Deepika Bhatia1 , Yogeeta1
, Yogeeta1 , Pradeep Goyal2
, Pradeep Goyal2  and Atul Kabra1*
and Atul Kabra1*
1University Institute of Pharma Sciences, Chandigarh University, Mohali, Punjab, 140413, India
2Saraswati institute of pharmacy, Mohali, Punjab, 140413, India
Corresponding Author E-mail: Bhatia.deepika89@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2595
Abstract
In the human body, the largest gland is the liver and does a lot of essential work of the body. Liver damage is the cause of mortality and increasing day by day. Liver disease is caused by multiple factors, such as an autoimmune condition, toxic chemical exposure, viral infection, and dietary factors. Drug-induced liver injury (DILI) is a critical issue in drug development because DILI causes failures in clinical trials and the withdrawal of approved drugs from the market and leading to pathological changes result, including increase in SGOT, SGPT and bilirubin as well as the free radical generation. In this review, contains the animal model of hepatotoxicity with a different cause, their action mech., and procedure with dose. These models include the toxicity caused by chemical, drug, radiation, metal, diet, and high-fat this will lead to pathological changes resulting in hepatotoxicity.
Keywords
Animal Models; hepatotoxicity; liver; Liver Damage
Download this article as:| Copy the following to cite this article: Bhatia D, Yogeeta Y, Goyal P, Kabra A. Development on Animal Models for Drug/Chemical Induced Liver Injury. Biomed Pharmacol J 2023;16(1). |
| Copy the following to cite this URL: Bhatia D, Yogeeta Y, Goyal P, Kabra A. Development on Animal Models for Drug/Chemical Induced Liver Injury. Biomed Pharmacol J 2023;16(1). Available from: https://bit.ly/3Jv0prE |
Introduction
In the human body, the largest gland is the liver and has an important role in our body. From metabolism to the production of minerals, vitamins storage, homeostasis, proteins, storing glucose and fat from the diet, remove RBC(red blood cell) which are damaged or dead through the spleen. The main workofthe liver is to remove the toxins present in the human body while doing the metabolism of toxins; toxins accumulate in life as the accumulation rate is higher than the rate of metabolism. And that cause damage to the liver [1]. The damage of liver causes alteration in the functioning of the liver and causes pathological changes in it. Death of liver cell, increase in the oxidative stress, liver got fatty, accumulation of triglyceride in the form of vacuoles, impairment in bile production, inflammation in the liver, obstruction of small hepatic nerves, increase in the level of CRP(c-reactive protein),ESR (erythrocyte sedimentation rate),PV(Plasma viscosity), cancer of liver further these changes in the liver causes increased pressure into portal vein and liver failure.[2]
Etiology
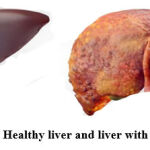 |
Figure 1: Healthy liver and liver with cirrhosis. |
There are many reasons for liver damage. It can be induced by the drug, by chemical, also by herbal or dietary supplements.
Drugs that cause toxicity: Non-steroidal anti-inflammatory Drugs i.e. Peracetamol, anti-cancer drugs, anti-Tuberculosis, antibiotics.
Chemical that causes toxicity: CCl4(Carbontertra chloride, thioacetamide),DEN (diethyl nitrosamine), aflatoxin B1.
Metals that cause toxicity: cadmium, mercury, arsenic.
Toxicity induced by radiations, alcohol, diet, high-fat diet.
Risk factors
Gender, age, concomitant medications, alcohol, nutrition, hepatitis B and C, and genetic factors.[3,4]
Experimental Models for Hepatotoxicity
Animal models are used as they have similarities with the function of the human body and play a major role in the experimentation. These models include the toxicity caused by chemical, drug, radiation, metal, diet, and high-fat food-induced.
Toxicity caused by chemicals
Some chemicals like CCL4, TTA, DEN cause hepatotoxicity. These metabolites leadto some changes in the biological function and physiology of the liver. GSH and neutrophils are found to have a major role in the induction of liver damage by chemicals.
CCl4
Carbon tetrachloride(CCl4) caused toxicity. It is an inorganic compound.
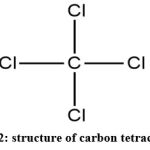 |
Figure 2: structure of carbon tetrachloride |
Mechanism of action
It is a well-known toxicant for liver toxicity. CCl4 after metabolism converts to CCl3 and it is produced by CYPE2E1 in the liver cell. This is a free radical that binds with the cellular proteins and lipids, and trichloromethyl peroxyl radical forms. This causes necrosis of centrilobular tissue present in the hepatic lobule[3]. It is studied in the histopathology that after 2-4 weeks fibrosis occurs significantly and after 5-7 weeks serious bridging fibrosis, and after 8-9 weeks onset of cirrhosis occurs.
Procedure
Rats of any gender are given a regular diet and water at the temperature of 25±2ºC. Animals are left empty stomach, before the experimentation. For causing hepatotoxicity, CCl4 with different doses through a different route and in different periods was administered. On the end day of experimentation animals were sedated, the sample of blood was collected, liver enzyme activity was carried out were killed after that histopathological and biochemical parameters were studied[5,9].
Table 1: Hepatotoxicity caused by CCl4
| Animal used | Dose | Route | references |
| Male Sprague-Dawley rats | 0.5 ml/kg for 3 days | i.p. | [6] |
| SpragueDawley rats (male) | 0.2 ml per 100gm for 2 weeks | i.p. | [7] |
| Wistar (male) rats | 0.5 ml per kg two time in 7 days(for 4 weeks) | i.p. | [8] |
| Wistar(male) rats | 0.125ml per kg for 7 days | i.p. | [9] |
| Albino rats of Wistar strain | 1.0 ml per kg after every 3rd day for a period of 10 days | i.p. | [10] |
|
Wistar(male) rats |
2 ml per kg, on every 3rd day for a period of 10 days | s.c. |
[11] |
TAA
TAA is anorgan ophosphorus solid, white crystalline compound, which is soluble in the water and is a source for sulfide ions in the production of inorganic and organic compounds [12]. It can also be used for causing fibrosis and also it causes injury to zone 3 and 1 hepatocytes[13].
