Abdelnaser Abdel Atti Gadallah1* , Hany Abdelbary Abdelaziz1, Waleed Abdelfattah Mousa2
, Hany Abdelbary Abdelaziz1, Waleed Abdelfattah Mousa2 , Mohamed Elsaeed Lashin3
, Mohamed Elsaeed Lashin3 , Mostafa Ahmed Al-Abyad1 and Anwar Abdelaleem Mohamed4
, Mostafa Ahmed Al-Abyad1 and Anwar Abdelaleem Mohamed4
1Internal Medicine, Gastroenterology & Hepatology, Faculty of Medicine - Menoufia University. Egypt
2Radiology, Faculty of Medicine, Menoufia University. Egypt
3Neurology, Faculty of Medicine- Menoufia University. Egypt
4Hepato-gastroenterology, National Liver Institute – Menoufia University. Egypt
Corresponding Author E-mail: ahmed_Naser2004@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2622
Abstract
Chronic Vascular Diseases (CVD) is a major health burden. Hepatitis C Virus (HCV) infection has been implicated in the development of carotid artery atherosclerosis and has recently been associated with poor prognosis in stroke patients. The purpose of this study is to predict the result of de novo cerebrovascular accidents in HCV-infected patients and to look for variables that may predict it. Case control, prospective study had been carried out on two groups, Group (A) of 32 HCV infected patients presented with newly onset cerebrovascular stroke and Group (B) of 32 patients with cerebrovascular stroke without HCV infection. After meticulous history taking and neurological examination for all patients, those presenting with cerebrovascular stroke confirmed by computerized tomography (CT) or Magnetic resonance imaging (MRI) of brain were included in this study. These patients were followed up for 2 weeks and then extended follow up for 3 months was done. The outcome and predictors of prognosis had been documented and estimated statistically. Hb, platelets, albumin, cholesterol, and Na showed significant decreases in the HCV patients than in the free group. However, direct bilirubin, total bilirubin, international normalized ratio (INR), and HbA1C showed significant increases in the case group. Age, National institute of health stroke scale (NIHSS), and Diabetes Mellitus (DM) showed significant increases in poor prognosis in HCV cases, whereas HDL showed a significant decrease. NIHSS, abnormal carotid intima thickness (CIT), and abnormal pulse showed significant increases in control group with poor prognosis. Regarding fate, no significant difference was found between HCV patients and the control group. Regarding prognosis by three-month Modified rankin score (MRS) a significant decrease in the HCV patient group in comparison to the free group. We found there is a significant association between chronic HCV infections and Ischemic Stroke severity and bad prognosis.
Keywords
Cerebrovascular stroke; HCV infected patients; Outcome; Prognosis
Download this article as:| Copy the following to cite this article: Gadallah A. A. A, Abdelaziz H. A, Mousa W. F, Lashin M. E, Al-Abyad M. A, Mohamed A. A. Prediction of Outcome of Newly Onset Cerebrovascular Stroke in HCV Infected Patients. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Gadallah A. A. A, Abdelaziz H. A, Mousa W. F, Lashin M. E, Al-Abyad M. A, Mohamed A. A. Prediction of Outcome of Newly Onset Cerebrovascular Stroke in HCV Infected Patients. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3HGZAt7 |
Introduction
The second biggest cause of death worldwide is stroke.1,2 In the ensuing decades, it will significantly worsen health burdens in most wealthy nations. Heart disease, high blood pressure, diabetes, alcohol use, poor diet, abdominal obesity, inactivity, psychosocial stress, and depression are all traditional risk factors for stroke.3
Hepatitis C is a liver condition brought on by HCV. Acute and chronic hepatitis, which can range from a mild condition that lasts a few weeks to a serious condition that lasts a lifetime, can both be brought on by the virus. One of the leading causes of liver cancer is hepatitis C.4
Numerous epidemiological studies have shown that chronic HCV infection increases the risk of stroke or cerebrovascular mortality independently.5,6 However, Younossi et al. found no connection between HCV and stroke.7
There are two primary stroke categories. A blockage is what causes an ischemic stroke. A blood vessel rupture is what leads to a hemorrhagic stroke. Both types of strokes deplete the brain’s tissues of blood and oxygen, which results in the death of brain cells. Acute Ischemic Stroke (IS) is the most frequent type of stroke which happens when a blood clot plugs a blood artery, stopping oxygen and blood from getting to certain areas of the brain. It can accomplish this in two ways. A stroke from embolism is one possibility. It happens when a blood clot develops elsewhere in the body and obstructs a brain blood artery. The second type of stroke is thrombotic, which happens when a blood clot develops in a brain blood vessel.8
How HCV increases the risk of an ischemic stroke is unknown. 20 to 30 percent of all instances of ischemic stroke are caused by carotid plaque instability, which is followed by rupture and erosion. In this regard, a solid causal relationship exists between chronic HCV infection and atherosclerosis. 8
The prognosis of a recent stroke may be influenced by patients who have cirrhosis and concomitant conditions. This study sought to identify factors that may predict the outcome of de novo cerebrovascular events in patients with HCV infection.
Patients and methods
A prospective study was conducted in two groups over ten months. Patients were categorized into two cohorts. Cohort I: HCV infected patients presented with newly onset cerebrovascular stroke were included in the study based on the presence of clinical, experimental, and imaging evidence of HCV virology. Cohort II: patients with cerebrovascular stroke without HCV infection. These patients were randomly selected through RCT from patients treated at Menoufia University Hospital’s Emergency Department and the Menoufia University National Liver Institute. Formal approval by the Research Ethics Committee, Menoufia University School of Medicine was received in June 2021 prior to study initiation under approval number (6/2021INTM29). Informed consent forms approved by the ethics committee of Menoufia University School of Medicine were obtained for all studied participants.
