Manuscript accepted on :08-12-2022
Published online on: 16-12-2022
Plagiarism Check: Yes
Reviewed by: Dr. Liudmila Spirina, Dr. B. Kirthika
Second Review by: Dr Jayanti Mukherjee
Final Approval by: Dr. Patorn Piromchai
Salma Bendiar 1 , Othman El Faqer 1
, Othman El Faqer 1 , Naima Benjelloun 1, Souada Hsseini 2, Hicham Bellaoui 2
, Naima Benjelloun 1, Souada Hsseini 2, Hicham Bellaoui 2 , Samira Rais 1
, Samira Rais 1 , Younes Zaid 1,2,3
, Younes Zaid 1,2,3 , El Mostafa Mtairag1
, El Mostafa Mtairag1 and Mounia Oudghiri 1*
and Mounia Oudghiri 1*
1Immunology and Biodiversity Laboratory, Faculty of Sciences Ain Chock, Hassan II University, Casablanca, Morocco.
2Department of biology, Faculty of Sciences, Mohammed V University, Rabat, Morocco.
3Research Center of Abulcasis University of Health Sciences, Rabat, Morocco.
Corresponding Author E-mail: mouniaoudghiri@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2572
Abstract
Background: The fruit of Ziziphus Lotus L. (ZL) is rich in bioactive components. It is known for its high content in polyphenols which gives it its immunomodulatory, antioxidant, and antimicrobial properties. Objective: The intent of the current study was to evaluate, in vivo, the effect of the aqueous extract of ZL fruit’s pulp on humoral immune response as well as its effect on neutrophils’ bactericidal activities, hemolytic and antioxidant and activities. Methods: The antioxidant activity of ZL’s aqueous extract’s was evaluated using DPPH. Hemmagglutination titer assay was used to evaluate the effect of the extract on humoral immune response. ZL extract’s hemolytic activity was assessed by enumerating hemoglobin rates. The effect of ZL extract on the bactericidal activity of Neutrophils was evaluated using MTT colorimetric assay. Results / Discussion: A significant (P<0.05) immunosuppressive effect on humoral immunity (6-fold) was observed. Significant suppression (P<0.05) of the bactericidal activity of neutrophils treated with 0.5 and 1 g/ml of the extract was observed compared to untreated neutrophils. The extract exhibited a high antioxidant activity determined by DPPH test with an IC50 value 10-fold higher (P<0.05) than the IC50 of ascorbic acid. The highest hemolytic activity was found with the lowest concentration of the extract while the higher concentrations tested seem to have an anti-hemolytic activity with a dose dependent effect. Conclusion: The aqueous extract of ZL’s fruit pulp possesses an immunosuppressive activity on both the innate and adaptive immunity responses. Our results demonstrate an anti-oxidative activity as well as an ability to decrease neutrophil bactericidal hemolytic activities as well as humoral immune responses.
Keywords
Antioxidant; Humoral immune response; Immunosuppressive; Neutrophil bactericidal activity; Ziziphus Lotus L (Desf.) fruits
Download this article as:| Copy the following to cite this article: Bendiar S El-Faqer O, Benjelloun N, Hsseini S, Bellaoui H, Rais S, Zaid Y, Mtairag E, Oudghiri M. Immunomosuppressive Effect of Ziziphus Lotus L. (Desf.) Fruit’s Extract on Neutrophil Bactericidal Activity and on in Vivo Humoral Immune Response in Mice. |
| Copy the following to cite this URL: Bendiar S El-Faqer O, Benjelloun N, Hsseini S, Bellaoui H, Rais S, Zaid Y, Mtairag E, Oudghiri M. Immunomosuppressive Effect of Ziziphus Lotus L. (Desf.) Fruit’s Extract on Neutrophil Bactericidal Activity and on in Vivo Humoral Immune Response in Mice. Available from: https://bit.ly/3FV1hni |
Introduction
The custom of using plants in traditional medicine has a significant role in Moroccan culture and has been an integral part of the Moroccan “public care” system. Indeed, many ethnobotanic inquiries led in various regions of Morocco, show that 70% to 80% of Moroccans resort to medicinal plants as treatment, for in spite of the development of modern treatments, they remain in most cases out of reach by their expensiveness and lack of proximity. Their use continues to progress partially because of the high price of medicine but also because of the notorious efficiency of traditional medicine 1-5.
