Shilpika Nagula1* , N. J. P. Subhashini1
, N. J. P. Subhashini1 , D. V. R. N. Bhikshapathi2
, D. V. R. N. Bhikshapathi2 , Palanati Mamatha2
, Palanati Mamatha2 and Sailaja Rao. P2
and Sailaja Rao. P2
1Department of Chemistry, University College of science, Osmania University, Hyderabad, Telangana-500 007, India.
2Teegala Ram Reddy College of Pharmacy, Pragathi Colony, Meerpet, Hyderabad-500097, Telangana, India.
Corresponding Author E-mail: shilpika047@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2667
Abstract
Background: In the Indian Traditional system of Medicine, the herbal drug Aerva lanata was widely used in the management of urolithiasis. In the present study, a bioenhancer was used to evaluate the potential of constituents quercetin and betulin isolated from the plant A.lanata. Objective: In the present study, the isolated compounds quercetin and betulin from the plant A.lanata were screened for in vivo anti-urolithiasis and nephroprotective activities. Methodology: In the Wistar albino rats, urolithiasis was induced by ethylene glycol (0.75% v/v) to study the anti-urolithiatic activity. The animals were divided into seven groups of 6 animals in each group (n=6). The study period was for 28-day treatment with quercetin and betulin in combination with piperine as bio enhancers in nephrolithiasis induced rats. The Nephroprotective effect was also investigated in Gentamicin induced nephrotoxicity animal model. Cystone (750 mg/kg) was used as a standard. Biochemical parameters such as blood urea nitrogen (BUN), uric acid, and creatinine in the serum were determined. Renal calculi were determined in kidney homogenate and histopathology was also examined. Results: With the test drug treatment, animals showed increased urine volume significantly, also the renal tissue exhibited a reduction in the calculi formation. In the disease control animals, there was an increase in the serum BUN, uric acid, and creatinine significantly. Serum analysis revealed a significant reduction (*p<0.001) in the levels of BUN, uric acid, and creatinine in treated rats. Histopathological studies disclosed an improvement in the anatomical aspect of renal tissue. Conclusion: It was concluded that quercetin and betulin exhibited anti-urolithiatic effect by a reduction in the formation of calculi. The current study provided a rationale for the combination of piperine, quercitin, and betulin.
Keywords
Aerva Lanata Flowers; Antiurolithiatic Activity; Betulin; Hydroalcoholic Extract; Kidney Stones; Quercetin
Download this article as:| Copy the following to cite this article: Nagula S, Subhashini N. J. P, Bhikshapathi D. V. R. N, Mamatha P, Rao P. S. Anti-Urolithiatic and Nephroprotective Activity of Quercetin and Betulin in Conjunction with a Bio Enhancer – An in Vivo Study. Biomed Pharmacol J 2023;16(2). |
| Copy the following to cite this URL: Nagula S, Subhashini N. J. P, Bhikshapathi D. V. R. N, Mamatha P, Rao P. S. Anti-Urolithiatic and Nephroprotective Activity of Quercetin and Betulin in Conjunction with a Bio Enhancer – An in Vivo Study. Biomed Pharmacol J 2023;16(2). Available from: https://bit.ly/3NHx2oa |
Introduction
Globally, the most pervasive disorder affecting the urinary system is Urolithiasis, which accounted for approximately 2–3% of the population.1 Renal calculus or kidney stones are hard, solid particles formed in the urinary system, when left untreated might cause very ill upshots like utmost obstruction, hydronephrosis, internal bleeding, and infection. A patient experiences nephrolithiasis as a burden and troublesome substantial health problem in the adult population, with serious medical repercussions.2 In a maximum number of cases, the stones may be smaller in size that gets eliminated from the body without any hindrance. Nevertheless, if a stone blocks the urine flow, there may be excruciating pain, that needs medical attention.3 Periodic occurrence of stone formation is highly concern and essential care is mandatory. Reoccurrence of stone forming tendency is common amongst the patients suffering from the disease. Additionally, the epidemiological studies revealed that relapse of nephrolithiasis has been raised by 50% in the patients.4,5 Globally, incidence of urolithiasis is quite high, and more than 80% of urinary calculi are calcium oxalate stones alone or calcium oxalate mixed with phosphate which reckons about 75-90% followed by magnesium ammonium phosphate (Struvite) (10- 15%).6 These crystals are now commonly recognized to cause kidney stones and systemic problems, such as chronic kidney disease and renal failure, metabolic disorders, and cardiovascular diseases. Kidney stones might increase the chance of developing chronic kidney disease.7 In the present days, the management of nephrolithiasis is cost-effective and also related to patient compliance. The drugs used in the treatment may lead to adverse effects and even drug-drug interactions in case of comorbid conditions like diabetes, hypertension, and so on.8 As a part of treatment, several invasive procedures are employed, which might cause complications imposing a great load on the health care system. Such invasive procedures include lithotripsy, surgery, and disruption of calculus locally through laser techniques with high intensity in order to get rid of the calculi.9 Tragically, surgical procedures were always associated with terrible complications such as infections, hemorrhage, acute renal injury, renal fibrosis, diminished renal function, and perforations in ureters. Furthermore, despite being less invasive, the fragmentation treatment (lithotripsy) is linked to a higher rate of stone recurrence and a higher cost.10 As a result, novel therapy and preventative strategies for recurrent kidney stone disease are urgently required. Hence it is mandated that a drug might be chosen in such a way that it has some sort of protective action on renal structure and function, and possess a potent nephroprotective activity.11 As an exemplification, whenever a patient undergoes any pharmacological (conventional) treatment or surgery for kidney stones, it becomes essential to follow dietary and lifestyle changes.12 In this regard sometimes it is imperative to rely on chronic use of medications like potassium citrate, thiazides and allopurinol.13 This may eventually lead to implicit problems like cost effectiveness, patient compliance and, side effects.14 A preventive medicine that is simple to use, affordable, safe, and effective would therefore be greatly desired.
Ayurvedic practitioners treat urolithiasis with a set of medicinal plants known as Pashanabheda (stone breaking) which were utilized as phytotherapeutic agents in the Indian system of traditional medicine. 15 Herbal drugs have coherent pharmacological actions with minimal side effects and are considered to be used in terms of efficacy and safety.16 Over use or long-term use of synthetic drugs might dispose of a high-rise of adverse drug effects and adverse events, eventually making scientists to create a research platform for the progress of safer drugs with minimal side effects.17 Relatively, both in vitro and in vivo models have been employed for several pharmacological investigations of medicinal plants used for urolithiasis therapy.18 Consequently, a search for herbal preparations is still in progress.