Sample size estimation
Based on previous study by Lee et al.9 who reported that the cumulative risk of cerebrovascular deaths was 2.7% for anti-HCV seropositive. Minimum calculated sample size is 29 anti-HCV seropositive patients with cerebrovascular disease (group 1), with std error 1.96, Expected proportion of cumulative risk of cerebrovascular deaths=0.027, Absolute error or precision= 0.05. This study was conducted over 2 groups of patients (HCV Sero-positive and sero-negative), each group included 32 patients after adding drop-out rate (10% of calculated sample size). Thus, a total sample size was 64 patients to get a power of the study 80%.
Randomization technique
142 stroke patients were eligible for the study, 38 of which were HCV positive (26%) and the remaining 104 were negative. 32 participants were randomly assigned following simple randomization procedures (computerized random numbers) from each of the 2 groups to be included in the study.
Patients with Ischemic Stroke were included in the study based on the presence of an acute episode of focal neurologic deficit of new onset lasting for more than 24 hours with no apparent non-vascular cause & no signs of primary intracerebral hemorrhage on brain imaging.
Patients with strokes of causes other than ischemic etiology were excluded from the study. When a blood clot stops or narrows an artery leading to cerebral ischemia, an IS ensues. Blood clots can occur in plaque formation (atherosclerosis), hemorrhagic stroke, systemic vasculitis of any cause, malignancies including Hepatocellular carcinoma (HCC), known bleeding or clotting diathesis except those associated with cirrhosis, transient ischemic attacks, traumatic brain injury. Causes of metabolic coma such as lesions, meningitis, and hypoglycemia, hyperglycemia.
All patients had an adequate and detailed medical history, complete neuropsychiatric examination, and presence of neurological deficits, with emphasis on size, liver and spleen consistency, presence of ascites, dilated veins, etc. A local abdominal examination was performed.
The lab includes random blood glucose levels, liver function tests (LFTs), renal function tests (RFTs), serum electrolytes, lipid profiles, viral markers, and complete blood count (CBC).
All patients underwent electrocardiography, and abdominal ultrasonography and all stroke patients underwent brain CT on admission. Brain MRI was repeated 24 hours later for all stroke patients. The same radiologist reported using brain imaging to diagnose ischemic stroke, determine its location and size, and rule out other causes of stroke. Two-dimensional transthoracic echocardiography. An echocardiogram was also performed to look for valve warts, intramural thrombi, or wall motor dysfunction as precipitators for the development of IS.10 Color Duplex Doppler Ultrasound of Carotid and Spine-Basilar System and comment on complex of the intimal and medial thickness (more than 0.8 mm thickness indicates atherosclerosis), Sub-intimallucency (lost in patients with atherosclerosis), degree of carotid artery stenosis (more than 90 percent is significant carotid artery stenosis) and atherosclerotic plaque (soft or calcified).
Child-Pugh scoring and grading were used in all patients studied in the cirrhosis group to detect liver disease severity. The subtype classification of acute ischemic stroke was defined by Trial of Org 10172 in Acute Stroke Treatment TOAST.11 Stroke patients were assessed on admission using the Menoufia University Hospital Stroke Sheet. This was based on (patient identification, logistic parameters, risk factors, previous stroke history, assessment of cardiac status, type of stroke, MRS,11 NIHSS12, treatment, imaging I parenchymal, imaging II vascular, new events after discharge.
Statistical analysis
Our data were tabulated and analyzed statistically using MICROSOFT EXCEL 2019 (Microsoft Corporation, Redmond, WA, USA) and Statistical Package for Social Science (SPSS version 25, (IBM Corporations, USA). The qualitative data were represented in number and percentage, while non-parametric quantitative data represented as median. The Chi-square test, Wilcoxon Mann-Whitney, Kruskal Wallis test (K), The Kaplan-Meier curve were used. Values were considered significant when p value <0.05.
Results
A CONSORT flow chart of the study population is shown in Figure 1. Of 75 patients with newly onset cerebrovascular stroke included in this study, they attended to Menoufia University Hospital, 11 patients excluded from our study, 8 of them did not meet the inclusion criteria and 3 declined consent, 64 patients were willing to participate and consented for participation in the study and were analyzed statistically 32 ischemic stroke patients with HCV infection as the group A and remind 32 ischemic stroke patients without HCV infection as the group B.
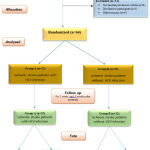 |
Figure 1: Flowchart of the studied patients with Ischemic stroke patients with and without HCV infection |
The study was conducted on 32 HCV-infected patients with newly onset ischemic stroke (cases), and 32 cerebrovascular accident patients without HCV infection (controls). Statistical analysis revealed highly significant esophageal varices in the group that had the virus in comparison to the free group, whereas smoking, DM, and hypertension (HTN) were not significant differences between patient groups. Statistical analysis revealed highly significant MRI with moderate lesions in the group that had the virus compared with the free group. A highly significant increase in serum cholesterol in the stroke without HCV group compared to the virus group. HbA1c significantly increased in the group that had the virus compared to the free group (Table 1).
Table 1: Descriptive comparison of demographic, risk factors and investigations in studied groups.
