies were mostly reported because bioactive compounds were discovered to be highly effective against bacterial pathogens.
In the literature, protein denaturation is reported only due to inflammatory processes like arthritis. The major role of NSAIDs is to protect against denaturation of protein molecules. In other words, a decline in the rate of protein denaturation may play a very crucial role, especially seen in the antirheumatic activity of NSAIDs. Various studies were conducted with reference to plant components and protein denaturation. In short, protein denaturation based studies were applied to understand the anti-inflammatory action of Momordica charantia. In literature, inflammation is directly associated with protein denaturation [18] and the results from these studies showed that Momordica charantia significantly inhibited the protein at higher doses as compared to the control. In other words, Momordica charantia had the highest anti-inflammatory potential (strong inhibition of protein denaturation) in the case of the typhoid vaccine as compared to BSA. In addition, proteinases are directly involved or associated with arthritic reactions because leukocytes containing proteinases may directly impact the development of tissue damage during inflammatory processes. Protease inhibitors have also been shown in the literature to provide significant protection against infectious diseases. Therefore, the existence of molecules present in Momordica charantia may directly contribute to its anti-inflammatory activity. In short, Momordica charantia in the form of an aqueous extract has antioxidant, antimicrobial, and anti-inflammatory potential.
Conclusion
Maximum antioxidant, antimicrobial, and anti-inflammatory activities were observed in an aqueous extract of Momordica charantia, which showed strong positive correlations with phenolic content. The results from this study revealed that Momordica charantia contains a substantial phenolic content, which was suggested to be the major contributor to its antioxidant, antibacterial, and anti-inflammatory activities. Future research work will be focused on the active metabolite of Momordica charantia to discover effective pharmacological agents.
References
- Molinski TF, Dalisay DS, Lievens SL, Saludes JP. Drug development from marine natural products. Nat. Rev. Drug Discov 2009; 8: 69-85.
CrossRef - Yousuf B, Qadri OS, Srivastava AK. Recent developments in shelf-life extension of fresh-cut fruits and vegetables by application of different edible coatings: A review, LWT. Food Sci Technol 2018; 89: 198-209.
CrossRef - Jiang Q, Zhang M, Xu B. Application of ultrasonic technology in post harvested fruits and vegetables storage: A review. Ultrason Sonochem 2020; 69: 105261.
CrossRef - Akram M, Tahir IM, Shah SMA, Mahmood Z, Altaf A, Ahmad K, Munir N, Daniyal M, Nasir S, Mehboob H. Antiviral potential of medicinal plants against HIV, HSV, influenza, hepatitis, and coxsackievirus: a systematic review. Phytother Res 2018; 32: 811-822.
CrossRef - Ramos B, Miller FA, Brandão TRS, Teixeira P, Silva CLM. Fresh fruits and vegetables—An overview on applied methodologies to improve its quality and safety. Innov Food Sci Emerg Technol 2013; 20: 1-15.
CrossRef - Oliveira M, Abadias M, Usall J, Torres R, Teixido N, Vinas I. Application of modified atmosphere packaging as a safety approach to fresh-cut fruits and vegetables – A review. Trends Food Sci Technol 2015; 46 (1): 13-26.
CrossRef - Xu B, Tiliwa ES, Yan W, Azam SMR, Wei B, Zhou C, Ma H, Bhandari B. Recent development in high quality drying of fruits and vegetables assisted by ultrasound: A review. Food Res Int 2021; 110744.
CrossRef - Jose JFBS, Andrade JN, Ramos AM, Vanetti MCD, César PS, Chaves PJB. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014; 45: 36-50.
CrossRef - Senguttuvan J, Paulsamy S, Karthika K. Phytochemical analysis and evaluation of leaf and root parts of the medicinal herb, Hypochaeris radicata for in vitro antioxidant activities. Asian Pac J Trop Biomed 2014; 4(1): S359–S367.
CrossRef - Yan SW, Ramasamy R, Alitheen NBM, Rahmat A. A comparative assessment of nutritional composition, total phenolic, total flavonoid, antioxidant capacity, and antioxidant vitamins of two types of Malaysian underutilized fruits (Averrhoa bilimbi and Averrhoa carambola) International Journal of Food Properties 2013;16(6):1231–1244.
CrossRef - Grover JK., Yadav SP. Pharmacological actions and potential uses of Momordica charantia: A review. J Ethnopharmacol 2004; 93:123–132.
CrossRef - Prasad V, Jain V, Girish D, Dorle AK. Wound-healing property of Momordica charantia fruit powder. J Hreb Pharmacother 2006; 6:105–115.
CrossRef - Raman A, Lau C. Anti-diabetic properties and phytochemistry of Momordica charantia (Cucurbitaceae) Phytomedicine 1996; 2:349–362.
CrossRef - Virdi J, Sivakami S, Shahani S, Suthar AC, Banavalikar MM, Biyani MK. Antihyperglycemic effects of three extracts from Momordica charantia. J Ethnopharmacol 2003; 88:107–111.
CrossRef - Bajpai M, Pande A, Tewari SK, Prakash D. Phenolic contents and antioxidant activity of some food and medicinal plants. Int J Food Sci Nutr 2005; 56:287–291.
CrossRef - Lin JY, Tang CY. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem 2007; 101:140–147.
CrossRef - Zhang PP, Liu JF, Wang CL, Ye YT, Xie JH. Study on the antimicrobial activities of the extracts from Momordica charantia Nat Prod Res 2008; 20:721–724.
- Gunathilake KDPP, Ranaweera KKDS, Vasantha Rupasinghe HP. In Vitro Anti-Inflammatory properties of selected green leafy vegetables. Biomedicines 2018; 6(4): 107.
CrossRef - Gupta A, Khajuria A, Singh J, Bedi KL, Satti NK, Dutt P, Suri KA, Suri OP, Qazi GN. Immunomodulatory activity of biopolymeric fraction RLJ-NE-205 from Picrorhiza kurroa. International Immunopharmacology 2006; 6(10): 1543-1549.
CrossRef - Gupta A, Khajuria A, Singh J, Singh S, Suri KA. Immunological adjuvant effect of Boswellia serrata (BOS 2000) on specific antibody and cellular response to ovalbumin in mice. International Immunopharmacology 2011; 11(8): 968-975.
CrossRef