Aerva lanata Juss. belongs to the family Amaranthaceae, is a common weed, and shrub which is erect and found all over India, and grows in fields.19 This plant has medicinal properties like a diuretic, anti-diabetic, and anti-tumor and is also used as an anti-lithiatic agent. The roots have a demulcent effect, also serves as a remedy for headaches.20 The phytochemical constituents responsible for biological activities present in A.lanata are alkaloids (ervine, methylervine, ervoside, aervine, methylaervine, aervoside, ervolanine, and aervolanine), flavanoids (kaempferol, quercetin, isorhamnetin, persinol, persinosides A and B), methyl grevillate, lupeol, lupeol acetate benzoic acid, b-sitosteryl acetate and tannic acid.21 A previous study was carried out by same investigators using column chromatography technique, two fractions of the flowers of A.lanata were subjected to isolation, ensuing purification of the isolated constituents by preparative HPTLC (high performance thin layer chromatography) and then the individual components were characterized by IR spectrophotometery.21 The constituents quercetin and betulin present in the flowers of A.lanata were used for the present investigation. However, there can be every chance that these new molecules possess an inimical pharmacokinetic profile due to impoverished solubility and membrane permeability.21 Concomitantly, whenever new chemical entities turn up, it is mandated to identify novel constituents which bear elevated therapeutic potential. There might exist a few reasons which affect permeability such as metabolic degradation, first-pass effect, and systemic drug delivery.22, 24 In order to enhance drug absorption, bio-enhancers have come into limelight, recognized as causation to improvise the quantity of unchanged drug that enters into systemic circulation alongside regulating the membrane permeability or metabolism. Bioenhancers are pharmacological facilitators, sometimes known as “bioavailability enhancers,” which are compounds that, when administered alone, do not exhibit the conventional drug activity.24 They enhance the drug’s molecule’s activity in a variety of ways, including improving the drug’s transmembrane bioavailability, interacting conformationally with the medication molecule to increase its potency, serving as drug receptors, and creating target cells more amenable to medication.25 A “bioenhancer” will always be capable of increasing a drug’s bioefficacy and bioavailability-specific medication that is used with it, bearing without any specific pharmacological action by itself independent of the administered dose.26
The species of Piper produced a pungent alkaloid named Piperine or 1-peperoyl piperidine. Piperine alters the lipid milieu and membrane dynamics at the site of absorption to improve permeability. The molecular nature of piperine makes it appropriate for inhibiting enzymes.27 Piperine is considered one of the top bio-enhancers and is used for both allopathic and ayurvedic drugs/formulations.28 It acts by intensifying the bioavailability of therapeutic drugs either by strengthening the absorption process or retarding the metabolism of drugs.29 In the year 2009, a study was conducted on the marketed product of Rifampicin, an Anti-tubercular drug, which was combined with other Anti-TB drugs (Tubercular), along with the piperine manufactured by Cadila Pharma in India. By using piperine, the bioavailability of rifampicin was elevated by roughly 60%.22 Thus, the dosage of rifampicin is decreased from 450 to 200 mg as a result of the addition of bioenhancer piperine. When combined with different medications, piperine lowers dosage, lessens negative effects, and boosts bioavailability.29 Hence in the present study, the test drugs quercetin and betulin were evaluated in conjunction with piperine for pharmacodynamic activity. Cystone was used as a standard drug, and had been reported as a prototype drug in the Ayurvedic practice, for the treatment of kidney stones. In addition to this, cystone is considered a safe compound and several studies emphasized that it possesses anti-urolithiatic effect.30 This investigation was focused on the in vivo anti-urolithiatic activity and nephroprotective effect of test compounds in animals.
Materials and Methods
Collection and authentication of plant
The flowers of Aerva lanata were collected from Medchal District, Hyderabad, Telangana. The flowers were authenticated by the Botanical Survey of India, Hyderabad, Telangana state. As a continuation of the previous study, the isolated compounds quercetin and botulin were evaluated for anti-urolithiatic effect.
Animals
The current study used Wistar albino rats (150-230g) of the male sex, the animals were housed under standard conditions,- and maintained on a 12 h light/dark cycle with free access to food and water up to the time of experimentation. The protocol bearing number 1447/PO/Re/S/11/41/A was reviewed and approved by the Institutional Animal Ethics Committee (IAEC), according to the guidelines of the Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA).
Acute oral toxicity study
Toxicity studies were carried out as per the Organization for Economic Cooperation and Development (OECD) guidelines no 423.31 Rats were used as experimental animals. All the animals were kept at overnight fasting before the experiment with free excess to water. The animals were divided into 3 groups, each comprising of 5 animals. The 1st group served as a negative control, while 2nd and 3rd were considered as tested groups that received orally the test drugs in normal saline with a starting dose of 2 mg/kg. The dose was calculated according to the body weight before the drug administration. The animals were observed for any toxic effects for the first 4 h after the treatment period. Further animals were investigated for a period of 3 days for any toxic effects. Behavioral changes and other parameters such as body weight, urination, food intake, water intake, respiration, convulsion, tremor, temperature, constipation, changes in eye and skin colors, etc. Therefore, the test compounds seem to be safe at a dose level of 2000 mg/kg, and the LD50 was considered to be >2000 mg/kg. The LD50 cut–off dose for Quercetin and Betulin was determined. To evaluate the anti urolithiatic activity a dose of 2 mg/kg body weight was selected for the study.
Induction of Urolithiasis by Ethylene glycol
With the purpose of induction of Urolithiasis, ethylene glycol (0.75% v/v) was used, an acceptable model to evaluate the anti-urolithiatic activity in animals.32 The animals were divided into 7 groups of six animals each (n=6). Group I served as control, administered with saline (0.9% Nacl). For groups II to VII from day 1 to day 28, they were given ethylene glycol (0.75 percent v/v) orally, mixed in drinking water, for the induction of renal calculi (day 1 to day 14-induction period). Group II was positive control, from 14th to 28th day (treatment period) group III animals were treated with a standard drug Cystone (750 mg/ kg b.w). Group IV to VII were treated with quercetin-2 mg/kg b.w., betulin-2 mg/kg b.w, Quercetin 2mg/kg + Piperine 10 mg/kg, betulin 2mg/kg + Piperine 10 mg/kg from 14th day to 28th day (equivalent dose) respectively.33
Grouping of animals
| Groups | Drugs and treatment |
| I | Normal control |
| II | Disease control (Ethylene Glycol 0.75% v/v) |
| III | Cystone 750 mg/kg |
| IV | Quercetin 2 mg/kg |
| V | Betulin 2 mg/kg |
| VI | Quercetin 2 mg/kg + Piperine 10 mg/kg |
| VII | Betulin 2 mg/kg + Piperine 10 mg/kg |
Urine samples were taken from all of the animals on the 14th and 28th days to determine the biochemical factors such as calcium, oxalate, and phosphate. The samples were stored at 4°C by adding one drop of conc. Hcl. On the same days, using polypropylene cages, the volume of urine from each group of animals was measured. On the 28th day the microscopic examination of urine samples for all animals was carried out for further microscopic study at 100 X under a microscope. Photographs were taken by using a digital camera attached to a microscope. The change in the body weight of animals was recorded individually. Under anesthetic ether, the blood samples were collected from the retro-orbital plexus on the 28th day of treatment, thus collected samples were centrifuged for 10 min (15000 rpm), serum was separated and estimated for (blood, urea, and nitrogen), uric acid, and creatinine. At the end of the experiment, animals were sacrificed and kidneys were examined for the presence of calculus.