Ziziphus Lotus L. (Desf) (ZL), also called jujube, is part of the angiosperm Rhamnaceae family. In Morocco, ZL is a fruit growing shrub commonly called “Sedra” and is found in many arid and semi-arid regions. ZL is one of the traditional plants commonly used in folk medicine in Morocco. The fruits, which have an edible endocarp, called “Nbag” is a valuable source of nutrients still consumed by local population and contains an important number of phenols, carbohydrate, tanins and flavonoids 6-10. ZL fruits are rich in vitamins E and C, fatty acids, calcium, amino acids, fibers and magnesium 11.
In herbal medicine, bioactive compounds’ properties will differ depending on the type of extract and the part of the plant used (root barks, leaves, or fruit).
For example, the oral administration of ZL’s fruits aqueous extract inhibited significantly and, in a dose dependent way, the HCL/ethanol induced ulcer and reduction of the secretion of gastric juice in pylorus legated rats as reported by Wahida et al. 12.
The methanolic extracts of leaves and fruits of ZL exhibited strong antibacterial, antifungal and antioxidant activities 9, 13.
ZL fruit was found to improve glucose tolerance, dyslipidaemia and fatty liver disease, but not the severity of obesity in mice induced by a high-fat-diet 14.
Aqueous extract of root barks of ZL administered intraperitoneally has shown dose-dependent and significant analgesic and anti-inflammatory activities [15] and has caused significant relaxation of spontaneous contractions induced by spasmogenic agents in rodents 16. It was also reported that the ethyl acetate and chloroformic extracts were less active than the polar extracts (aqueous and methanolic) 16. Hence, the presence of the saponin and flavonoid fractions in the methanolic extracts of root bark and leaves of ZL was responsible of inhibition of algesia, nitrite production and paw edema 8.
It is acknowledged that ZL is rich in polyphenols, displaying antimicrobial, antioxidant, immunomodulatory and antidiabetic properties. 11, 17, 18. Fruit, leaves and seeds are also rich in different vitamins that are responsible of in vitro antioxidant properties 19, 20.
All parts of ZL are rich in the members of polyphenol family and the fruit’s major compounds are total phenols. Natural plant polyphenols exhibited great inhibitory effects on abnormal human lymphocytes cells 21. It was also reported that antimicrobial activities of ZL fruits were attributed to its phenolic compounds content 22.
Polyphenols are also known to modulate human immune cell signaling. An immunosuppressive effect, in vitro, on human T cells and Jurkat cells proliferation by ZL polyphenols was reported 19; 11. T-cells signaling mechanisms were assessed by Chan et al. who were able to provide the confirmation that a combination of herbs rich with Zizyphus extracts were able to induce the expression of mitogen-activated protein kinases (i.e. JNK, p38 and ERK) in T-cells. This indicated that the effects of Zizyphus on immunomodulatory activities involves the activation of second messenger cascade 23.
The immune system is the body’s defense system against pathogens. On the one hand, immune deficiency can be described as a situation where the immune system is less active than it should, leading to recurrent and life-threatening affections. Additionally, a hyperactive immune system can lead to autoimmune diseases or allergies.
A limited number of studies have explored the effect of the fruits of Ziziphus lotus on the immune system, in vivo, or on other expressions of the immune responses other than T lymphocytes proliferation in vitro.
In the current study, the objective was to evaluate the effect of ZL fruit’s pulp aqueous extract on humoral immune response, NF-κB signaling pathway, as well as its effect on others non-specific immune responses such as the bactericidal activity of neutrophils, hemolytic and antioxidant activities.
Materials and Methods
Preparation of Ziziphus lotus aqueous extract
The pulp of Ziziphus lotus (ZL) fruit was first dried in shade, and later crushed into a powder and kept in a dry and dark place before use. The extract was gathered by boiling under reflux for 1h at 60°C (decoction) 100 g of pulp powder in 1 L of distilled water. The solution was then centrifuged and filtered before it was evaporated using rotary vacuum evaporator at 40°c. The extract was then saved at -20°C until the time it had to be used.