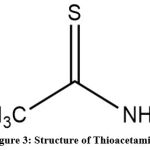 |
Figure 3: Structure of Thioacetamide |
Mechanism of action
It is not harmful to the liver, but thioacetamide-s-oxide, a TAA intermediate metabolite, covalently binds to hepatic macromolecules, altering cell permeability and increasing intracellular Ca2+ concentrations, causing cellular damage and necrosis in both zone 1 and zone 3 hepatocytes. At low doses, TTA induces the formation of cirrhosis and portal central septa or portal-portal septa. When it is compared with the other hepatotoxins, TAA therapy took much time to generate substantial fibrosis, whereas other hepatotoxins increase the chance of test animals dying prematurely by the progression of cholangiocarcinoma and HCC(hepatocellular carcinoma) [13,14].
Procedure
Animal of any gender were given the proper diet and given adequate water before the experiment, and they were kept in an environment where the temperature is being controlled with a 12-hour of the dark and light cycle. The rats were given TAA in various concentrations, methods, and periods to induce TAA induced hepatotoxicity. The animals were sedated on the final day; the sample of blood was obtained, centrifuged. After that, the liver serum enzyme activities were performed.
Table 2: Hepatotoxicity caused by TTA
| Animal | Dose | Route | References |
| Wistar rat (male) | TTA 200 mg per kg, for the time period of 12 weeks | i.p. | [15,16] |
| Wistar rat (male) | Thioacetamide 300 mg per kg, on 14th day | i.p. | [17] |
| Wistar rat (male) | Thioacetamide 400 mg per kg, for 2 weeks | i.p. | [18] |
DEN(diethyl nitrosamine)
The majority of nitrosamines, which have the chemical structure R1 N(– R2)–N=O, are cancer-inducing agents. It’s utilized for the production of insecticides, in the majority of rubber products, and cosmetic products. It can cause cancer in a wide range of animal species, implying that it may also cause cancer in humans [19].
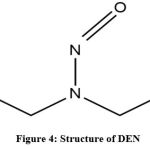 |
Figure 4: Structure of DEN |
Mechanism of action
DEN is frequently used for inducing cancer. Diethyl nitrosamine is hydroxylated by CYP2E1 in the liver to produce ethyl diazonium ion, a bioactive intermediate that causes alteration in DNA by interacting with the nucleophiles, resulting in hepatocyte necrosis around the vein and periportal areas, as well as Centro-portal fibrotic septa. HCC is caused by low doses of DEN given for a long time period [20,21]. As a result, this model is particularly useful for research on the development of liver HCC from fibrosis.
Procedure
Animal of any gender was maintained in cages and given free access to food and tap water in a controlled environment (25º C and with the 12 h dark and light cycle). Animals will be administered by varying doses of DEN to develop hepatotoxicity (50mg –200 mg per kg, by route i.p. for a period of 4–12 weeks) to create hepatotoxicity [22–24]. The animals were sedated on the last day, blood samples were obtained, and centrifuged, and serum samples were separated for the assessment of hepatotoxicity indices. After that, animals were euthanized, and the livers of animals were extracted for studying various morphological and molecular analyses.
Table 3: Hepatotoxicity caused by DEN.
| Animal | Route | Dose | References |
| Albino rat | I.p. | 50-200mg per kg for 4to 12 wk | [22-24] |
Hepatotoxicity caused by drug
Toxicity caused to the liver by the drug can be severe, life-threatening, and can create a severe condition to hospitalization. These reactions can be predicted or unpredicted. Predictable reactions are the reaction noted before or have the possibility to occur inpatient due to the dose of the drug or the drug exposure. Unpredictable hepatotoxicity is the unusual response of drugs that are not noted in clinical trials, they can occur in a few days to months, not compulsory to occur by dose. There are two broad categories of hepatotoxicity- 1st is the injury caused by the drug or by its metabolites(critical cellular functions are altered by causing injury to hepatic cell. Second is the condition in which drug metabolites directly cause toxicity by sensitizing the hepatic cell to cytokines-induced injury.
Among the drug which causes hepatotoxicity NSAIDs are the most commonly causing liver injury. These drugs are mostly prescribed to the patient for pain including arthritis, acute musculoskeletal disorder, a wide range of inflammatory conditions, and trauma resulting from painful conditions [25]. The mechanism of these drugs on which they work is anti-inflammation, antipyretic, and analgesic. These drugs mostly cause toxicity by overdose or long-term use and show their side effects to the liver, GIT, and kidneys [26,27].
PCM
PCM is also known as acetaminophen. It is mainly used as an analgesic, antipyretic. But at the same time at higher doses, it causes toxicity to the body.
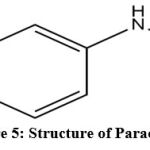 |
Figure 5: Structure of Paracetamol. |
Mechanism of action
PCM converts to inactive compounds through Phase 2 metabolism by coupling with glucuronide and sulfate. A portion of PCM was oxidized through enzyme cyt P450. Then CYP2E1 & CYP3A4 convert it into NAPQI. This Semiquinone radical is formed by the reduction of one electron of NAPQI, covalently bond to cellular membrane macromolecules, and enhance lipid peroxidation, causing tissue damage. NAPQI and acetaminophen at higher doses can oxidize and alkylate the intracellular GSH (glutathione), which causes depletion of the GSH pool in the liver that leads to liver oxidation by increased lipid peroxidation. [28, 29].