Histopathology of Kidney
Kidneys were removed from each animal and rinsed in ice cold physiological solution. By using 10% buffered formalin, right kidney was fixed. After the processing, finally paraffin wax was (5 µm section) used to fix the tissue. Hematoxylin and eosin was used as staining agent. The deposition of calcium oxalate was observed under microscopic examination.
Induction of Nephrotoxicity by Gentamicin
Six groups of six rats in each group (n=6) were used in this model. Group I administered with normal saline (1.0 ml) for 23 days. Group II treated with gentamicin (40 mgkg-1 b.w, s.c) for 13 days and normal saline (1.0 m) from 14-23 days by oral route. The rats of groups III, IV, V and VI were administered with gentamicin at the dose of 40 mg kg-1 b.w, s.c, for first 13 days and treated with quercetin-2 mg/kg b.w., betulin-2 mg/kg b.w, Quercetin 2mg/kg + Piperine 10mg/kg, betulin 2mg/kg + Piperine 10mg/kg from 14th day onwards to 23rd day. On the 24th day blood was withdrawn through a tail vein to assess for renal function tests for all groups.19 The serum samples were estimated for blood urea, uric acid, and creatinine. Histological changes in kidney tissues of different treatment groups were also evaluated.20
Grouping of animals
| Groups | Drugs and treatment |
| I | Normal control |
| II | Gentamicin (40 mg/kg SC) |
| III | Gentamicin induced + Quercetin 2 mg/kg |
| IV | Gentamicin induced + Betulin 2mg/kg bw,p.o |
| V | Gentamicin induced + Quercetin 2mg/kg + Piperine 10mg/kg |
| VI | Gentamicin induced + Betulin 2mg/kg + Piperine 10mg/kg |
Statistical analysis
All the data were expressed as mean ± S.E.M., was analyzed using with GraphPad Prism 4.0 (GraphPad software, USA) one-way analysis of variance test (ANOVA), followed by Dunnett’s t-test, values less than p < 0.001 were considered as statistically significant.
Results
The test compounds Quercetin and betulin were screened for Anti-Urothiliatic and nephroprotective activity on Wistar Albino rats by using suitable models.
Acute Oral Toxicity Studies
The results of acute toxicity studies showed no mortality, also other side effects were not observed with the selected dose of 2 mg /kg body weight.
Urine Volume studies
In negative control animals, the urine volume was found to be 10.5 ± 0.71 and 11.33 ± 0.21 ml on 14th and 28th day respectively. In diseased control animals, there was a decline in the volume of urine levels with 3.83 ± 0.31 and 3.16 ± 0.30 ml on day 14 and 28, along with a significant reduction (*p<0.01) in the weight of the animals (Fig 1). In quercetin and betulin treated animals as individually and in combination with bio enhancer, there was an increase in weight significantly (*p<0.01) as compared with the normal animals. Additionally, the volume of urine was also increased significantly (*p<0.01) as compared to both the control and diseased rats (Table 1). The measurement of calcium, phosphate and oxalate crystals was done, found to be higher in diseased induced rats significantly (*p<0.01) as compared to normal rats. The standard drug treated animals revealed a significant reduction in the formation of crystals. The test drugs treated animals in combination with piperine exhibited a significant effect on lowering the levels of calcium, phosphate and oxalate crystals as compared to the diseased animals and individually test drug treated animals (Table 2).
Table 1. Effect of Test drugs treatment on Urine volume
| Groups | Treatment and Dose | Day 14 (ml) | Day 28 (ml) |
| I | Normal control | 10.5 ± 0.71 | 11.33 ± 0.21 |
| II | Disease control | 3.83 ± 0.31*** | 3.16 ± 0.30** |
| III | Standard drug Cystone | 6.83 ± 0.30*** | 10.17 ± 0.31*** |
| IV | Quercetin 2mg/kg | 7.16 ± 0.31** | 9.0 ± 0.71** |
| V | Betulin 2mg/kg | 6.17 ±0.31** | 6.66 ± 0.33** |
| VI | Quercetin 2 mg/kg + Piperine 10 mg/kg | 6.66 ± 0.33*** | 9.5 ± 0.31*** |
| VII | Betulin 2 mg/kg + Piperine 10 mg/kg | 5.33 ± 0.33ns | 7 ± 0.45*** |
*Normal group of animals as comparable to the positive control, and the groups IV, V, VI and VII. p < 0.01 was considered as significant.
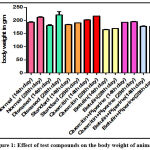 |
Figure 1: Effect of test compounds on the body weight of animals. |
*Normal group of animals as comparable to the positive control, and the groups IV, V, VI and VII. p < 0.01 was considered as significant.
Table 2: Effect of test drugs treatment on urinary Calcium, Phosphate and Oxalate levels
| Groups | Treatment and Dose | Calcium | Phosphate |
Oxalate |
| I | Normal control | 4.6 ± 0.12 | 145.0 ± 1.6 | 1.8 ± 0.05 |
| II | Disease control | 8.25 ± 0.50*** | 348.2 ± 1.9*** | 4.96 ± 0.09*** |
| III | Standard drug Cystone | 5.26 ± 0.09 | 187 ± 3.4*** | 2.06 ± 0.11** |
| IV | Quercetin 2mg/kg | 6.91 ± 0.20 | 219 ± 2.06 | 2.35 ± 0.06 |
| V | Betulin 2mg/kg | 6.8 ± 0.09 | 217.8 ± 2.04 | 2.35 ± 0.06 |
| VI | Quercetin 2mg/kg + Piperine 10 mg/kg | 6.0 ± 0.15** | 196 ± 5.1** | 2.05 ± 0.05** |
| VII | Betulin 2mg/kg + Piperine 10 mg/kg | 6.0 ± 0.17** | 201± 2.06** | 2.08 ± 0.09** |
*Normal group of animals as comparable to the positive control, and the groups IV, V, VI and VII. p < 0.01 was considered as significant.