Yields percentages were calculated using the following formula:
Wr = weight of plants residues
Ws = weight of raw sample plant.
Qualitative Phytochemical screening
ZL extract was exposed to phytochemical screening to highlight the presence or absence of phenols, flavonoids and tannins:
The detection of phenols was carried out by adding 3 ml of the extract was to a mix of 1 ml of Folin Ciocalteu and 1 ml of 20% of Na2CO3. A blue coloration indicated the presence of phenols.
The detections of flavonoids was carried out by adding 3 ml of the extract to 1 ml of a solution of NAOH at 10%. A blue coloration indicated the presence of flavonoids.
The detection of tannins was carried out by adding 2 ml of a solution of FeCl3 at 5% to 5 ml of extract. A dark green coloring indicated the presence of catechic tannins. The presence of a dark blue color indicated the presence of gallic tannins.
Antioxidant activities assay
DPPH free radical scavenging method (also known as 2, 2-diphenyl-1-picrylhydrazyl) was the one used to determinate the antioxidant activities of the aqueous extract of Ziziphus Lotus fruit pulp. The DPPH assay was adapted according to the method described by Roy et al. 24. Fifty µl of ZL’s extract at different concentrations (10, 15, 20, 25, 30, 50 ml/ml) or ascorbic acid (positive standard) at the following concentrations (0.075; 0.09; 0.2; 0.4; 0.6; 0.8 and 1 mg/ml) were added to 1.950 ml of a methanolic solution of DPPH. The mixture was kept for 30 minutes at room temperature and in the dark. The absorbance was later measured at 517 nm. All the experiences were performed three times. A methanolic DPPH solution was used as the blank solution. The antiradical activity was expressed as IC50 in mg/ml; which represented the sample’s concentration needed to scavenge 50% DPPH free radicals.
Inhibition percentage was assessed using the following equation:
Where: – A DPPH = DPPH’s absorbance,
A sample = Sample’s absorbance (sample or ascorbic acid).
Assessment of Ziziphus Lotus’ aqueous extract on humoral immune activity
Hemagglutination antibody titer assay
The mice were immunized by injecting 200 μL of Rat Red Blood Cells (RRBC) diluted in PBS (30% v/v) on day 0 via intra-peritoneal route. Two groups of 5 mice each were used. The first group received the extract via oral route, at a concentration of 5 g/kg body weight, starting 3 days before immunization for a period of 10 days in total. The second group received the vehicle only (control group). Seven days after immunization, blood was collected from the mice and the serum recovered after an incubation at 4 °C, overnight. It was then centrifuged, incubated at 56 °C for 30 minutes to inactivate the complement and kept until use at –20 °C. The hemagglutination technique described by Bin Hafeez et al. [25] was used to determine antibody levels. Briefly, 25 μl of RRBC diluted in a physiological solution to a concentration of 1% (v/v) were added to the serum that was serially diluted in 25μl PBS two-fold in 96-well microplates. The mix was incubated for 2h at room temperature. The reciprocal of the highest dilution of the positive test serum agglutination was taken as the antibody titer. This experiment was repeated three times (3 independent experiments n = 3).
In vitro hemolytic activity assay
Hemolytic activity assay was performed by following the method used by Bulmus et al. [26].
Blood from human volunteers having signed an informed consent was drawn in EDTA tubes.
The blood was centrifuged (15 min at 200 g), to collect the plasma. The human red blood cells (HRBCs) were then washed three times with a solution of 150 mM of NaCl, suspended in a 100 mM of PBS (phosphate buffer solution)) and diluted to a final concentration of 108 HRBCs per 200ml (to 10% of their initial concentration). Then, 800 ml of PBS was mixed to 200 ml of HRBC and 50 ml of different concentrations (10, 50, 100, 200, 500 and 1000 mg/ml) of the extract of ZL. The tubes were centrifuged for 5 min at 13500 g after an incubation of 1h at a temperature of 37°C. The absorbance of the supernatant was measured at 540 nm. HRBC were incubated with Triton-X100 (1 wt %) and considered as positive control. A solution of PBS was used as negative control.