| Parameters | Cases
(CVS with HCV) (n=32) |
Controls
(CVS without HCV) (n=32) |
P-value | |
| Age (Years) | Mean ± SD | 63.6 ± 10.0 | 62.1 ± 11.4 | 0.572 |
| Gender
|
Male
Female |
18 (54.5%)
14 (45.5%) |
24 (75%)
8 (25%) |
0.085 |
| Smoking | Yes
No |
14 (45.5%)
18 (54.5%) |
17 (53.1%)
15 (46.9%) |
0.536 |
| Diabetes Mellitus
(DM) |
Yes
No |
14 (45.5%)
18 (54.5%) |
17 (53.1%)
15 (46.9%) |
0.097 |
| Hypertension
(HTN) |
Yes
No |
21 (63.6%)
11 (36.4%) |
20 (62.5%)
12 (37.5%) |
0.924 |
| Hb (gm/dl) | Mean ± SD | 11.3 ± 1.8 | 12.6 ± 1.5 | 0.002* |
| TLC (cells/cum) | Mean ± SD | 8.3 ± 3.5 | 290.3 ± 3.1 | 0.182 |
| Platelets (cells/cum) | Mean ± SD | 177.5 ± 129.5 | 190.5 ± 116.5 | <0.0001* |
| ALT (U/L) | Mean ± SD | 44.7 ± 19.9 | 39.3 ± 11.6 | 0.171 |
| AST (U/L) | Mean ± SD | 56.3 ± 36.9 | 45.0 ± 10.9 | 0.101 |
| Urea (mg/dl) | Mean ± SD | 64.3 ± 32.7 | 71.2 ± 24.2 | 0.340 |
| Creatinine (mg/dl) | Mean ± SD | 1.3 ± 0.7 | 1.2 ± 0.5 | 0.560 |
| Total bilirubin(mg/dl) | Mean ± SD | 1.8 ± 0.9 | 0.9 ± 0.2 | <0.0001* |
| Direct bilirubin(mg/dl) | Mean ± SD | 0.6 ± 0.4 | 0.3 ± 0.1 | <0.0001* |
| INR | Mean ± SD | 1.4 ± 0.3 | 1.1 ± 0.2 | <0.0001* |
| Serum Albumin(gm/dl) | Mean ± SD | 2.6 ± 0.4 | 3.3 ± 0.3 | <0.0001* |
| Na (mmol/l) | Mean ± SD | 133.6 ± 8.4 | 140.4 ± 4.7 | <0.0001* |
| K (mmol/l) | Mean ± SD | 4.0 ± 0.7 | 3.9 ± 0.6 | 0.465 |
| HDL (mg/dl) | Mean ± SD | 34.1 ± 10.8 | 32.4 ± 9.1 | 0.491 |
| LDL (mg/dl) | Mean ± SD | 115.3 ± 52.1 | 142.7 ± 65.5 | 0.066 |
| Triglyceride (mg/dl) | Mean ± SD | 129.7 ± 75.9 | 163.0 ± 59.3 | 0.054 |
| Serum cholesterol (mg/dl) | Mean ± SD | 177.5 ± 52.0 | 202.8 ± 48.1 | 0.046* |
| HbA1c | Mean ± SD | 6.4 ± 1.7 | 4.3 ± 1.0 | 0.007* |
Chi2 square test; Student’s t test; Mann–Whitney U test; Student’s t test
Hb: Hemoglobin, TLC: Total leucocytic count, INR: international normalized ratio, ALT : Alanine transferase, AST : Aspartate transferase, HDL: High Dense Lipoprotein, LDL: Low Dense Lipoprotein
Statistical analysis revealed that highly significant Carotid intimal thickness by duplex in Affection in prognosis in HCV free patients. Inclusion in prognosis after a three-month follow-up with MRS yields significant results for NIHSS at admission correlations. Statistical analysis showed that patients with a normal pulse had a favorable prognosis, as significant pulse abnormalities such as atrial fibrillation with emotional instability were found in the virus-free group. There was a significant association influencing the prognosis of diabetic patients in the group that had the virus, with unfavorable three-month MRS compared with nondiabetic patients. High HDL levels in the virus group contributed to a favorable prognosis at MRS follow-up. Among patients in the virus group, there was a highly significant association between DM and stroke prognosis, with uncontrolled DM in the virus group due to peripheral insulin resistance and glycogen deficiency in patients with cirrhosis being associated with the group that had the virus & stroke contributes to this poor prognosis in patients (Table 2).
Table 2: Stroke scales of the studied group.
| Parameters | Cases
(CVS with HCV ) (n=32) |
Controls
(CVS without HCV ) (n=32) |
P value | |
| NIHSS | Mean ± SD | 10.4 ± 3.8 | 10.3 ± 5.6 | 0.945 |
| Degree of stroke according to NIHSS | Minor
Moderate Moderate to severe Severe |
5 (15.2%)
25 (78.8%) 2 (6.1%) 0 |
5 (15.6%)
24 (75.0%) 2 (6.3%) 1 (3.1%) |
0.786 |
| MRS
|
Mean ± SD | 2.8 ± 1.3 | 2.8 ± 1.3 | 0.983 |
| MRS categories | 0
1 2 3 4 5 |
1 (3.0%)
6 (18.2%) 4 (12.1%) 11 (36.4%) 8 (24.2%) 2 (6.1%) |
0
7 (21.9%) 6 (18.8%) 9 (28.1%) 7 (21.9%) 3 (9.4%) |
0.827 |
| MRS after 3 months | Mean ±SD | 1.6 ± 1.2 | 2.5 ± 1.8 | 0.025* |
| Prognosis according to MRS after 3 months | Favorable
(MRS 0-2) Unfavorable (MRS 3-6) |
18 (56.2%)
14 (43.8%) |
28 (87.5%)
4 (12.5%) |
0.011* |
National Institutes of Health Stroke Scale (NIHSS)
Modified Ranken Score (MRS scale)
Stroke scales in patients with MRS scores were recognizable at 3 months in the virus group, with a worse prognosis than in the virus-free group and higher prevalence in patients with cirrhosis (Table 3).