Urine Analysis
The microscopic studies of urine samples in normal rats revealed the absence of crystal formation as evident from the sections observed. The excretion of crystals of calcium oxalate was found to be remarkable in rectangular-shaped in disease-induced groups of animals. In the standard drug- treated animals, there was absence or slight visibility of calcium oxalate crystals as apparent from the examination. Quercetin and betulin treated group of animals showed a significant moderate reduction in the formation of calcium oxalate crystals, whereas quercetin and betulin in combination with piperine treated animals showed maximum reduction in the calcium oxalate crystals. 34, 35 (Fig 2).
 |
Figure 2: Effect of treatment of test drugs on microscopic observation of urine samples. |
Serum Analysis for biochemical parameters
Effect of test drug treatment on serum biochemical parameters (BUN, Creatinine, Uric acid).
Serum analysis of biochemical parameters were analyzed in all the groups of animals. For examination of drug-induced nephrotoxicity in humans and animals, elevated levels of biochemical markers such as plasma or serum urea, uric acid, and creatinine were regarded as reliable. 36 In the positive control rats, there was a significant rise (*p<0.01) in serum BUN, uric acid, and creatinine levels. The quercetin treated group of animals showed a significant improvement (*p<0.01) in the levels of serum BUN, uric acid, and creatinine. Similarly, betulin treated rats showed a prominent effect on the serum levels of BUN, uric acid and creatinine as compared to quercetin. The animals treated group with quercetin and betulin in combination with piperine showed diminished (*p<0.01) levels of BUN, creatinine, and uric acid as compared to the positive control animals. (Fig 3).
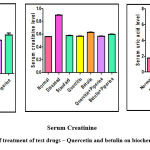 |
Figure 3: Effect of treatment of test drugs – Quercetin and betulin on biochemical parameters. |
Histopathological Examination
In normal animals, the histopathological studies of kidneys revealed that there was an absolute absence of renal calculi and associated deformities. In the positive control group, there was a deposition of calcium oxalate crystal deposits, desquamation of epithelium, cellular inflammation, and blood vessel congestion which is discernible from the sections. A significant recovery of such abnormalities was noticed in animals with test drug-treated animals in combination with piperine (Fig 4).
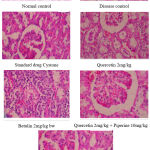 |
Figure 4: Effect of test drug treatment on histopathological sections of kidneys in Ethylene Glycol induced Urolithiasis in rats |
Effect of test drugs treatment in Gentamicin induced toxicity
There was an increased level of blood urea and creatinine in disease induced group of animals. Nevertheless, after the treatment, a significant protective effect of test drugs was remarkably noticed in gentamicin induced nephrotoxic rats. The blood urea concentrations were found to be reduced in a significant manner (*p < 0.01) in test drug treated animals individually and also in combination with piperine. The values in combined treatment of test drugs with piperine treated animals were found to be lessened as compared to the individualized drug treated groups. Similarly, with regard to serum creatinine concentrations, same observations were noted in the groups treated with quercetin and botulin along with piperine (Table. 3). In the positive control animals, the histopathological sections of the kidney showed a damaged proximal tubule with a definite sign of nephrotoxicity and pronounced renal dysfunction as evidenced by the elevated levels of serum creatinine (Fig 5). Also, it was a striking observation that there was an improvement in the anatomical feature of the proximal tubule in animals treated with a combination of test drugs along with bio enhancers.
Table 3: Effect of test drugs treatment on serum biochemical parameters in Gentamicin – induced nephrotoxicity
| Groups | Treatment and Dose |
Blood Urea (mg/dl) |
Uric acid (mg/dl) |
Serum Creatinine (mg/dl) |
| I | Normal | 23.23 ± 1.45 | 2.08 ± 0.07 | 0.33 ± 0.05 |
| II | Gentamicin induced
(40 mg/kg) |
97.46± 1.57** | 3.84 ± 0.09** | 4.46 ± 0.06** |
| III | Quercetin (2 mg/kg) | 42.78± 1.61* | 2.49 ± 0.05* | 1.35 ± 0.09* |
| IV | Betulin (2 mg/kg) | 46.8± 1.98* | 2.72 ± 0.08** | 1.38 ± 0.05* |
| V | Quercetin 2 mg/kg + Piperine 10 mg/kg | 27.94± 1.78** | 2.35 ± 0.07** | 0.53 ± 0.06** |
| VI | Betulin 2 mg/kg + Piperine 10 mg/kg | 26.99± 1.25** | 2.80 ± 0.05** | 0.46 ± 0.03** |
*Normal group of animals as comparable to the positive control, and the groups IV, V, VI and VII. p < 0.01 was considered as significant.