The experiments were performed three times and the extract’s inhibitory activity was calculated using the below equation. The results were expressed as percentage of hemolytic activity.
Abs: absorbance.
SDS–PAGE and immunoblotting
Proteins were resolved in SDS-PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) gels at 12 % and later transferred to nitrocellulose membranes. The latter were blocked for 1 h using 5% non-fat dry milk before being washed three times with a solution of TBS/T (150 mM NaCl, 20 mM Tris, pH 7.4, 0.1% Tween-20) and incubated overnight, at 4 °C, with the primary antibody p-IκBαSer32/36. Enhanced chemiluminescence (PerkinElmer Life Sciences) was used to detect bound peroxidase activity. Finally, membranes were stripped and blocked using 5% milk and later blotted for β-actin to assess equal protein loading.
Neutrophil bactericidal activity assay
Bacterial strain
The antibacterial strength of the plant extract was evaluated on Staphylococcus aureus ATCC43300 strain at different concentrations (2, 1 and 0.5 g/ml) in view to determine the one that has no effect on the viability of the strain.
Determination of the concentration of Antimicrobial activity of ZL extract on Staphylococcus aureus
The Disk diffusion method was used to assess the antimicrobial activity of Ziziphus lotus extract on Staphylococcus aureus ATCC43300 strain. The bacteria were cultured in Nutritive broth overnight, at 37°C and diluted to a final concentration of 107 CFU/ml.
Müeller Hinton agar plates were inoculated using a cotton swab. Sterile paper discs embedded with 20 µl of different concentrations of the plant extract (at 2; 1 or 0.5 g/ml) were then placed on the plates. Sterile discs embedded with sterile distilled water were used as negative control. Positive control consisted of Amoxicillin at 0.05%.
After an incubation for 24h at 37 °C, the inhibition’s zones diameters were measured. This enabled to classify the sensitivity of the bacteria to ZL extract (<8 mm diameter, non-sensitive; sensitive for diameters between 9 mm and 14 mm; very sensitive for diameters > 15 mm).
Isolation of human neutrophils
Human Neutrophils were extracted from blood collected from volunteers using the method described by Boyum 27 and adjusted by Kobayashi et al. 28. Human blood collected in heparinized tubed was obtained from healthy donors after they provided a signed informed consent.
The blood was mixed with a 0.9% solution of sodium chloride containing 3% Dextran T-500 (Pharmacia) and incubated at room temperature for 20 minutes to enable sedimentation of erythrocytes. The supernatant (rich with leukocytes) was centrifuged for 10 min at 550 g, then the pellet was suspended in 35 ml of a solution of sodium chloride at 0.9 % and undeplayed with 10 ml of Ficoll-Paque (1.077 g/l, Pharmacia). The mix was then centrifuged for a duration of 30 minutes to extract the PMNs. The interface layer was removed and the residual red blood cells were destroyed by hypotonic lysis. PMNs were finally suspended in RPMI 1640 medium (GIBCO), buffered with Hepes at a concentration of 10 mM, and kept on ice. PMNs were numbered in 2% acetic acid, using a hemacymeter. Slides were prepared and stained with a modified Wright-Giemsa (Sigma). Each preparation of PMNs had a final rate of 95–98% neutrophils.
In vitro treatment of neutrophils with ZL aqueous extract
A volume of 50 ml of 1 x 107 neutrophils /ml in RPMI containing Fetal Bovine Serum (5%) and 50 ml of ZL extract at the following final concentrations: 1g/ml; 0.5 g/ml and 0.1 g/ml was incubated 37°C for a duration of 30 minutes in wells of a flat bottom 96 well plates. The control neutrophils were only incubated in RPMI. All experiments were performed in triplicate.