Procedure
Rats of any gender are fed with regular diet and given access to unlimited water. Before use, they were given a 12-hour light/dark cycle to adapt to. PCM was given at various levels (3 mg per kg, by p.o. for a period of 7 days for 3 weeks) to induce hepatotoxicity [30–34]. Parameters for toxicity were tested. On the last day of experimentation, animals were sedated, the sample of blood was collected, liver enzyme activity was carried out.
Table 4: Hepatotoxicity caused by PCM.
| Animal | Dose | Route | References |
| Rat, mice | 500 mg per kg | Orally on the 3rd and 5th day | [31] |
| Wistar rat | 3 gm per kg | Per orally | [32] |
| Rat | 3 mg per kg for 7 days for 3 weel | Per orally | [33] |
| Pregnant mice | 300-400mg per kg | i.p. | [34] |
Hepatotoxicity induced by anticancer drug
Cancer causes numerous physiological changes in the liver that further cause malignant tumors. The occurrence of neoplasia is the endpoint of cancer, biologically[35]. Many new cytotoxic chemotherapeutic agents have been developed in recent decades to prolong the lives of patients with advanced or metastatic tumors. Along with chemotherapy biological agents and specifically targeted antibodies are given in combination to increase the life of the patient[36]. The anticancer drug inducestum or cell apoptosis by the production of secondary ROS and causes liver injury [37].
Cisplastin
It is commonly used as an anticancer agent.
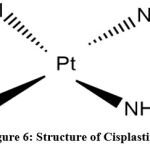 |
Figure 6: Structure of Cisplastin |
Mechanism of action
Cisplastin is commonly used as an anti-cancer agent. Cisplastin causes toxicity by mainly two mechanisms drug metabolism or oxidative stress. Some genes and proteins help in the metabolism of the drug and cause hepatotoxicity, Like CYP 450. Proteins like CYP2D5, CYP2D1, AND CYP2C13 and genes like CYP4A1 and CYP2E1. Elevation in this enzyme and genes causes an increase in oxidative stress and ROS which causes hepatotoxicity[38].
Procedure
Animals were taken into the 12 hrs of the day and light cycle at the room temp (23±2ºC) and access to water and food is free. Hepatotoxicity caused by given different concentration conc. of 3.5mg -7 mg per kg via i.p. route for one to 5 days [39-42]. At the end of the experimentation, animals were sedated and blood samples were collected. After that liver is taken out from the animal body to estimate the antioxidant parameters and histopathological studies were done.
Table 5: hepatotoxicity caused by Cisplastin.
| Animal | Dose | Route | References |
| Albino rat | 3.5 mg per kg | i.p. | [39] |
Methotrexate
It is earlier known as am ethopterin, it is effective as an immunity suppressant and chemotherapeutical agent. It is also used in the treatment of autoimmune disease, medical abortion, and autoimmune disorders.
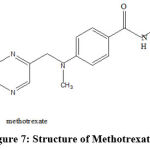 |
Figure 7: Structure of Methotrexate |
Mechanism of action
It acts by inhibiting the enzymes that are , phosphoribosyl transferase, dihydrofolate reductase
It inhibits cell division by inhibiting nucleotide synthesis.
By causing cellular arrest it inhibits the synthesis of the DNA and RNA and by fibrosis.
Procedure
Animals were taken into the 12 hrs of the day and light cycle at the room temp (23±2ºC) and access or water and food are free. Hepatotoxicity caused by given diff. conc. Of20mg/kg/ip in single dose. At the end of the experimentation, animals were sedated and blood samples were collected. After that liver is taken out from the animal body to estimate the antioxidant parameters and histopathological studies were done[43].
Table 6: hepatotoxicity caused by Methotrexate.
| Animal | Route | Dose | References |
| Albino rat | i.p. | 20mg/ kg/ single dose | [43] |
| Rabbit | i.p. | 20 mg/kg for 3 days | [43] |
| Rat | p.o | 100ug per kg for 4 weeks | [43] |
Hepatotoxicity induced by Antibiotics
Antibiotics are microorganisms produced. Antibiotics are effective on the low concentration that selectively kill or inhibit the growth of the microorganism. They are also classified on the mech of action antibiotic which causes cell death care called bactericidal antibiotic and those which inhibit the growth of bacteria are called as a bacteriostatic antibiotic [44].
Erythromycin
It belongs to the macrolide antibiotic class. It is the most common family used against gram-positive bacteria like Streptococcus pneumonia, Staphylococcus aureus, and Hemophilusin fluenza[45].
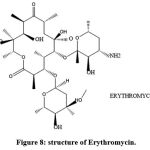 |
Figure 8: structure of Erythromycin. |
Mechanism of action
Erythromycin A is a compound that is 14- membered and ring in shape. It shows both activities bacteriostatic and bactericidal. At low concentration, it is bacteriostatic and at high conc. it is bactericidal. it interferes in protein production by binding the 50S subunit of the ribosome and translocation of susceptible cells is inhibited[46].
Procedure
Animals were taken in 12 hrs a day and light cycle at the room temp (23±2ºC) and access or water and food are free. 100 mg per kg, viap.o. given for a period of 14 days to induce hepatotoxicity[47,48]. On the end day collection of blood sample is done and biomarker enzymes of the liver are estimated. After that liver of the animal is removed to estimate the antioxidant parameter and for the study of histopathological parameters.