Table 3: Correlation between Demographic, Risk factors, and Investigation with prognosis in studied groups
|
Parameters |
Cases
(n=32) |
Controls
(n=32) |
|||||
| Favorable
(MRS 0-2) |
Unfavorable
(MRS 3-6) |
P value | Favorable
(MRS 0-2) |
Unfavorable
(MRS 3-6) |
P value | ||
| Age (years) | Mean ± SD | 60.3 ± 9.1 | 68.1 ± 10.0 | 0.028* | 62.0 ± 11.3 | 63.0 ± 13.6 | 0.873 |
| Sex | Men
Women |
10 (55.6%)
8 (44.4%) |
8 (57.1%)
6 (42.9%) |
0.928 | 22 (78.6%)
6 (21.4%) |
2 (50%)
2 (50%) |
0.217 |
| NIHSS at admission | Mean ± SD | 9.0 ± 3.4 | 12.0 ± 3.8 | 0.025* | 9.2 ± 4.0 | 18.3 ± 9.4 |
0.001* |
| Infarction size by CT | Small
Moderate |
11 (61.1%)
7 (38.9%) |
8 (57.1%)
6 (42.9%) |
0.821 | 23 (82.1%)
5 (17.9%) |
2 (50%)
2 (50%) |
0.146 |
| Infarction size by MRI | Mild
Moderate |
9 (50%)
9 (50%) |
8 (57.1%)
6 (42.9%) |
0.688 | 23 (82.1%)
5 (17.9%) |
2 (50%)
2 (50%) |
0.146 |
| Carotid intimal thickness by duplex | Normal
mild moderate severe |
2 (6.25%)
10 (31.2%) 6 (18.7%) 0 |
5 (35.7%)
7 (50%) 1 (7.1%) 1 (7.1%) |
0.025* | 16 (57.1%)
9 (32.1%) 2 (7.1%) 1 (3.6%) |
1 (25%)
0 3 (75%) 0 |
0.821 |
| HDL (mg/dl) | Mean ± SD | 37.2 ± 9.0 | 29.5 ± 11.7 | 0.043* | 32.4 ± 9.2 | 32.3 ± 9.0 | 0.971 |
| LDL (mg/dl) | Mean ± SD | 127.9 ± 52.8 | 103.1 ± 49.2 | 0.185 | 142.3 ± 67.8 | 145.5 ± 54.0 | 0.928 |
| Triglyceride
(mg/dl) |
Mean ± SD | 130.7 ± 58.2 | 131.6 ± 98.0 | 0.972 | 160.2 ± 59.8 | 182.8 ± 59.2 | 0.485 |
| Serum cholesterol
(mg/dl) |
Mean ± SD | 174.5 ± 58.1 | 183.9 ± 45.8 | 0.625 | 205.0 ± 49.6 | 187.5 ± 37.7 | 0.506 |
| Diabetes Mellitus (DM) | Yes
No |
4 (25%)
12 (75%) |
10 (62.5%)
6 (37.5%) |
0.032* | 12 (63.1%)
7 (36.8%) |
5 (38.4%)
8 (61.5%) |
0.169 |
| Hypertension | Yes
No |
12 (66.7%)
6 (33.3%) |
9 (64.1%)
5 (35.7%) |
0.888 | 18 (64.3%)
10 (35.7%) |
2 (50%)
2 (50%) |
0.581 |
| Pulse \ min | Normal
AF |
13 (72.2%)
5 (27.8%) |
11 (78.6%)
3 (21.4%) |
0.681 | 24 (85.7%)
4 (14.3%) |
1 (25%)
3 (75%) |
0.006* |
AF : Atrial Fibrillation; NIHSS : National Institutes of Health Stroke Scale; MRS scale : Modified Ranken Score; CT : computerized tomography; MRI : Magnetic resonance imaging; HDL : High Dense Lipoprotein; LDL : Low Dense Lipoprotein
The fate of patients in the study after three months with more bad prognosis and affection of HCV positive patients more than HCV free patients. A non-significant result, even though there was a higher mortality rate in patients with the virus than in patients without virus. At the three-month follow-up, we found that there were five deaths in the virus group versus only one death in the virus-free group, so there was significant survival advantage between the two groups. Therefore, compared with the virus-free group, the survival prognosis of the group that had the virus was not favorable (Table 4-5). Fate of patients in study after 3 months was with more bad prognosis and affection in stroke with HCV infected patients more than stroke without HCV patients. Causes of death in HCV patients were (2 cases died with pneumonia, 2 cases died with attacks of hematemesis and melena and one case died on hepatic coma. The HCV negative case died with chest infection on aspiration pneumonia, (Table 4). There was insignificant affection of survival rates between the studied group although there was increased number of died cases in HCV positive group, (Table 5). In this context, there was unfavorable prognosis in stroke with HCV positive group in survival in relation to the stroke without HCV group, (Figure 2).
Table 4: Fate of the studied group after 3 months
| Fate | Cases
CVS with HCV (N= 32) |
Controls
CVS without HCV (N= 32) |
*P-value |
| Alive
Died |
27 (84.4%)
5 (15.6%) |
31 (96.9%)
1 (3.1%) |
0.086 |
*Fate of patients was statistically analyzed among cases and control using Chi-square test.
Table 5: The overall survival of the studied group in relation to the virology status (N=64).
| Mean | Median | Log rank | P value | |||
| Value | 95%CI | value | 95%CI | |||
| Positive | 4 | 4 – 4 | 4 | —- | 0.004 | 0.953 |
| Negative | 3.4 | 1.6 – 5.2 | 3 | 0.85 – 5.15 | ||
| Overall | 3.5 | 2 – 3 | 3 | 0.6 – 5.4 | ||
* The overall survival of the studied group was statistically analyzed using Mann-Whitney U test.
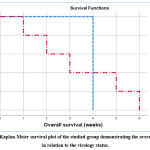 |
Figure 2: Kaplan-Meier survival plot of the studied group demonstrating the overall survival in relation to the virology status. |
Discussion
HCV infection is a global problem affecting approximately one hundred eighty-five million people, with the highest reported prevalence in Egypt.14 Cerebrovascular disease is a major health burden in most developed countries. One of the extrahepatic symptoms of HCV infection is IS. 15
This study showed that stroke patients with significant cirrhotic characteristics also have positive chronic HCV antibodies. Liao and her colleagues demonstrated that the cumulative risk of stroke was noticeably increased in her HCV-positive participants in a sizable population-based prospective cohort.16 Similar findings were made by a community-based prospective cohort study, which discovered that chronic HCV infection with higher serum HCV RNA levels is not only a predictor of stroke severity but also a risk factor for stroke-related death.15
According to Adinolfi et al., stroke patients had a significantly higher prevalence of HCV infection than the general population. This finding, along with others, adds to the mystery surrounding the relationship between HCV and stroke (high prevalence, incidence, rates, and mortality), strongly indicating a strict association. Chronic HCV infection and stroke-related symptoms. 17
In contrast to HCV-negative stroke patients, chronic HCV antibody-positive stroke patients had significantly lower serum triglyceride levels and marginally lower serum cholesterol levels. Serfaty, 18 noted in 2000 that HCV patients’ serum cholesterol levels were considerably lower than those of controls. Another investigation revealed that patients with HCV infection had more frequently occurring fatty liver and age-matched hypocholesterolemia. 19 Additionally, Adinolfi and associates, 20 and stroke patients with chronic heart diseases (CHD) had reduced cholesterol levels.