Colorimetric bactericidal assay
Staphylococcus aureus was first pre-cultured at 37°C for 18h in Nutrient Broth to obtain a concentration of 1 x 108 Bacteria /ml. It was then opsonized in RPMI 1640 (Dubelco) at 37°C for a duration of 20 minutes using autologous inactivated human serum. The neutrophils treated with ZL as well as the non-treated Neutrophils were supplemented with a volume of 50 ml/well of S. aureus. Plates were then incubated at 37°C for a duration of 1h under agitation to permit bactericidal activity of neutrophils. The previously opsonized bacteria were diluted to concentrations of 0, 30, 60 and 90% in RPMI and co-incubated in plates as a base to enable the construction of a standard curve of bactericidal activity. Neutrophils were lysed by using 50 ml of a solution of Triton X-100 at 0.2% in PBS and kept on ice for 5 min. The neutrophils and bacteria were triturated in the well. Later, each well was supplemented with a solution of 2 mg/ml of MTT. The plates were then incubated at room temperature for a duration of 10 minutes and then centrifuged for 5 minutes at 1600 g. The pellet was dissolved with a solution of DMSO (150 ml). After an incubation at room temperature for 10 minutes, the plates were shaken to solubilize the formazan and 50 ml of PBS was added to dissolve the residual formazan.
The OD measured at 560 nm enabled the quantification of bacteria produced formazan. OD equivalent to 0 – 90% killing bacteria was established by linear regression analysis using a standard curve. Neutrophils incubated with opsonized bacteria alone were used as positive control. The percentage of killed bacteria was determined using the following formula:
Statistical analysis
All experiments were performed 3 times and the results are represented as mean ± SEM (n ³3)
The significance of the difference between all values was determined using ANOVA, with a significance level of P<0.05.
Results
Yields percentages and qualitative Phytochemical screening
The yield percentage of ZL aqueous extract was 54, 08% ± 3,57. Preliminary quantitative phytochemical screening of aqueous extract of Ziziphus lotus fruit pulp showed the presence of gallic tannins, phenols and flavonoids.
Effect of ZL extract on humoral immunity
The effect of Ziziphus lotus’ aqueous extract on the production of antibodies in mice was carried out. Results are shown in figure 1. A decrease of 6-times, in titer values, of antibodies of mice treated with ZL extract (8192) when compared to those of the untreated mice (54613) was highlited. The extract of Ziziphus lotus has shown a significant (P<0.05) immunosuppressive effect on humoral immune response in vivo.
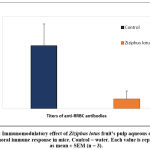 |
Figure 1: Immunomodulatory effect of Ziziphus lotus fruit’s pulp aqueous extract on the humoral immune response in mice. |
Control = water. Each value is represented as mean ± SEM (n = 3).
Effect of ZL extracts on NF-kB signaling pathway activation
The effect Ziziphus lotus fruit’s aqueous extract on NF-kB signaling pathway activation was evaluated. NF-kB is a transcription factor that plays key roles in many cellular phenomena, including cell proliferation and immunity. As shown in figure 2, ZL extract activates NF-kB pathway by phosphorylating IκBα. When phosphorylated, IκBα is conducted to proteasome degradation which allows NF-kB activation.
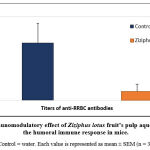 |
Figure 2: Ziziphus lotus fruit’s pulp aqueous extract activates NF-kB pathway activation |
Figure represents the mean of data of overlay blots, expressed as arbitrary units in optical density, as compared to control (n = 3, * and **P<0.05 vs. control).
Antioxidant activity of ZL extract
In the present study, the extract of Ziziphus lotus fruit pulp showed a great antioxidant activity with an IC50 value of 11.26 mg/ml which was 10-times higher (P<0.05) than IC50 of the positive control, ascorbic acid (0,152 mg/ml) (Table 1).
Table 1: IC 50 (µg/ml) values of aqueous extract of Ziziphus lotus fruit pulp compared to ascorbic acid
| IC 50 values | |
| ZL extract | 11.26 mg/ml |
| Ascorbic acid | 0.152 mg/ml |
In vitro hemolytic activity
The aim of this experiment was to evaluate whether ZL’s extract had a hemolytic effect, in vitro, on erythrocytes. Results are shown in figure 3. The extract shows anti-hemolytic effects at high concentrations. Indeed, maximum percentage hemolytic activity on HRBC (75.96 ± 1.75%) was obtained in the presence of the lowest concentration of the extract (10mg/ml), while the higher concentrations of ZL extract tested (50-1000 mg/ml) seems to have an anti-hemolytic activity that was dose dependent. The highest anti-hemolytic activity (12.9 ± 1.72 %) was observed in the presence of a concentration of 500 mg/ml of the extract.