Table 7: Hepatotoxicity caused by Erythromycin.
| Animal | Dose and route | References |
| Rat | 10mg per kg by orally | [47] |
Hepatotoxicity induced by anti TB drug
A patient suffering from tuberculosis have to take medicine for a period of 4 to 8 months, it is a long duration and these drugs may cause side effect in the body. The most common drug used for TB is isoniazid and rifampicin and these causes side effect too. Isoniazid has a side effect on life and further causes hepatitis[49].
Isoniazid: Also known as is onicotinyl hydrazine, is a TB treating drug.
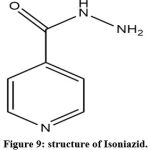 |
Figure 9: structure of Isoniazid. |
Mechanism of action
Isoniazid metabolized in acetyl isoniazid, hydrolyzed to acetyl hydrazine in the existence of N acetyltransferase. CYP2E1 metabolizes acetyl hydrazine to the acyclic molecule which covalently binds to hepatocytes and causes hepatocytic damage[50]. Isoniazid elevates ALP, SGOT, SGPT, and bilirubin levels while lowering albumin and total protein levels[51].
Procedure
Wister rat of both gender of wt. 150-200 gm, kept in a temp. 25±2ºC and in light and dark cycle of 12 hrs, and free access to water and food before one week of experimentation. Rats were co-administered with isoniazid and rifampicin at the dose 50mg-100 mg per kg via i.p./o.p. for 10 days to 28 days[52-55].A sample of blood is taken at the end to estimate the biomarker enzyme of the liver. To estimate antioxidant parameter liver is removed and histopathological studies were done.
Table 8: Hepatotoxicity caused by Isoniazid.
| Animal | Dose | Route | References |
| Wistar rat | 50mg-100mg perkg for 10 days to 28 days | Per oral ori.p. | [55] |
Hepatotoxicity caused by Radiation
Radiation is a form of energy. It is produced by machines in the form of waves particles. Radiations are of two types ionizing and non-ionizing.
Mechanism of action
Acute liver injury progresses to radiation-induced liver damage. The sinusoidal endothelial cells and central vein endothelium were thought to activate the coagulation cascade in radiation exposure, resulting in fibrin build-up and clot formation in the hepatic sinusoids and central veins. The loss of centrilobular hepatic cells and reduction inside of the inner liver plate may result in liver dysfunction [56, 57].
Procedure
Standard conditions were maintained for keeping animals, including providing a standard diet and clean water. Radiation was used to cause hepatotoxicity in the animals. An evaluation of liver biomarker enzymes is performed at the end of the experiment. It is required to remove liver tissue for assessment of antioxidant parameters (GSH, SOD, lipid peroxidation) and histopathological analysis.
Table 9: hepatotoxicity caused by Radiation.
| Animal | Dose | References |
| Male Wistar Albino rats | Gamma rays were introduced in a single dose for 15 days regularly(6Gy). | [58] |
| Male Sprague Dawley rats | Gamma rays of 5Gy were induced for 2 days | [59] |
| Wistar male rat | Level 3 to 6Gy of an acute single dose for 7 days | [60] |
Hepatotoxicity induced by Metal
In the environment, the production of toxic and carcinogenic compounds is mainly due to human activity.
Mercury
Poisoning is common among other metals. It is a transitional metal. It increases the level of ROS in the body[61]. Mercury has side effects or toxic effects like CVS disease, kidney or liver damage, cancer, autoimmune disease, neurobehavioral disorder, anemia, and diseases that are associated with experimental animals and human death[62].
Mech of action
Mercury is present in different forms as it is a transitional metal; like metallic mercury, mercurous(Hg2₊₊), mercury vapor(Hg), mercuric(Hg₊₊)are inorganic mercury[63].It promotes the generation of ROS. This increase in ROS increases the formation of a highly reactive radical(hydroxyl) and the formation of the lipid peroxides[64]. Production of these radicals causes damage to the cell membrane and destroys it. It also inhibits the activity of the free radical SOD, GPX, and catalase [65].
Procedure
Rats of both genders were given diet and free access to water. They are taken in 12hrs a light and dark cycle. Hepatotoxicity is caused by mercury. Histopathological parameters were studied. On the last day of experimentation, animals were sedated, a sample of blood was collected, liver enzyme activity was carried out.
Table 10: Hepatotoxicity caused by metal
| Animal | Route and dose | References |
| Wistar rat(male) | 80 mg per l as water for 4 weeks(HgCl2) | [66] |
| Albino rat (male) | Hg-5mg per kg by injecting subcutaneously | [67,68] |
Alcohol
It is fermented by sugar. Alcohol is stimulant at lower doses but at a higher rate, it causes drowsiness and respiratory depression. It showsa toxic sedative effect at high doses, it has a bad effect on body organs depending on the BAC (blood alcohol conc.)[69,70].
 |
Figure 10: Structure of Alcohol. |
Mechanism of action
As soon as alcohol leaves the small intestine, it travels to the liver where it is converted into acetate by two enzymes: (a) alcohol dehydrogenase(ADH) in the mitochondria of hepatocytes [71]. As soon as alcohol leaves the small intestine, it travels to the liver where it is converted into acetate by two enzymes: (a) ADH in the mitochondria of hepatocytes (b) ADH present in the cytosol.
Alcohol is converted into acetaldehyde by following three pathways in the liver: (a) by ADH in the cytosol; (b) by microsomal enzyme P-450; (c) by catalase such as H2O2. Through the generation of highly ROS, acetaldehyde may membrane damage and necrosis of cells [72]. ADH is oxidized into acetaldehyde by the liver, which is relatedto the NAD that reduces to NADH. As a result, NADH level got increases the activity of xanthine oxidase, which is responsible for rising superoxide levels and lowering glutathione levels in the liver [73].