Since serum cholesterol levels are negatively correlated with the severity of fatty liver, this metabolic condition may be caused by a shared route. 21 According to a Japanese study, compared to HBV infection, HCV genotype one b infection caused higher levels of hypocholesterolemia and lower levels of abetalipoproteinemia. The low-density lipoprotein receptor has been hypothesized as a recognition receptor for HCV entrance into hepatocytes, even though the mechanism of this interaction is unknown. 22
Our study’s findings that HCV positive stroke patients had considerably lower serum cholesterol may help to explain why carotid artery atherosclerosis is more common in this population. We concur with Adinolfi and colleagues’ findings, which showed that in chronic hepatitis cirrhotic (CHC) patients; greater serum triglyceride levels were substantially related to carotid atherosclerosis but not cholesterol. 20
According to this study’s findings on mean serum cholesterol levels, there was a highly significant rise in the stroke group compared to the HCV group. These outcomes were in line with those reported by Ali et al. 24, who found no connection between cholesterol and the risk of stroke. 23, 24
Their distinct associations with ischemic stroke subtypes help to explain some of the debate surrounding the connection between lipids and stroke risk. Tirschwell et al.26, Imamura et al.27, Choi et al.28 reported a strong association between large artery atherosclerotic subtypes and elevated cholesterol levels from ischemic stroke. Tirschwell et al.26, Amarenko et al.29 demonstrated Dyslipidemia is also a recognized risk factor for coronary artery disease.
These findings in the same line with Cicognani et al.30, who found that cholesterol and its fractions decreased gradually with the severity of the liver disease.
One of the most significant results of the current investigation was a somewhat higher prevalence of carotid artery atherosclerosis (CA) in stroke patients with HCV antibody positivity compared to stroke patients without HCV antibody positivity. These findings are in line with those of earlier research, the largest of which revealed a prevalence of atherosclerosis despite younger ages and better cardiovascular risk profiles among HCV-infected people. The rate was revealed to be considerably higher. The significantly lower serum cholesterol among HCV positive stroke patients, found in our study, could explain the increased prevalence of carotid atherosclerosis in this group.
Also in our study Carotid Intimal Thickness was insignificant result in comparison between the two studied groups as imaging routine in stroke survey, but it was significant as a predictor in prognosis of stroke in HCV negative patients as we found unfavorable prognosis according to MRS score in moderate IMT, there was more unfavorable prognosis.
Adinolfi and colleagues, 20 reported a significantly higher prevalence of CA in CHC patients compared with matched controls. Furthermore, among young HCV-positive patients (e.g., less than fifty years), approximately thirty-four percent showed CA compared with controls (sixteen percent) and a significant proportion (twenty-four percent) had plaques, a rare event. The control group (three-point nine percent). Their data suggest that despite a more favorable cardiovascular risk profile with lower lipid levels, lower prevalence of metabolic syndrome, and possibly lower prevalence of hypertension, chronic HCV infection is associated with CA and progressive disease. supported the view that it predisposes to the development of lesions. 25
Our understanding of the pathogenic processes connecting HCV infection to early and severe atherosclerosis has improved due to several prior discoveries. Researchers have discovered an independent relationship between serum HCV RNA levels and both the early stages of CA, particularly IMT damage, and the advanced stages of plaque. 23
HCV RNA sequences were discovered in carotid plaques in 2000, indicating that the CA is where HCV replicates.22 Collectively, these results suggest that HCV has a direct proatherogenic effect, which may speed up the aging process of the artery wall. Unaffected by HCV genotype, age, sex, or the severity of histologic liver damage, evidence suggests that HCV patients with steatosis had the highest prevalence of atherosclerosis (77.7%). A separate risk factor for atherosclerosis was HCV-associated steatosis. Effective specificity and sensitivity make HCV-associated steatosis an excellent predictor of atherosclerosis. 21
According to Berzigotti et al. 31 and Chen et al. 32, patients with cirrhosis had a lower risk of having an ischemic stroke if they have coagulopathy, thrombocytopenia, or abnormal hemodynamic drift patterns. Although ischemic stroke became considerably less likely in all cirrhotic patients, the liver-related mortality and morbidity were significantly better compared to ischemic stroke in non-cirrhotic patients, according to Solyimanni et al.33 and Goyal et al. 34 which come agreed with our results. Contrarily, Parikh et al.35 and Wu et al.36 found that patients with cirrhosis had a higher risk of having an ischemic stroke as well as higher rates of post-stroke epilepsy, intensive care unit (ICU) admission, and bleeding problems (among those who received antiplatelets and anticoagulants for these conditions). The severity of liver cirrhosis, gastrointestinal bleeding, and HCC were all linked to the full-size thirty days put up stroke mortality in those patients. Additionally, Sogaard et al.37 reported a tenfold increased risk of in-hospital mortality in cirrhotic patients with venous thromboembolism. Additionally, Zhang et al.38 reported that the in-hospital mortality in ischemic stroke cirrhotic patients became significantly elevated due to multiple organ failure and gastrointestinal bleeding; the patients in the latter study were significantly older and had systemic hypertension to a significant degree, that are agreed with our results.
In our research, we discovered portal vein thrombosis (PVT) in patients with HCV infection. The development of PVT, a frequent complication in individuals with cirrhosis from HCV, can have a negative impact on prognosis, particularly if liver function is impaired and portal hypertension emerges. 39
For the onset of stroke and portal vein thrombosis in cirrhosis, there seem to be several shared pathophysiological pathways or risk factors. First off, both are characterized by decreased protein C (PC) and elevated factor eight (FVIII) and are connected to hypercoagulability in cirrhosis. 40, 41 Second, portal hypertension is linked to both stroke and portal vein thrombosis. Common characteristics of portal hypertension that aggravate the onset of portal vein thrombosis include portal vein depression and portal-systemic collateral shunt. 42
Important clinical symptoms of portal hypertension leading to hypovolemia and organ hypovolemia leading to ischemic stroke include ascites and esophageal variceal haemorrhage.43 Third, compared to people with normal blood sugar levels, diabetics have a two- to four-fold increased risk of having an ischemic stroke. 44 As of late, diabetes has emerged as a separate risk factor for portal vein thrombosis. 45 Fourth, ischemic stroke and portal vein thrombosis risk may be elevated by the antiphospholipid syndrome. Significant stroke risk factors include antiphospholipid syndrome, Irregular thickening of the valve leaflets secondary to antiphospholipid syndrome and lupus anticoagulants are significant risk factor of stroke.