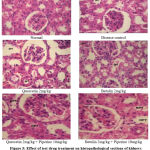 |
Figure 5: Effect of test drug treatment on histopathological sections of kidneys in Gentamicin induced Nephrotoxic animals. G-glomerulus, DPT-damaged proximal tubule, NPT-normal proximal tubule. |
Discussion
The use of any type of drug, whether a conventional or an alternative depends on causing minimum side effects, reduced cost, and patient compliance.37 In the Indian System of Medicine, Ayurveda was found to be most prominent and might be considered perpetual in the treatment of chronic diseases such as diabetes, hypertension, central nervous system disorders, and certain cancers.38 According to reports, kidney stones have been a problem for humans for a very long time and were one of the causes of renal failure. Surgery was seen to be the best option because there is currently not even a single medication that may effectively treat urolithiasis, especially when previous treatments were ineffective.39,40 However, it is high-priced and out of reach for an average person. As a result, natural medicines were seen as the best option.41 In the present research, urolithiasis had been evaluated with chronic treatment in animals. The rationale behind this type of investigation depended on the test drug that possesses medicinal values, which eventually inclined to a good therapeutic outcome. Globally the prevalence of renal stone formation had been tremendously increased ranging from 1 to 15%, along with time, affecting with more eminent hallmark as reoccurrence of stone formation.42 Several treatment strategies were heeded for ages, but there was a lacuna of proper display of successful triumphant therapy.43 Hence the current study emphasized a phytotherapeutic investigation of active constituents of the herbal drug in the treatment of urolithiasis. In the present study, the role of a bio enhancer played was so crucial that the combination of test drugs with a bio enhancer, piperine was investigated for anti-urolithiatic activity.44,45,46 There were studies that explored the concomitant use of bio enhancers with the test drugs,- and reported a favorable outcome with respect to the therapy.47 Concurrently, it is mandated to consider the absorption, when given in conjunction with other drugs, as absorption reflects the bioavailability of the test drug administered. Piperine was used as a bio enhancer, as it is commonly accepted, to affect the absorption process from the gut, decelerate the biotransformation, inactivation and elimination of drugs.48,49 Eventually, the concentration and sustainability of the drug in the blood are retained thus making the availability and utilization of drug to a certain extent by the tissues.50 Since ancient times, the concept of bio-enhancers with herbal origin was found to have importance in the Ayurvedic system of medicine.51 The species belonging to ‘Piper’ which contains the active component piperine was found to improvise the bioavailability of drugs, vitamins, and nutrients.52 The combination of piperine with drugs is more beneficial in drugs that are poorly available, long-term usage, hazardous and are costly.53 Piperine might have a mechanism of inhibiting the enzymes responsible for biotransformation thus preserving the drug stay for longer period of time. In the therapy of kidney stone formation, there might be a requirement of chronic use of medicines as a part of therapy, or a need for a surgery that becomes burdensome for a common man. Hence the use of herbal bio-enhancer in combination with drugs may have a promising effect in the successful treatment.54 Additionally, piperine was also found to act by increasing the drug absorption in the GIT, reduces the drug elimination from cells, inhibits the production of glucuronic acid in the intestine, thus making the active drug enter the body.55
In the current study, the body weight, urine analysis for calcium, oxalate, and phosphate estimation, serum concentrations of BUN, urea, creatinine, and microscopic examination of urine samples for calcium oxalate crystal formation and the histopathology of the kidney were analyzed.56 The test drugs quercetin and betulin were combined individually with piperine and were evaluated for the above-mentioned activities. In their previous study by the same authors the Hydroalcoholic (80%-water, 20%-alcohol) extract of A. lanata flowers was prepared and fractionation with different organic solvents were carried out. The two fractions (ethyl acetate and n-butanol) were subjected to isolation of active constituents using column chromatography technique, followed by preparative high performance thin layer chromatography (HPTLC) and then the individual components were characterized by IR spectrophotometry.21 Urolithiasis in experimental animals was induced using ethylene glycol, administration of this led to calcium oxalate monohydrate formation through the metabolism process and got reflected in both urine and serum.57,58 The chronic treatment with Quercetin solely and in combination with piperine revealed a significant intrinsic diuretic like effect by increasing the output of urine, thus diluting the electrolyte concentration. The effect on the output of urine might be due to the possible action of A.lanata on the excessive secretion or reduction in the concentration of urinary salts, consistent with the previous studies of A.lanata as a diuretic.59
In the genesis of calculi, urinary super saturation with regard to the stone-forming constituents was considered one of the causative factors. The excretions of calcium and phosphorus in urine were also elevated in animals which possessed a tendency of stone formation. In the test drug treated animals in combination with piperine there was a significant lowering effect in the levels of calcium, phosphate and oxalate crystals.60 In the positive control animals, there was an exceptional formation of rectangular shaped calcium oxalate crystals in the urine sample. Additionally, the microscopic analysis of urine samples revealed that the test drugs mitigated the intensity of formation of crystals of calcium which was conspicuously noted. This may be also due to the inhibition of the Oxalate oxidase enzyme activity that is responsible for the stone formation.61
Urolithiasis occurs when calculi build up in the urinary tract, obstructing the glomerular filtration rate (GFR) and lowering urine output and also due to the damage to renal parenchyma. In consequence, waste products, compounds like BUN, creatinine, and uric acid, may build up in the bloodstream.62 Due to this, waste products, particularly nitrogenous substances such as urea, creatinine, and uric acid, get accumulated in the blood. The treatment with quercetin and betulin in combination with piperine considerably reduced the high BUN, creatinine, and uric acid levels in the blood. This lowered the danger of waste debris obstructing urine flow in the urinary system.63The increased quantity of nitrogenous compounds in serum also signals a risk of kidney injury, which was dramatically reduced in rats given with the chronic treatment of test drugs in conjunction with piperine. 64,65,66 Microscopically the slides of kidneys in disease control animals showed accumulation of calcium oxalate crystal deposits with cellular inflammation; test drug treated animals exhibited recovery from abnormalities after the treatment in combination with piperine.
An aminoglycoside, Gentamicin being nephrotoxic was used for induction of nephrotoxicity in animals in order to evaluate the test drugs.67 It produced necrosis followed by a hampered function of kidney which might be a clear evident from the histopathological study of kidney sections.68 In the current study, the serum samples were determined for urea, uric acid and creatinine in diseased and test drug treated groups. In positive control animals’ the histopathological sections revealed an upsurge of three parameters which indicated a dysfunction and nephrotoxic effect, also manifested by the proximal tubule damage and marked renal dysfunction as observed with the levels of serum creatinine. The test drug treated animals with bio enhancer had significantly reduced the biochemical parameters- urea, uric acid and serum creatinine and restored the anatomical features of renal tissue to normalcy. It is quite important to note that whenever there is an alteration in creatinine, it might enhance the stone formation, deposition of nitrogenous waste as proven with the urea and uric acid levels, and eventually a kidney damage. There was an improvement in the structural features of proximal tubule in animals treated with combination of test drugs along with piperine. Several studies have been reported on bioenhancing effect of piperine that it improvised the absorption of many drugs.69,70,71 The same principle might be responsible for the mode of action of isolated test compounds quercetin and botulin in urolithiasis and nephrotoxicity.
In regard to the betulin mechanistic effect, there had been a study conducted on mice and was derived that betulin diminished liver injury induced by alcohol, probably by activating sirtuin 1 (SIRT1) signaling pathway.72 The NAD+ (Nicotinamide adenine dinucleotide) -dependent deacetylase sirtuin 1 (SIRT1) significantly protects the kidneys by controlling fibrosis, apoptosis, senescence, oxidative stress, inflammation, and the aging process. Numerous kidney disorders, including diabetic kidney disease and HIV-related kidney disease, have been linked to Sirt1’s renal protective properties. SIRT1 may be developed as a treatment for both CKD (Chronic Kidney disease) and CKD complications because it has protective effects against arterial calcification.73 Hence there is also a possibility of the test compounds quercetin and betulin acting as sirtuin 1 activators and thereby might have a promising therapeutic effect on nephrolithiasis and kidney stone formation.74
Keeping in view of these above considerations, the test drugs quercetin and betulin may be suitable for the treatment of calculi formation and renal damage, hence considered as nephroprotective agents.