Procedure
The male and female rats were given a standard chew diet and water in free access. We allowed them to adjust for 12 hours in a light/dark cycle before use. Alcohol is introduced to the animal for inducing toxicity Animals. Blood was collected, on the last day from retro-orbital punctures, after animals were sedated with aesthetic ether. All parameters are studied.
Table 11: Hepatotoxicity caused by Alcohol
| Animal | Dose | Route | References |
| WistarAlbino rat (male) | 7.9gm per kg | Ethanol Per oral daily for 45 days | [74] |
| Albino Wistar rat | 2ml per 100 gm | Ethanol Per oral for 21 days | [75] |
| Albino Wistar rat (male) | 5g per kg | Ethanol Per oral daily for 60 days | [76] |
| Wistar rat (female) | 3.76gm per kg twice a day | Ethanol per oral for 25 days | [77] |
In-vesive model
ligation of the bile duct
Rats are more suitable for this model as they do not have a gallbladder. Extracellular deposition of matrix components occurs in the liver and this condition is characterized as liver fibrosis[78].
Mechanism of action
It is a classic secondary biliary fibrotic experimental model. Basically human liver consists of the epithelial cell which is of two types; one is biliary epithelial cell and another is hepatic cell.[79]. Biliary epithelial cell transports the bile acid to the hepatic cell and helps in their survival through the biliary system[80]. So, obstruction in the biliary system causes cholestasis and this leads to injury in the liver and causes apoptosis by the generation of the free radical and necrosis in hepatic cells [81].
Procedure
To perform this procedure, one needs to make an incision to the midline abdominal and isolate the common bile duct above the duodenum, and then the distal and proximal parts of the bile ducts are ligated. To minimize the risk of formation of cyst in bile, each lobule and the biliary duct are separately ligated. This (Ligation of the bile duct) stimulates bile duct proliferation, causing cholestasis,portal inflammation, and fibrosis, resulting in secondary cirrhosis of the biliary system and liver failure[78,81].
Ligation of portal-vein
Portal blood carries material that has physiological effects that result in hyperplasia and hypertrophy [82]. Early on after the ligation, inflammation, and edema accompany tissueinjury. Cirrhosis and other intrinsic liver diseases are usually accompanied by portal-systemic shunting and decreased hepatic blood flow, mainlyblood flow of the portal vein [83].
Mechanism of action
Cirrhosis is caused by portal-systemic shunting and a reduction in hepatic, specifically blood flow of portal vein. Some pathophysiological changes seen in people with chronic liver disease are caused, at least in part, by a decrease in portal venous flow and its shunting across the liver [84].
Procedure
incision in the upper abdomen is done. An anterior lobe PVL involves the ligation of the portal vein leading to the left side of the heart. The portal vein was held with a needle of 10-20 gauge and two to three silk ligatures were tied around it. After carefully slipping out of the ligatures, the needle opened the portal vein and the abdomen was closed before the needle was reinserted [84].
Genetic model
The role of the specific factors in fibrogenesis has been evaluated using genetically modified models. It would be reasonable to conclude that the increased fibrosis in knockout mice (KO) upon exposure to pro-fibrotic stimuli suggests that this gene product has direct and indirect pro-fibrotic and anti-fibrotic effects.In transgenic animals, exogenous genes can be introduced into the germline. This technology allows for the in vivo analysis of the gene function. Exogenous genes can be implanted into the germline of transgenic animals [85].
Transgenic mice TGF-1
Overexpression of TGF-1 in the liver of transgenic mice plays an important role inliver fibrogenesisby stimulating HSCs(hepatic satellite cells) that grow intothe myofibroblasts and increase the production of the extracellular matrix proteins [86].
Mice/ Bcl-xL
The removal of specific anti-apoptotic gene Bcl-xL-per se induces liver fibrosis by preventing apoptosis in hepatocytes [87].
Transgenic mice PDGF
In PDGF family members overexpressing PDGF-A, B, or C was found to upregulate TGF-b1 and therefore result in liver fibrogenesis [88,89].
Mice/Abcb4(Mdr2/mice)
The removal of phospholipid transporter from bile causes chronic cholangitis and severe biliary fibrosis in ABCB4-KO mice. This results in intoxication-based liver and bile duct damage [90].
Conclusion
Liverinjury can be caused by many reasons it can be genetic, by thegeneration of the free radical, by metal by drug most commonly, or by radiationwhich will lead to pathological changes in body. Although significant progress has been made in the last few years, there is clearly a need to further improve insight into the molecular mechanisms of liver injury and cell death in all experimental models. In this review, our main aim is on animal models for hepatotoxicity and pathological changes resulting in hepatotoxicity. There are still huge gaps in knowledge concerning the animal model, in understanding the diseases in human and in the translation of the model to the human disease.
Conflict of Interest
There is no conflict of interest.
References
- Bigoniya P, Singh CS, Shukla A. A comprehensive review of different liver toxicants used in experimental pharmacology. Int J Pharm Sci Drug Res. 2009;1:124–35
- Singh A, Bhat KP,Om P. Clinical biochemistry of hepatotoxicity. J ClinToxicol. 2011, S4:001.
- Devarbhavi, H., Dierkhising, R., Kremers, W.K. Antituberculosis therapy drug-induced liver injury and acute liver failure.Hepatology 52, 2010, 798–799.
- Devarbhavi, H., Karanth, D., Prasanna, K.S., Adarsh, C.K., Patil, M., 2011. Drug-induced liver injury with hypersensitivity features has a better outcome: a single-center experience of 39 children and adolescents. Hepatology 54,2011,1344–1350
- Recknagel RO. Carbon tetrachloride hepatotoxicity. Pharmacol Rev. 1967,19,145–208.
- Xu J-Y, Su Y-Y, Cheng J-S, et al. Protective effects of fullerenol on carbon tetrachloride-induced acute hepatotoxicity and nephrotoxicity in rats. Carbon. 2010,48,1388–96.