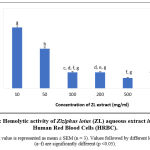 |
Figure 3: Hemolytic activity of Ziziphus lotus (ZL) aqueous extract in vitro on Human Red Blood Cells (HRBC). |
Each value is represented as mean ± SEM (n = 3). Values followed by different letters (a–f) are significantly different (p <0.05).
No significant difference was observed (P>0.05) in the hemolytic activity of the extract when HRBC were treated with ZL extracts concentrations higher than 200 mg/ml.
ZL’s aqueous extract seems to have an anti-hemolytic activity in a dose dependent way.
Effect of ZL extract on Staphylococcus aureus
Ziziphus lotus’ fruit aqueous extract was explored to evaluate its antibacterial activity against Staphylococcus aureus using the disk diffusion method. At a concentration of 2 g/ml, the extract showed an antibacterial effect on the bacterial strain with inhibition diameters of 12.67 ± 0.6 mm. This antibacterial activity is weaker than the one displayed by the positive control, amoxicillin (18.3 ± 1.5 mm) (Table 2). Of note that at lower concentrations (1 and 0.5 g/ml), the extract did not show any antibacterial activity on Staphylococcus aureus.
Table 2: Inhibition diameters values of ZL aqueous extract and amoxicillin on Staphylococcus aureus. Amoxicillin was considered as positive control.
| Inhibition diameters | |
| ZL extract (2mg/ml) | 12.67 ± 0.6 mm |
| Amoxicillin | 18.3 ± 1.5 mm |
Neutrophil bactericidal activity
The incubation of neutrophils for a duration of 30 min with 0.1; 0.5 and 1 g /ml in of Ziziphus lotus’ fruit pulp extract displayed an inhibition of the bactericidal activity of Neutrophils. Results are shown in figure 4. The inhibitive effect was dose dependent as it increased with the concentrations of ZL. Indeed, neutrophils treated with 0.5 g / ml were able to kill 51.06 ± 2.5 % of bacteria and the ones treated with 1g/ml showed 4.99 ± 0.9 % of killed bacteria. A treatment with 0.1 g/ml (67.8 ± 8% of killed bacteria) did not show any significant effect on the bactericidal activity of neutrophils compared to untreated neutrophils (74.43 ± 2.6 % of killed bacteria).
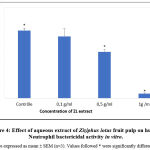 |
Figure 4: Effect of aqueous extract of Ziziphus lotus fruit pulp on human Neutrophil bactericidal activity in vitro. |
A significant suppression (P£0.05) of the bactericidal activity (compared to the non-treated neutrophils) was reached in the presence of 0.5 and 1 g/ml of the aqueous extract,
Discussion
In the current study, we evaluated the effect of ZL’s fruit pulp aqueous extract on multiple aspects of the immune responses in vivo and in vitro. Antioxidants have been considered as immunomodulatory molecules 29. Reactive oxygen species (ROS) and free radicals are a group of molecules resulting from the partial reduction of oxygen. They serve many physiological functions by participating to the defense of the body against foreign particles and by controlling biological processes like apoptosis and cell proliferation. ROS also play an important role in the immune system. Indeed, phagocytosis by macrophages and neutrophils is made possible by the production of ROS. However, to maintain body’s homeostasis, ROS levels need to be balanced. When the levels increase and accumulate beyond the needs of the cells, their signaling pathways get altered, causing pathologies like cardiomyopathies and auto-immune pathologies 30, 31.
Antioxidants are molecules capable of suppressing ROS actions by inhibiting specific oxidizing enzymes. Researchers have shown an increasing interest in natural antioxidants because of their abundance and their potency. A wide range of antioxidants can be found in medicinal plants and foods 32.