The HCV group had a significantly higher rate of strokes compared to the HCV group, according to this study’s analysis of mean HbA1C levels. The significant insulin resistance of HCV-positive patients served as the basis for this conclusion. It can be explained that a glucose metabolism deficit is brought on by HCV infection. According to earlier research, the prevalence of diabetes in HCV patients is roughly equal to the age-standardized prevalence (9.7%) of diabetes, while the prevalence of prediabetes in HCV patients is higher than the age-standardized prevalence (15.5%). 46
In chronic HCV-infected patients, increased insulin resistance is a key factor in the emergence of type 2 diabetes. In fact, moderate fibrosis patients with chronic HCV infection already have insulin resistance, which is far more common in infected people than in uninfected people. Additionally, the degree of fibrosis and portal vein inflammation are positively connected with its existence. 47 According to the history and onset of diabetes involvement in our study, there were no significant differences between the two groups studied; however, HbA1c results in HCV-infected patients with uncontrolled DM were significant, even more so in HCV-infected patients with insulin resistance and metabolic syndrome linked to HCV-infected cirrhosis. 46
According to the results of the current investigation, stroke patients without HCV had significantly higher mean systolic and diastolic blood pressure values than HCV-positive patients. Vascular resistance is promoted by both the direct and indirect effects of chronic hepatitis C infection. HCV infection, on the other hand, results in consequences including cirrhosis and cardiomyopathy and is characterized by a hyperactive state with decreased peripheral vascular resistance. 43
Compared to patients in the control group, patients in the HCV positive group had significantly lower mean systolic and diastolic blood pressure readings, which suggests that there may have been a considerable rise in Child’s grade C compared to control group. This outcome supported the idea that advanced cirrhosis causes progressive visceral and systemic vasodilation, which is mediated by nitric oxide and other vasodilators. As a result, the mean arterial pressure is decreased, and the effective central blood volume is decreased. Our findings agreed with those of Simons et al.48 regarding the significant relation between hypertension & stroke risk but disagreed with Simons et al. regarding other significant risk factors (older age, male sex, and dyslipidemia). 48 Additionally, silent hypertension was listed as a substantial risk factor for ischemic stroke by Hasan et al.49 However, our findings diverged from those of Willy et al.50, who found that hypertension does not increase the chance of having a stroke.
In our study, patients with cirrhosis decompensation had critical characteristics that were entirely different from those in the HCV-negative control group, including skin characteristics, clinical examination, abdominal ultrasonography, and abdominal examination. Additionally, there were notable differences in thrombocytopenia and anemia between the two groups, which may have an impact on the outcome of a stroke. We found that size of lesion in MRI was significant as size of lesions were moderate in number of HCV positive patients more than HCV free group that may be significantly higher association with CA and less severe stroke than those with negative HCV antibodies. We found that MRI was more significant in detection of lesions more than CT brain due to high sensitivity of MRI to stroke lesion.51 In our research, we discovered a highly substantial correlation between stroke patients’ NIHSS at admission and MRS at the conclusion of a three-month follow-up. A 3-month MRS follow-up analysis of both groups revealed notable findings, including a better prognosis for the HCV-negative group than the HCV-positive group.
Conclusion
This study indicates a strong connection between chronic HCV infection and severity of ischemic stroke and a bad prognosis result from it. No significant correlation between the severity of HCV cirrhosis and the severity of Ischemic Stroke. However, the presence of cirrhosis exposed patients with acute Ischemic Stroke to a prolonged hospital stay, higher morbidity & mortality compared to HCV negative patients.
Risk factors of stroke that may contribute prognosis of Ischemic Stroke in HCV patients were significant like HbA1c and uncontrolled diabetes, hyperlipidemia with HCV patients, carotid intimal thickness affection and carotid atherosclerosis in HCV patients, portal vein thrombosis and coagulopathy state in HCV affected patients, all these risk factors were significant in HCV patients and so affect prognosis of newly onset Ischemic stroke in HCV positive patients.
Prognosis of stroke in HCV positive patient is more unfavorable than in HCV negative groups, and mortality rate among HCV patients with newly onset Ischemic Stroke is higher than negative group due to more comorbidities and bad general condition of HCV cirrhotic patients.
Conflict of Interest
There is no conflict of interst.
References
- Sacco, R.L., S.E. Kasner, J.P. Broderick, L.R. Caplan, J. Connors, A. Culebras, et al., An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2013. 44(7): p. 2064-2089.
CrossRef - Counsell, C. and P. Sandercock, Anticoagulants for acute ischaemic stroke. Cochrane Database of Systematic Reviews. Issue, 1999. 2.
- Lisman, T., J. Adelmeijer, P. De Groot, H. Janssen, and F. Leebeek, No evidence for an intrinsic platelet defect in patients with liver cirrhosis–studies under flow conditions. Journal of Thrombosis and Haemostasis, 2006. 4(9): p. 2070-2072.
CrossRef - Hou, M.C., H.C. Lin, T.T. Liu, B.I.T. Kuo, F.Y. Lee, F.Y. Chang, and S.D. Lee, Antibiotic prophylaxis after endoscopic therapy prevents rebleeding in acute variceal hemorrhage: a randomized trial. Hepatology, 2004. 39(3): p. 746-753.
CrossRef - Lisman, T., F.W. Leebeek, and P.G. de Groot, Haemostatic abnormalities in patients with liver disease. Journal of hepatology, 2002. 37(2): p. 280-287.