- Bahashwan S, Hassan MH, Aly H, Ghobara MM, El-Beshbishy HA, Busati I. Crocin mitigates carbon tetrachloride-induced liver toxicity in rats. J TaibahUniv Med Sci. 2014,14(9): 955.
- Eidi A, Mortazavi P, Bazargan M, Zaringhalam J. Hepatoprotective activity of cinnamon ethanolic extract against CCL 4-induced liver injury in rats. EXCLI J. 2012;11:495–507.
- Moreira PR, Maioli MA, Medeiros HCD, Guelfi M, Pereira FvTV, MingattoFbE. Protective effect of bixin on carbon tetrachloride-induced hepatotoxicity in rats. Studies. 2014;6:10.
- Shahjahan M, Sabitha KE, Jainu M, Devi CSS. Effect of Solanumtrilobatum against carbon tetra chloride induced hepatic damage in albino rats. Indian J Med Res. 2004;120:194–8.
- Palanivel MG, Rajkapoor B, Kumar RS, et al. Hepatoprotective and antioxidant effect of Pisoniaaculeata L. against CCl4-induced hepatic damage in rats. ScientiaPharmaceutica. 2008;76:203.
- Singh R, Kumar S, Rana AC, Sharma N. Different model of hepatotoxicity and related liver diseases: a review. Int Res J Pharm. 2012;3:86–94.
- Starkel P, Leclercq IA. Animal models for the study of hepatic fibrosis. Best Pract Res ClinGastroenterol. 2011;25:319–33.
- Liu Y, Meyer C, Xu C, et al. Animal models of chronic liver diseases. Am J PhysiolGastrointest Liver Physiol. 2013;304:G449–68.
- Shirin H, Sharvit E, Aeed H, Gavish D, Bruck R. Atorvastatin and rosuvastatin do not prevent thioacetamide induced liver cirrhosis in rats. World J Gastroenterol WJG. 2013;19:241.
- Alkiyumi SS, Abdullah MA, Alrashdi AS, Salama SM, Abdelwahab SI, Hadi AHA. Ipomoea aquatica extract shows protective action against thioacetamide-induced hepatotoxicity. Molecules. 2012;17:6146–55.
- Nafees S, Ahmad ST, Arjumand W, Rashid S, Ali N, Sultana S. Carvacrol ameliorates thioacetamide-induced hepatotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in liver of Wistar rats. Human ExpToxicol. 2013:32(12):1292-304
- Kabiri N, Setorki M, Darabi MA. Protective effects of kombucha tea and silimarin against thioacetamide induced hepatic injuries in wistar rats. World ApplSci J. 2013;27:524–32.
- Swann PF, Magee PN. Nitrosamine-induced carcinogenesis. The alkylation of N-7 of guanine of nucleic acids of the rat by diethylnitrosamine, N-ethyl- N-nitrosourea and ethyl methanesulphonate. Biochem J. 1971;125:841–7.
- Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM. Experimental models of hepatocellular carcinoma. J Hepatol. 2008;48:858–79.
- Schiffer E, Housset C, Cacheux W, et al. Gefitinib, an EGFR inhibitor, prevents hepatocellular carcinoma development in the rat liver with cirrhosis. Hepatology. 2005;41:307–14.
- Park D-H, Shin JW, Park S-K, et al. Diethylnitrosamine (DEN) induces irreversible hepatocellular carcinogenesis through overexpression of G1/S-phase regulatory proteins in rat. ToxicolLett. 2009;191:321–6.
- Wan T-C, Chen C-M, Lin L-C. Hepatoprotective effects of natural Calculus Bovis against diethylnitrosamine induced hepatic injury in rats. J PharmacognPhytother. 2013;5:189–95.
- Rezaie A, Fazlara A, Karamolah MH, Shahriari A, Zadeh HN, Pashmforosh M. Effects of Echinacea purpurea on Hepatic and Renal Toxicity Induced by Diethylnitrosamine in Rats. Jundishapur J Nat Pharm Prod. 2013;8:60.
- Abdel Wahhab MA. Ahmed H, Hagazi MM. Prevention of aflatoxin B1-initiated hepatotoxicity in rat by marine algae extracts. J ApplToxicol. 2006;26:229–38.
- Meek IL, Van de Laar MAFJ, Vonkeman HE. Non-steroidal anti-inflammatory drugs: an overview of cardiovascular risks. Pharmaceuticals. 2010;3:2146–62.
- Vane JR, Botting RM. The mechanism of action of aspirin. Thromb Res. 2003;110:255–8.
- Abraham P, Wilfred G, Cathrine SP. Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. ClinicaChimicaActa. 1999;289:177–9.
- Kauppinen T, Toikkanen J, Pedersen D, et al. Occupational exposure to carcinogens in the European Union. Occup Environ Med. 2000;57:10–8.
- Parmar SR, Vashrambhai PH, Kalia K. Hepatoprotective activity of some plants extract against paracetamol induced hepatotoxicity in rats. J Herbal Med Toxicol. 2010;4:101–6.
- Shenoy S, Kumar H, Thashma NV, Prabhu K, Pai P. Hepatoprotective activity of Plectranthusamboinicus against paracetamol induced hepatotoxicity in rats. Int J PharmacolClin Sci. 2012;2:32–8.
- Kanchana N. SadiqAM. Hepatoprotective effect of Plumbagozeylanica on paracetamol induced liver toxicity in rats. Int. J Pharm Pharm Sci. 2011;3:151–4.
- ZakiPhd HF. Potential protective effect of honey against paracetamol-induced hepatotoxicity. Archives Iranian Med. 2012;15:674.