Conclusion
The current study results showed depletion in the formation of calcium, phosphate, and oxalate crystals in the test drug-treated animals with the enhanced functional capacity of the kidneys as evident with the parameters like BUN, urea, and creatinine respectively. Additionally, there were no renal calculi noted in the histopathological sections. Hence it can be concluded that chronic treatment with the combination of quercetin and betulin with piperine had a probable anti-urolithiatic activity combating stone formation which supported the rational use of a bio enhancer. As a nephroprotective agent, the test compounds presented a significant improvement in the serum parameters and restored the renal structure. Thus, Quercetin and betulin were proven to be nephroprotective and could be considered favorable drugs in kidney related diseases. The concept of the use of bio enhancers inclined innovative research in the traditional system of medicine. This eventually leads to cost reduction, minimal side, toxic effects, and safer drug usage in patients. However, the development of new potent Piperine derivatives and novel bio enhancers must be continued. Extreme care is necessary when piperine is combined with such drugs whose levels get influenced by it. Hence, furthermore, elaborated pharmacokinetic studies are required to evaluate the crucial role of bio enhancers.
Conflicts of Interest
There are no conflicts of interest.
Fundig Sources
There is no funding sources.
References
- Bahmani M, Baharvand-Ahmadi B, Tajeddini P, Rafieian-Kopaei M, Naghdi N Identification of medicinal plants for the treatment of kidney and urinary stones. J Renal Inj Prev.;5(3):129-133 (2016). Published 2016 Jul 27. doi:10.15171/jrip.2016.27.
- Ramello A, Vitale C, Marangella M. Epidemiology of nephrolithiasis. Nephrol.,13 Suppl 3: S45-50 (2000). PMID: 11132032.
- Bouanani S, Henchiri C, Migianu-Griffoni E, Aouf N, Lecouvey M. Pharmacological and toxicological effects of Paronychia argentea in experimental calcium oxalate nephrolithiasis in rats. J Ethnopharmacol.,129(1):38-45 (2010). doi: 10.1016/j.jep.2010.01.056. Epub 2010 Feb 4. PMID: 20138208.
- Shoag J, Tasian G, Goldfarb D, Eisner B. The new epidemiology of nephrolithiasis. Chronic. Kidney. Disease., 22(4):273–8 (2015). Available from: https://www.sciencedirect.com/science/article/pii/ S1548559515000592 https://doi.org/10.1053/j.ackd.2015.04.004 PMID: 26088071 3.
- Sener TE, Sener G, Cevik O, Eker P, Cetinel S, Traxer O, Tanidir Y, Akbal C. The Effects of Melatonin on Ethylene Glycol-induced Nephrolithiasis: Role on Osteopontin mRNA Gene Expression. Urology., 99:28 (2017). e9–287.e15. Available from: https://doi.org/10.1016/j.urology.2016.09.032 PMID: 27717860
- Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. Int., 92(1):137-40 (2003). doi: 10.1046/j.1464-410x.2003.04289x. PMID: 12823398.
- Touhami M, Laroubi A, Elhabazi K, Loubna F, Zrara I, Eljahiri Y, Oussama A, Grases F, Chait A. Lemon juice has protective activity in a rat urolithiasis model. Urol., 7:18 (2007). Published 2007 Oct 5. doi:10.1186/1471-2490-7-18.
- Butterweck V, Khan SR. Herbal medicines in the management of urolithiasis: alternative or complementary? Med., l75(10):1095-103 (2009). Epub 2009 May 14. PMID: 19444769; PMCID: PMC5693348.
- Kumar KV, Naidu MU, Shifow AA, Ratnakar K S. Probucol protects against gentamicin-induced nephrotoxicity in rats. J. Pharmacol., 32:108-13 (2000).
- Pani SR, Mishra S, Sahoo S, Panda PK. Nephroprotective effect of Bauhinia variegate (linn.) whole stem extract against cisplatin-induced nephropathy in rats. J. Pharmacol.,43:200-2 (2011).
- Kosalge SB, Fursule RA. Investigation of ethnomedicinal claims of some plants used by tribals of Satpuda Hills in India. Ethnopharmacol., 121(3):456-61 (2009). doi: 10.1016/j.jep.2008.11.017. Epub 2008 Nov 28. PMID: 19100321.
- Goldfarb DS. Increasing prevalence of kidney stones in the United States. Int., 63:1951 (2003). [PubMed: 12675877]
- Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC. Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int., 63:1817 (2003).
- Trinchieri A. Diet and renal stone formation. Minerva Med., 104(1):41-54 (2013). PMID: 23392537.
- Veronika B, Saeed RK. Herbal medicines in the management of urolithiasis: alternative or complementary? Medica.,7:1095e103 (2012).
- Herbs: an alternative approach in nephroprotection. Res. J. Pharmacognosy. Phytochem .;5:15e21 (2013).
- Soundararajan P, Mahesh R, Ramesh T, Begum V H. Effect of Aerva lanata on calcium oxalate urolithiasis in rats. J. Exp. Biol.,44(12):981-6 (2006). PMID: 17176671.
- Dinnimath BM, Jalalpure SS, Patil UK. Antiurolithiatic activity of natural constituents isolated from Aerva lanata. Ayurveda. Integr. Med., 8(4):226-232 (2017). doi: 10.1016/j.jaim.2016.11.006. Epub 2017 Nov 21. PMID: 29169771; PMCID: PMC5747499.
- Shirwaikar A, Issac D, Malini S. Effect of Aerva lanata on cisplatin and gentamicin models of acute renal failure. J Ethnopharmacol., 90(1):81-6 (2004). doi: 10.1016/j.jep.2003.09.033. PMID: 14698513.
- Mitragotri S. Synergistic effect of enhancers for transdermal drug delivery. Pharm Res. ,17(11):1354-9 (2000). doi: 10.1023/a:1007522114438. PMID: 11205727.
- Naikal James Prameela S, Nagula S. Antiurolithiatic activity of natural constituents isolated from aerva lanata flowers. J. Pharm. Sci. Drug Res.,12(6):668-73 (2020). http://www.ijpsdr.com/index.php/ijpsdr/article/view/1949
- Choudhary N, Khajuria V, Gillani Z H, et al., Effect of Carum carvi, a herbal bioenhancer on pharmacokinetics of antitubercular drugs: A study in healthy human volunteers. Clin. Res., 5, 80 (2014).