CrossRef - Forner, A., M. Reig, and J. Bruix, Hepatocellular carcinoma. Lancet, 2018. 391(10127): p. 1301-1314.
CrossRef - Mohammed, H.I., K.H. Afifi, R.S. El Zaiat, Y.A. Alghalban, and A.A. Sakr, Study of von Willebrand factor as a risk for thrombotic cerebrovascular stroke in cirrhotic patients. Menoufia Medical Journal, 2021. 34(3): p. 839.
- Sharara, A.I. and D.C. Rockey, Gastroesophageal variceal hemorrhage. New England Journal of Medicine, 2001. 345(9): p. 669-681.
CrossRef - Lee MH, Yang HI, Wang CH, Jen CL, Yeh SH, Liu CJ, You SL, Chen WJ, Chen CJ. Hepatitis C virus infection and increased risk of cerebrovascular disease. Stroke. 2010 Dec 1;41(12):2894-900.
CrossRef - Lang, R.M., M. Bierig, R.B. Devereux, F.A. Flachskampf, E. Foster, P.A. Pellikka, et al., Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr, 2005. 18(12): p. 1440-63.
CrossRef - Yilmaz, V.T., D. Dincer, A.B. Avci, and R. Cetinkaya, Significant association between serum levels of von Willebrand factor (vWF) antigen with stages of cirrhosis. The Eurasian Journal of Medicine, 2015. 47(1): p. 21.
CrossRef - Warun Kumar, M. and E. Harisha, Assessment of lipid profile changes with respect to severity of liver dysfunction in cirrhosis of liver. Indian J Basic Appl Med Res.(internet), 2015. 4: p. 56-63.
- Nangliya, V., A. Sharma, D. Yadav, S. Sunder, S. Nijhawan, and S. Mishra, Study of trace elements in liver cirrhosis patients and their role in prognosis of disease. Biol Trace Elem Res, 2015. 165(1): p. 35-40.
CrossRef - Vivas, S., M. Rodriguez, M.A. Palacio, A. Linares, J.L. Alonso, and L. Rodrigo, Presence of bacterial infection in bleeding cirrhotic patients is independently associated with early mortality and failure to control bleeding. Digestive diseases and sciences, 2001. 46(12): p. 2752-2757.
CrossRef - Powers, W.J., C.P. Derdeyn, J. Biller, C.S. Coffey, B.L. Hoh, E.C. Jauch, et al., 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2015. 46(10): p. 3020-3035.
CrossRef - Zhang, X., X. Qi, E.M. Yoshida, N. Méndez-Sánchez, F. Hou, H. Deng, et al., Ischemic stroke in liver cirrhosis: epidemiology, risk factors, and in-hospital outcomes. European Journal of Gastroenterology & Hepatology, 2018. 30(2): p. 233-240.
CrossRef - Xian, Y., J.J. Federspiel, M. Grau-Sepulveda, A.F. Hernandez, L.H. Schwamm, D.L. Bhatt, et al., Risks and benefits associated with prestroke antiplatelet therapy among patients with acute ischemic stroke treated with intravenous tissue plasminogen activator. JAMA neurology, 2016. 73(1): p. 50-59.
CrossRef - Serfaty, L., T. Andreani, P. Giral, N. Carbonell, O. Chazouillères, and R. Poupon, Hepatitis C virus induced hypobetalipoproteinemia: a possible mechanism for steatosis in chronic hepatitis C. Journal of hepatology, 2001. 34(3): p. 428-434.
CrossRef - Saver, J.L. and H. Altman, Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke, 2012. 43(6): p. 1537-1541.
CrossRef - Adinolfi, L.E., R. Nevola, G. Lus, L. Restivo, B. Guerrera, C. Romano, et al., Chronic hepatitis C virus infection and neurological and psychiatric disorders: an overview. World Journal of Gastroenterology: WJG, 2015. 21(8): p. 2269.
CrossRef - Kurth, T., B.M. Everett, J.E. Buring, C.S. Kase, P.M. Ridker, and J.M. Gaziano, Lipid levels and the risk of ischemic stroke in women. Neurology, 2007. 68(8): p. 556-562.
CrossRef - Tziomalos, K., V.G. Athyros, A. Karagiannis, and D.P. Mikhailidis, Dyslipidemia as a risk factor for ischemic stroke. Curr Top Med Chem, 2009. 9(14): p. 1291-7.
CrossRef - Bots, M., P.C. Elwood, Y. Nikitin, J. Salonen, A.F. de Concalves, D. Inzitari, et al., Total and HDL cholesterol and risk of stroke. EUROSTROKE: a collaborative study among research centres in Europe. Journal of Epidemiology & Community Health, 2002. 56(suppl 1): p. i19-i24.
CrossRef - Castilla-Guerra, L., M. del Carmen Fernández-Moreno, and J. Álvarez-Suero, Secondary stroke prevention in the elderly: new evidence in hypertension and hyperlipidemia. European journal of internal medicine, 2009. 20(6): p. 586-590.
CrossRef - Ali, I., M. Abuissa, A. Alawneh, O. Subeh, A. Abu Sneineh, S. Mousa, et al., The prevalence of dyslipidemia and hyperglycemia among stroke patients: preliminary findings. Stroke research and treatment, 2019. 2019.
CrossRef - Tirschwell, D.L., N.L. Smith, S.R. Heckbert, R.N. Lemaitre, W.T. Longstreth, Jr., and B.M. Psaty, Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology, 2004. 63(10): p. 1868-75.
CrossRef - Imamura, T., Y. Doi, H. Arima, K. Yonemoto, J. Hata, M. Kubo, et al., LDL cholesterol and the development of stroke subtypes and coronary heart disease in a general Japanese population: the Hisayama study. Stroke, 2009. 40(2): p. 382-8.
CrossRef - Cui, R., H. Iso, K. Yamagishi, I. Saito, Y. Kokubo, M. Inoue, and S. Tsugane, High serum total cholesterol levels is a risk factor of ischemic stroke for general Japanese population: the JPHC study. Atherosclerosis, 2012. 221(2): p. 565-9.