Among the identified phytochemical molecules, flavonoids and phenolics molecules show the most effective antioxidant properties. Flavonoids possess a structure that is considered ideal for scavenging free radicals as they present hydroxyls that can act as hydroxyl-donors, making them antioxidant agents. Phenolic compounds’ antioxidant activity rests on their redox activities.
DPPH free radical scavenging test is one among the most widely employed tests to evaluate the antioxidant activity of extracts from plants due to its stability and reproducibility. This test is based on the aptitude of the tested compound to donate an atom of hydrogen, reducing to yellow the purple color of DPPH. In the current study, this test highlited that the aqueous extract of ZL’s fruit pulp has an antioxidant activity ten times higher than that of ascorbic acid. Abdelhafidh et al. 20 have shown that ZL’s fruit extract has a protective activity against oxidative stress induced by Cypermethrin in mice. This protection might be due to the antoxidant properties of the extract its antioxidant property as well as its ability to scavenge active free radicals. Our study confirms this ability of ZL’s fruit aqueous extract.
The results can be attributed to the presence of flavonoids and phenolic components in the aqueous extract of Ziziphus lotus’s fruit pulp which was assessed by a phytochemical screening and described in other studies 33.
Benammar et al.19 have highlited comparable results, attributing the antioxidant activity of aqueous extract of different parts of ZL (leaves, roots, stem, seed and fruit pulp) to the presence of Vitamins A and C. Dahiru and Obidoa 34 have shown that flavonoids, tannins and phenolic compounds of Ziziphus mauritania have protective effects against oxidative damage induced by alcohol administration in rats. Ziziphus mauritania Lam and Ziziphus oenoplia (L) were also described as having strong antioxidant activities 35. These preliminary results suggest that Ziziphus lotus L. (Desf.) can be considered as a good source of antioxidants.
The inhibition of neutrophils’ bactericidal activity highlited in our study can also be linked to the presence in ZL’s extract, of antioxidant components. These results are in accordance with those highlited of Ciz et al. 36 who demonstrated comparable data by showing the ability of flavonoids to inhibit neutrophil’s respiratory burst.
Neutrophils represent an essential part of the body’s defense against foreign pathogens. These molecules use different mechanisms to destroy invading microorganisms, among which, production of ROS. Just like the phagocytosis of bacteria, neutrophils are able to release reactive chemical species: it’s the oxidative burst. The latter generates H202 that forms with Cl– and myeloperoxidase effective bactericidal agents 37, 38.
It was also highlighted in the current study that ZL extract shows an anti-hemolytic activity. The membrane of erythrocytes is mainly composed of proteins and lipids, disposed in such a way as to enable a reversible deformation of red blood cells while maintaining their integrity [40]. One of the mechanisms that could cause the hemolysis of erythrocytes is the oxidation of proteins and lipids, and more specifically, lipids peroxidation. Lipid peroxidation is a reaction in chain of free radicals, resulting in the deterioration of RBC’s membranes, leading to hemolysis. Costa et al. 39 highlited that free radicals could be inactivated in presence of flavonoids and phenols, leading to the decrease of oxidative hemolytic activity. These results are in accordance with the anti-hemolytic activity of ZL extract highlighted here.
Another mechanism used by flavonoids and phenols to achieve their anti-hemolytic effect is by interfering in the conformity of the membrane of erythrocytes. Different studies highlited that certain flavonoids and polyphenols interact with the membrane of erythrocytes, decreasing its fluidity and reducing the diffusion of free radicals. 40, 41.
Benammar et al. [19] have observed that aqueous extract of different parts of ZL exerted immunosuppressive effect on T-cell proliferation with seed extracts showing the most effective immunosuppressive activity on IL-2 mRNA expression and T cell proliferation in vitro.