- Janakiraman K, Manavalan R. Studies on effect of piperine on oral bioavailability of ampicillin and norfloxacin. Afr J Tradit Complement Altern Med., 5(3):257-262 (2008). doi:10.4314/ajtcam.v5i3.31281.
- Badmaev V, Majeed M, Prakash L. Piperine derived from black pepper increases the plasma levels of coenzyme Q10 following oral supplementation. Nutr. Biochem.,11:109–113 (2000).
- Bajad S, Bedi KL, Singla AK, Johri RK. Piperine inhibits gastric emptying and gastrointestinal transit in rats and mice. Med., 67: 176-179 (2001).
- Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. Pharmacol. Exp. Ther., 232: 258-262 (1985).
- Mhaske DB, Sreedharan S, Mahadik KR. Role of Piperine as an Effective Bioenhancer in Drug Absorption. Analytica. Acta., 591 (2018). doi: 10.4172/2153-2435.1000591
- Atal N, Bedi KL. Bioenhancers: Revolutionary concept to market. Ayur. Int. Med. 1: 96-99 (2010).
- Amar S, Pawar VK, Vikash J, Parabia MH, Rajendra A, Sharma G. In-vivo assessment of enhanced bioavailability of metronidazole with piperine in rabbits. J. Pharm. Biol. Chem. Sci., 1: 273-278 (2010).
- Erickson SB, Vrtiska TJ, Lieske JC. Effect of Cystone® on urinary composition and stone formation over a one-year period. Phytomedicine., 18(10):863-7 (2011). doi: 10.1016/j.phymed.2011.01.018. Epub 2011 Mar 17. PMID: 21419609; PMCID: PMC3925635.
- OECD guidelines for testing chemicals. Acute Oral Toxicity – Acute Toxic Class Method. 423 (2001).
- Hadjzadeh MA, Khoei A, Hadjzadeh Z. Ethanolic extract of nigella sativa L seeds on ethylene glycol-induced kidney calculi in rats. Urol J., 4(2):86-90 (2007) PMID: 17701927.
- Huang HS, Ma MC, Chen J, et al. Changes in the oxidant-antioxidant balance in the kidney of rats with nephrolithiasis induced by ethylene glycol. J Urol., 167(6):2584-93 (2002). PMID: 11992092.
- Lulat SI, Yadav YC, Balaraman R, Maheshwari R. Antiurolithiatic effect of lithocare against ethylene glycol-induced urolithiasis in Wistar rats. Indian. Pharmacol., 48(1):78-82 (2016). doi: 10.4103/0253-7613.174564. PMID: 26997728; PMCID: PMC4778213.
- Jagannath N, Chikkannasetty SS, Govindadas D, Devasankaraiah G. Study of antiurolithiatic activity of Asparagus racemosus on albino rats. J. Pharmacol., 44(5):576-9 (2012). doi: 10.4103/0253-7613.100378. PMID: 23112416; PMCID: PMC3480787.
- Naushad M, Urooj M, Ahmad T, Husain GM, Kazmi MH, Zakir M. Nephroprotective effect of Apium graveolens L. against Cisplatin-induced nephrotoxicity. Ayurveda. Integr. Med., 12(4):607-615 (2021). doi: 10.1016/j.jaim.2021.06.005. Epub 2021 Nov 11. PMID: 34774409; PMCID: PMC8642661.
- Patle A, Hatware KV, Patil K, Sharma S, Gupta G. Role of Herbal Medicine in the Management of Urolithiasis- A Review for Future Perspectives. J Environ Pathol Toxicol Oncol., 38(2):97-118 (2019). doi: 10.1615/JEnvironPatholToxicolOncol.2019029075. PMID: 31679274.
- Monti E, Trinchieri A, Magri V, Cleves A, Perletti G. Herbal medicines for urinary stone treatment. A systematic review. Arch Ital Urol Androl., 31;88(1):38-46 (2016). doi: 10.4081/aiua.2016.1.38. PMID: 27072174.
- Alelign T, Petros B. Kidney Stone Disease: An Update on Current Concepts. Adv Urol., 4 (2018). doi: 10.1155/2018/3068365. PMID: 29515627; PMCID: PMC5817324.
- Nagal A, Singla RK. Herbal resources with antiurolithiatic effects: a review. Indo Glob J Pharm Sci.,3(1):6-14 (2013).
- Saigal CS, Joyce G, Timilsina AR. Urologic Diseases in America Project. Direct and indirect costs of nephrolithiasis in an employed population: opportunity for disease management? Kidney Int., 68(4):1808-14 (2005). doi: 10.1111/j.1523-1755.2005.00599.x. PMID: 16164658.
- Hollingsworth JM, Rogers MA, Kaufman SR, Bradford TJ, Saint S, Wei JT, Hollenbeck BK. Medical therapy to facilitate urinary stone passage: a meta-analysis. Lancet., 368(9542):1171-9 (2006). doi: 10.1016/S0140-6736(06)69474-9. PMID: 17011944.
- Kesarwani K, Gupta R, Mukerjee A. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed.;3(4):253-266 (2013). doi:10.1016/S2221-1691(13)60060-X.
- Peterson B, Weyers M, Steenekamp JH, et al. Drug Bioavailability Enhancing Agents of Natural Origin (Bioenhancers) that Modulate Drug Membrane Permeation and Pre-Systemic Metabolism. Pharmaceutics., 11(1):33 (2019). doi: 10.3390/pharmaceutics11010033. PMID: 30654429; PMCID: PMC6359194.
- Khatri S, Awasthi R. Piperine containing floating microspheres: an approach for drug targeting to the upper gastrointestinal tract. Drug Deliv Transl Res., 6: 299-307 (2016).
- Di X, Wang X, Liu Y. Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J Pharm Biomed Anal., 115: 144-149 (2015).
- Shaikh J, Ankola DD, Beniwal V, Singh D, Kumar MN. Nanoparticle encapsulation improves oral bioavailability of curcumin by at least 9-fold when compared to curcumin administered with piperine as absorption enhancer. J. Pharm. Sci.,37;223-30 (2009).
- Patel S, Chopra S, Chaurasia S, Sarwat M. Plant based Bioavailability Enhancers. Curr Pharm Des., 28(8):642-654 (2022). doi: 10.2174/1381612828666220112141355. PMID: 35023453.
- Dudhatra GB, Mody SK, Awale MM, Patel HB, Modi CM, Kumar A, Kamani DR, Chauhan BN. A comprehensive review on pharmacotherapeutics of herbal bioenhancers. Scientific World Journal.,637953 (2012). doi: 10.1100/2012/637953.
- Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. Pharmacol. Exp. Ther.,302:645-50 (2002).
- Myung JK, Jae YC, Byung HS, Duk KK, Jaehwi L. Bioavailability enhancing activities of natural compounds from medicinal plants. J of Med Plan Res., 31;3(13):1204-11 (2009).
- Shinkar DM, Amrutkar SV, Pingale PL. Case study: Indian herbal bioenhancers. Drug Delivery Technology: Herbal Bioenhancers in Pharmaceuticals., 21:239 (2022).
- Gülçin I. The antioxidant and radical scavenging activities of black pepper (Piper nigrum) seeds. Int J Food Sci Nutr.,56(7):491-9 (2005). doi: 10.1080/09637480500450248. PMID: 16503560.
- Khan IA, Mirza ZM, Kumar A, Verma V, Qazi GN. Piperine, a phytochemical potentiator of ciprofloxacin against Staphylococcus aureus. Antimicrobial agents and chemotherapy.,50(2):810-2 (2006).
- Patil UK, Singh A, Chakraborty AK. Role of piperine as a bioavailability enhancer. Int Recent. Adv. Pharm. Res., 4: 16-23 (2011).
- Ramachandran S, Vijayakumar TM, Saisandeep V, et al. Anti-lithiatic activity of polyherbal extract on ethylene glycol-induced lithiasis in rats. J. Biomed. Sci., 3:36e9 (2011).
- Karadi RV, Gadge NB, Alagawadi KR, Savadi R V. Effect of Moringa oleifera Lam. root-wood on ethylene glycol induced urolithiasis in rats. J Ethnopharmacol. 105(1-2):306-11 (2006). doi: 10.1016/j.jep.2005.11.004.
- Patel PK, Patel MA, Vyas BA, Shah D R, Gandhi T R. Antiurolithiatic activity of saponin rich fraction from the fruits of Solanum xanthocarpum Schrad. & Wendl. (Solanaceae) against ethylene glycol induced urolithiasis in rats. Ethnopharmacol., 144(1):160-70 (2012). doi: 10.1016/j.jep.2012.08.043. Epub 2012 Sep 5. PMID: 22981722.
- Amarnath S, Narayanan K R, Venkataraman R, Dasarathan P. Anti-Diuretic impact of Aerva lanata extracts and their active compounds in Furosemide administered Diuretic rats. Ind. J., 14(6): 183 (2018).
- Ashok Sharma, S.C. Sharma, J.S. Vaghela, Phytopharmacological Investigation of Aerva Lanata Flowers with Special Emphasis on Diuretic Activity. J., 2 (17); 59-62 (2010).
- Mishra S, Pani SR, Sahoo S. Anti-nephrotoxic activity of some medicinal plants from tribal rich pockets of Odisha. Pharmacognosy Res.,6(3):210-7 (2014). doi: 10.4103/0974-8490.132598. PMID: 25002801; PMCID: PMC4080501.
- Kumar R, Kumar T, Kamboj V, Chander H. Pharmacological evaluation of ethanolic extract of Kigelia pinnata fruit against ethylene glycol induced urolithiasis in rats. J. Plant. Sci. Res., 2:63e72 (2012).
- Atmani F, Slimani Y, Mimouni M, Hacht B. Prophylaxis of calcium oxalate stones by Herniaria hirsuta on experimentally induced nephrolithiasis in rats. Int. 92:137e40 (2003).
- Gadge NB, Jalalpure SS. Curative treatment with extracts of Bombax ceiba L. (Bombacaceae) fruit reduces risk of calcium oxalate urolithiasis in rats. Biol., 50:338e43(2012).
- Zhang W, Zheng Q, Song M, Xiao J, Cao Y, Huang Q, Ho CT, Lu M. A review on the bioavailability, bio-efficacies and novel delivery systems for piperine. Food Funct., 4;12(19):8867-8881 (2021). doi: 10.1039/d1fo01971f. PMID: 34528635.
- Divakar K, Pawar AT, Chandrasekhar SB, Dighe SB, Divakar G. Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem Toxicol., 48(4):1013-8 (2010).
- Lulat SI, Yadav YC, Balaraman R, Maheshwari R. Anti-urolithiatic effect of lithocare against ethylene glycol-induced urolithiasis in Wistar rats. Indian J Pharmacol., 48(1):78-82 (2016). doi: 10.4103/0253-7613.174564. PMID: 26997728; PMCID: PMC4778213.
- Silan C, Uzun O, Comunoğlu NU, Gokçen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull., 30(1):79-83 (2007). doi: 10.1248/bpb.30.79. PMID: 17202664.
- Yarijani ZM, Najafi H, Hamid Madani S. Protective effect of crocin on gentamicin-induced nephrotoxicity in rats. Iran J Basic Med Sci.,19(3):337-43 (2016). PMID: 27114805; PMCID: PMC4834125.
- Huang T, Liu Y, Zhang C. Pharmacokinetics and Bioavailability Enhancement of Baicalin: A Review. Eur J Drug Metab Pharmacokinet., 44(2):159-168 (2019). doi: 10.1007/s13318-018-0509-3. PMID: 30209794.
- Ajazuddin, Alexander A, Qureshi A, Kumari L, Vaishnav P, Sharma M, Saraf S, Saraf S. Role of herbal bioactives as a potential bioavailability enhancer for Active Pharmaceutical Ingredients. Fitoterapia., 97:1-14 (2014). doi: 10.1016/j.fitote.2014.05.005.
- Bai T, Yang Y, Yao YL, Sun P, Lian LH, Wu YL, Nan JX. Betulin alleviated ethanol-induced alcoholic liver injury via SIRT1/AMPK signaling pathway. Pharmacol Res., Mar;105:1-12 (2016). doi: 10.1016/j.phrs.2015.12.022. Epub (2016) Jan 15. PMID: 26776965.
- Ye QL, Wang DM, Wang X, Zhang ZQ, Tian QX, Feng SY, Zhang ZH, Yu DX, Ding DM, Xie DD. Sirt1 inhibits kidney stones formation by attenuating calcium oxalate-induced cell injury. Chem Biol Interact., 25;347:109605 (2021). doi: 10.1016/j.cbi.2021.109605. Epub 2021 Jul 29. PMID: 34333021.
- Hou J, Ding J, Li L, Peng Y, Gao X, Guo Z. Association of sirtuin 1 gene polymorphisms with nephrolithiasis in Eastern chinese population. Ren Fail,. Nov;41(1):34-41 (2019). doi: 10.1080/0886022X.2019.1568258. PMID: 30714469; PMCID: PMC6366414.