- Lebda MA, Taha NM, Korshom MA, Mandour AE-WA, Goda RI. Ginger (Zingiberofficinale) potentiate paracetamol induced chronic hepatotoxicity in Rats. J Med Plants Res. 2013;7:3164–70.
- Seyfried TN, Shelton LM. Cancer as a metabolic disease. NutrMetab (Lond). 2010;7:269–70.
- Maor Y, Malnick S. Liver injury induced by anticancer chemotherapy and radiation therapy. Int J Hepatol. 2013; 815105.
- Lim SC, Choi JE, Kang HS, Si H. Ursodeoxycholic acid switches oxaliplatin induced necrosis to apoptosis by inhibiting reactive oxygen species production and activating p53 caspase 8 pathway in HepG2 hepatocellular carcinoma. Int J Cancer. 2010;126:1582–95.
- Cho Y-E, Singh TSK, Lee H-C, et al. In-depth identification of pathways related to cisplatin-induced hepatotoxicity through an integrative method based on an informatics-assisted label-free protein quantitation and microarray gene expression approach. Mol Cell Proteom. 2012;11(1)
- Fasihi M, Ghodratizadeh M, Ghodratizadeh S. Protective effect of captopril on cisplatin induced hepatotoxicity in rat. American-Eurasian J Toxicol Sci. 2012;4:131–4.
- Ko J-W, Lee I-C, Park S-H, et al. Protective effects of pine bark extract against cisplatin-induced hepatotoxicity and oxidative stress in rats. Laboratory Animal Res. 2014;30:174–80.
- Naqshbandi A, Khan MW, Rizwan S, Yusufi ANK, Khan F. Studies on the protective effect of fish oil against cisplatin induced hepatotoxicity. Biol Med. 2011;3:86–97.
- Arhoghro EM, Kpomah DE, Uwakwe AA. Curative potential of aqueous extract of lemon grass (Cymbopogoncitratus) on cisplatin induced hepatotoxicity in albino Wistar rats. J Phys Pharm Adv. 2012;2:282–94.
- Bhatia D, Goyal P. methotrexate model of Hepatotoxicity: A review. Der Pharma chemical,2021;13(6):16-18.
- Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nat Rev Microbiol. 2010;8:423–35.
- Gaynor M, Mankin AS. Macrolide antibiotics: binding site, mechanism of action, resistance. Curr Top Med Chem. 2003;3:949–60.
- Garza-Ramos G, Xiong L, Zhong P, Mankin A. Binding site of macrolide antibiotics on the ribosome: new resistance mutation identifies a specific interaction of ketolides with rRNA. J Bacteriol. 2001;183:6898–907.
- Abdel-Hameid N-AH. Protective role of dimethyl diphenylbicarboxylate (DDB) against erythromycin induced hepatotoxicity in male rats. ToxicolIn Vitro. 2007;21:618–25.
- Sambo N, Garba SH, Timothy H. Effect of the aqueous extract of Psidiumguajava on erythromycin-induced liver damage in rats. Niger J Physiol Sci. 2009; 24.
- Huang YS, Chern HD, Su WJ, et al. Cytochrome P450 2E1 genotype and the susceptibility to antituberculosis drug induced hepatitis. Hepatology. 2003;37:924–30.
- Noda A, Hsu KY, Noda H, Yamamoto Y, Kurozumi T. Is isoniazid-hepatotoxicity induced by the metabolite, hydrazine? J UOEH. 1983;5:183–90.
- Bigoniya P, Singh CS, Shukla A. A comprehensive review of different liver toxicants used in experimental pharmacology. Int J Pharm Sci Drug Res. 2009;1:124–35.
- Jyothi B, Mohanalakshmi S, Anitha K. Protective effect of mirabilis Jalapa leaves on anti-tubercular drugs induced hepatotoxicity. Asian J Pharm Clin Res. 2013;6:221–4.
- Pal R, Vaiphei K, Sikander A, Singh K, Rana SV. Effect of garlic on isoniazid and rifampicin-induced hepatic injury in rats. World J Gastroenterol. 2006;12:636.
- Rao CV, Rawat AKS, Singh AP, Singh A, Verma N. Hepatoprotective potential of ethanolic extract of Ziziphusoenoplia (L.) Mill roots against antitubercular drugs induced hepatotoxicity in experimental models. Asian Pac J Tropical Med. 2012;5:283–8.
- Yue J, Peng R-X, Yang J, Kong R, Liu J. CYP2E1 mediated isoniazid-induced hepatotoxicity in rats. ActaPharmacologicaSinica. 2004;25:699–704.
- Lawrence TS, Robertson JM, Anscher MS, Jirtle RL, Ensminger WD, Fajardo LF. Hepatic toxicity resulting from cancer treatment. Int J RadiatOncolBiol Phys. 1995;31:1237–48.
- Dawson LA, Ten Haken RK, Lawrence TS. Partial irradiation of the liver. Seminars in radiation Oncology. Amsterdam: Elsevier; 2005;15(4):279-83
- Mansour HH, El Azeem MGA, Ismael NE. Protective effect of moringaoleifera on Iˆ3 -radiation-induced hepatotoxicity and nephrotoxicity in Rats. 2014.
- Park SH, Pradeep K, Choi MH, Ko K, Park H. Hesperidin and Curdlan treatment ameliorates Iˆ3 -radiation induced cellular damage and oxidative stress in the liver of Sprague-Dawley rats. RJPBCS. 2010;1:165–77.
- Ramadan LA, Roushdy HM, Abu Senna GM, Amin NE, ElDeshw OA. Radioprotective effect of silymarin against radiation induced hepatotoxicity. Pharmacol Res. 2002;45:447–54.