CrossRef - Amarenco, P., J. Labreuche, and P.J. Touboul, High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis, 2008. 196(2): p. 489-96.
CrossRef - Cicognani, C., M. Malavolti, A.M. Morselli-Labate, L. Zamboni, C. Sama, and L. Barbara, Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med, 1997. 157(7): p. 792-6.
CrossRef - Berzigotti, A., A. Bonfiglioli, A. Muscari, G. Bianchi, S. LiBassi, M. Bernardi, and M. Zoli, Reduced prevalence of ischemic events and abnormal supraortic flow patterns in patients with liver cirrhosis. Liver International, 2005. 25(2): p. 331-336.
CrossRef - Chen, C.Y., K.T. Lee, C.T. Lee, W.T. Lai, and Y.B. Huang, Effectiveness and safety of antiplatelet therapy in stroke recurrence prevention in patients with liver cirrhosis: a 2-year follow-up study. Pharmacoepidemiol Drug Saf, 2012. 21(12): p. 1334-43.
CrossRef - Solaymani-Dodaran, M., T.R. Card, G.P. Aithal, and J. West, Fracture risk in people with primary biliary cirrhosis: a population-based cohort study. Gastroenterology, 2006. 131(6): p. 1752-7.
CrossRef - Goyal, A., K. Chatterjee, N. Shah, and S. Singh, Is cirrhosis associated with lower odds of ischemic stroke: A nationwide analysis? World J Hepatol, 2016. 8(35): p. 1564-1568.
CrossRef - Parikh, N.S., A.E. Merkler, A. Jesudian, and H. Kamel, Association between cirrhosis and aneurysmal subarachnoid hemorrhage. Ann Clin Transl Neurol, 2019. 6(1): p. 27-32.
CrossRef - Wu, H.Y., C.S. Lin, C.C. Yeh, C.J. Hu, C.C. Shih, Y.G. Cherng, et al., Cirrhosis patients’ stroke risks and adverse outcomes: Two nationwide studies. Atherosclerosis, 2017. 263: p. 29-35.
CrossRef - Søgaard, K.K., E. Horváth-Puhó, H. Grønbaek, P. Jepsen, H. Vilstrup, and H.T. Sørensen, Risk of venous thromboembolism in patients with liver disease: a nationwide population-based case-control study. The American journal of gastroenterology, 2009. 104(1): p. 96-101.
CrossRef - Zhang, Y., J. Tuomilehto, P. Jousilahti, Y. Wang, R. Antikainen, and G. Hu, Total and high-density lipoprotein cholesterol and stroke risk. Stroke, 2012. 43(7): p. 1768-74.
CrossRef - Mandorfer, M., K. Kozbial, P. Schwabl, C. Freissmuth, R. Schwarzer, R. Stern, et al., Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J Hepatol, 2016. 65(4): p. 692-699.
CrossRef - Tripodi, A. and P.M. Mannucci, The coagulopathy of chronic liver disease. New England Journal of Medicine, 2011. 365(2): p. 147-156.
CrossRef - Tripodi, A., Q. Anstee, K. Sogaard, M. Primignani, and D. Valla, Hypercoagulability in cirrhosis: causes and consequences 1. Journal of thrombosis and haemostasis, 2011. 9(9): p. 1713-1723.
CrossRef - Maruyama, H., H. Okugawa, M. Takahashi, and O. Yokosuka, De novoportal vein thrombosis in virus-related cirrhosis: predictive factors and long-term outcomes. Official journal of the American College of Gastroenterology| ACG, 2013. 108(4): p. 568-574.
CrossRef - Garcia-Tsao, G., S. Friedman, J. Iredale, and M. Pinzani, Now there are many (stages) where before there was one: In search of a pathophysiological classification of cirrhosis. Hepatology, 2010. 51(4): p. 1445-9.
CrossRef - Cui, R., H. Iso, K. Yamagishi, I. Saito, Y. Kokubo, M. Inoue, and S. Tsugane, Diabetes mellitus and risk of stroke and its subtypes among Japanese: the Japan public health center study. Stroke, 2011. 42(9): p. 2611-2614.
CrossRef - Eshraghian, A., S. Nikeghbalian, K. Kazemi, M. Mansoorian, A. Shamsaeefar, H. Eghlimi, et al., Portal vein thrombosis in patients with liver cirrhosis and its impact on early and long‐term outcomes after liver transplantation. International Journal of Clinical Practice, 2019. 73(3): p. e13309.
CrossRef - Yuan, M., J. Zhou, L. Du, L. Yan, and H. Tang, Hepatitis C virus clearance with glucose improvement and factors affecting the glucose control in chronic hepatitis C patients. Scientific reports, 2020. 10(1): p. 1-10.
CrossRef - Drazilova, S., J. Gazda, M. Janicko, and P. Jarcuska, Chronic hepatitis C association with diabetes mellitus and cardiovascular risk in the era of DAA therapy. Canadian Journal of Gastroenterology and Hepatology, 2018. 2018.
CrossRef - Simons, L.A., J. McCallum, Y. Friedlander, and J. Simons, Risk factors for ischemic stroke: Dubbo Study of the elderly. Stroke, 1998. 29(7): p. 1341-6.
CrossRef - Hasan, Z.N., M.Q. Hussein, and G.F. Haji, Hypertension as a risk factor: is it different in ischemic stroke and acute myocardial infarction comparative cross-sectional study? Int J Hypertens, 2011. 2011: p. 701029.
CrossRef - Willey, J.Z., Y.P. Moon, E. Kahn, C.J. Rodriguez, T. Rundek, K. Cheung, et al., Population attributable risks of hypertension and diabetes for cardiovascular disease and stroke in the northern Manhattan study. J Am Heart Assoc, 2014. 3(5): p. e001106.
CrossRef - Kernan, W.N., B. Ovbiagele, H.R. Black, D.M. Bravata, M.I. Chimowitz, M.D. Ezekowitz, et al., Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke, 2014. 45(7): p. 2160-2236.
CrossRef