- Nicolli A, Basso E, Petronilli V, Wenger RM, Bernardi P. Interactions of cyclophilin with the mitochondrial inner membrane and regulation of the permeability transition pore, a cyclosporin A-sensitive channel. J Biol Chem. 1996;271:2185–92.
- Singh R, Kumar S, Rana AC, Sharma N. Different model of hepatotoxicity and related liver diseases: a review. Int Res J Pharm. 2012;3:86–94.
- Bernhoft RA. Mercury toxicity and treatment: a review of the literature. J Environ Public Health. 2012; 2012.
- Miller DM, Lund BO, Woods JS. Reactivity of Hg(II) with superoxide: evidence for the catalytic dismutation of superoxide by Hg(II). J BiochemToxicol. 1991;6:293–8.
- Benov LC, Benchev IC, Monovich OH. Thiol antidotes effect on lipid peroxidation in mercury-poisoned rats. ChemBiol Interact. 1990;76:321–32.
- Haouem S, Chargui I, Najjar MF, Sriha B, El Hani A. Liver function and structure in rats treated simultaneously with cadmium and mercury. Open J Pathol. 2013;3:26.
- Oda SS, El-Ashmawy IM. Protective effect of silymarin on mercury-induced acute nephro-hepatotoxicity in rats. Studies. 2012;35:36.
- Ekambaram M, Ramalingam K, Balasubramanian A. Effect of Portulacaquadrifida Linn on Mercury-Induced Hepatotoxicity in Swiss Albino Mice. Res Rev J PharmacolToxicol Stud. 2014;2:18–21.
- Roehrs T, Roth T. Sleep, sleepiness, and alcohol use. Alcohol Res Health. 2001;25:101–9.
- Zakhari S. Overview: how is alcohol metabolized by the body? Alcohol Res Health. 2006; 29(4): 245–254.
- Huang Y-W, Yang S-S, Kao J-H. Pathogenesis and management of alcoholic liver cirrhosis: a review. Hepat Med Evid Res. 2011;3:1.
- Gramenzi A, Caputo F, Biselli M, et al. Review article: alcoholic liver disease pathophysiological aspects and risk factors. Aliment PharmacolTher. 2006;24:1151–61.
- Lieber CS. Alcohol: its metabolism and interaction with nutrients. Annu Rev Nutr. 2000;20:395–430.
- Sivaraj A, Vinothkumar P, Sathiyaraj K, Sundaresan S, Devi K, Senthilkumar B. Hepatoprotective potential of Andrographispaniculata aqueous leaf extract on ethanol induced liver toxicity in albino rats.2011: 204-208.
- Sharma A, Sangameswaran B, Jain V, Saluja MS. Hepatoprotective activity of Adina cordifolia against ethanol induce hepatotoxicity in rats. IntCurr Pharm J. 2012;1:279–84.
- Reddy VD, Padmavathi P, Gopi S, Paramahamsa M, Varadacharyulu NC. Protective effect of Emblicaofficinalis against alcohol-induced hepatic injury by ameliorating oxidative stress in rats. Indian J ClinBiochem. 2010;25:419–24.
- Modi H, Patel V, Patel K. Hepatoprotective activity of Aeglemarmelos against ethanol induced hepatotoxicity in rats. Asian J Pharm Clin Res. 2012;5:164–7
- Tarcin O, Basaranoglu M, Tahan V, et al. Time course of collagen peak in bile duct-ligated rats. BMC Gastroenterol. 2011;11:45
- Tietz PS, Alpini G, Pham LD, Larusso NF. Somatostatin inhibits secretin-induced ductal hypercholeresis and exocytosis by cholangiocytes. Am J Physiol-Gastrointest Liver Physiol. 1995;269.
- Nathanson MH, Boyer JL. Mechanisms and regulation of bile secretion. Hepatology. 1991;14:551–66.
- Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction: a new experimental model for cirrhosis in the rat. Br J ExpPathol. 1984;65:305.
- Rozga J, Jeppsson B, Bengmark S. Hepatotrophic factors in liver growth and atrophy. Br J ExpPathol. 1985;66:669.
- Kahn D, Kajani M, Zeng Q, et al. Effect of partial portal vein ligation on hepatic regeneration. Investig Surg. 1988;1:267–76.
- Bulbuloglu E, A˚ zˇahin M, Kantarceken B, et al. The outcome of major hepatectomies following different durations of portal vein ligation in rats. 2005;2, 47-55.
- Leclercq IA, Farrell GC, Schriemer R, Robertson GR. Leptin is essential for the hepatic fibrogenic response to chronic liver injury. J Hepatol. 2002;37:206–13.
- Kanzler S, Lohse AW, Keil A, et al. TGF-Iˆ2 1 in liver fibrosis: an inducible transgenic mouse model to study liver fibrogenesis. Am J Physiol-Gastrointest Liver Physiol. 1999;276:G1059–68.
- Takehara T, Tatsumi T, Suzuki T, et al. Hepatocyte-specific disruption of Bcl-xL leads to continuous hepatocyte apoptosis and liver fibrotic responses. Gastroenterology. 2004;127:1189–97.
- Czochra P, Klopcic B, Meyer E, et al. Liver fibrosis induced by hepatic overexpression of PDGF-B in transgenic mice. J Hepatol. 2006;45:419–28.
- Campbell JS, Hughes SD, Gilbertson DG, et al. Platelet-derived growth factor C induces liver fibrosis, steatosis, and hepatocellular carcinoma. ProcNatlAcadSci USA. 2005;102:3389–94.
- Fickert P, Zollner G, Fuchsbichler A, et al. Ursodeoxycholic acid aggravates bile infarcts in bile duct ligated and Mdr2 knockout mice via disruption of cholangioles. Gastroenterology. 2002;123:1238–51.