The effects of ZL extract in vivo have never been tested on the immune responses. In the present study we show that the ZL fruit pulp extracts inhibited or decreased the production of antibodies in mice when these are challenged by an antigen with a dose dependent effect. This response is linked to the activation and amplification of T lymphocytes clones. If this extract has an anti-proliferative effect on T cells [19] that can explain the decrease of antibodies production in animals challenged with ZL fruit pulp extract compared to controls. Our results are in accordance with those of Adhvaryu et al. 42. Indeed, they have shown that extracts of Ziziphus exert immunomodulatory activities on guinea pigs. Here, we also showed that Ziziphus lotus fruit’s aqueous extract activates NF-kB signaling pathway NF-kB by phosphorylating IκBα. The latter, when phosphorylated is degraded by proteasome allowing NF-kB activation.
When activated, NF-kB a transcription factor, regulates many cellular phenomena including cell proliferation and immune response.
In conclusion, the ZL fruit pulp aqueous extract has an immunosuppressive activity on the innate and adaptive immunity by its anti-oxidative activity, its decreasing or inhibiting of neutrophil bactericidal activity, its hemolytic activities and the decrease of humoral immune responses.
To our best knowledge, no study has, been carried out as yet on the effects on autoimmune disease of Zizyphus lotus L. (Desf.). Additional studies should be undertaken to clarify the effects of the different plant extracts in the evolution of autoimmune diseases.
Acknowledgment
The authors thank the Moroccan Ministry of High Education and Hassan II University of Casablanca for their financial contribution.
Conflict of Interest
There are no conflict of interest.
Funding Source
This work was supported by grants N/Ref. 2587/22 from the Moroccan Ministry of Higher Education and Research.
References
- Bellakhdar J, Claisse R, Fleurentin J, Younos C. Repertory of standard herbal drugs in the Moroccan. J. Ethnopharmacol. 1991; 35: 123–143.
CrossRef - El Hilaly J, Hmammouchi M, Lyoussi B. Ethnobotanical studies and economic evaluation of medicinal plants in Taounate province (Northern Morocco). J Ethnopharmacol. 2003 86.
CrossRef - Kabbaj FZ, Meddah B, Cherrah Y, Faouzi MEA. Ethnopharmacological profile of traditional plants used in Morocco by cancer patients as herbal therapeutics. Phytopharmacology. 2012; 2(2): 243–256.
- Fakchich J, Elachouri M. Ethnobotanical survey of medicinal plants used by people in Oriental Morocco to manage various ailments. J Ethnopharmacol. 2014; 154:76-87.
CrossRef - Neffati M, Najjaa H, Mathé A, editors, Medicinal and Aromatic Plants of the World-Africa. Volume 3. Netherlands: Springer; 2017.
CrossRef - Ghedira K, Chemli R, Caron C, Nuzillard JM, Zeches M, Le Men-Olivier L. Four cyclopeptide alkaloids from Zizyphus lotus. Phytochemistry 1995; 38:767–72.
CrossRef - Renault JH, Ghedira H, Thepenier P, Lavand C, Zeches-Hanrot M, Le Men-Olivier L. Dammarane saponins from Zizyphus lotus. Phytochemistry 1997; 4:1321–7.
CrossRef - Borgi W, Recio MC, Ríos JL, Chouchane N. Anti-inflammatory and analgesic activities of flavonoid and saponin fractions from Zizyphus lotus (L.) Lam. S Afr J Bot. 2008; 74:320–4.
CrossRef - Ghazghazi H, Aouadhi C, Riahi L, Maaroufi A, Hasnaoui B. Fatty acids composition of Tunisian Ziziphus lotus (Desf.) fruits and variation in biological activities between leaf and fruit extracts. Nat Prod Res. 2014; 28(14):1106-10.
CrossRef - Adeli M, Samavati V. Studies on the steady shear flow behavior and chemical properties of water-soluble polysaccharide from Ziziphus lotus Int J Biol Macromol. 2015; 72:580-7.
CrossRef - Abdoul-Azize S, Bendahmane M, Hichami A. Effects of Zizyphus lotus (Desf.) polyphenols on Jurkat cell signaling and proliferation. International Immunopharmacology. 2013; 15(2):364–371.
CrossRef - Wahida B, Abderrahman B, Nabil C. Antiulcerogenic activity of Zizyphus lotus (L.) extracts. J Ethnopharmacol. 2007 Jun 13;112 (2):228-31.
CrossRef - Lahlou M, El Mahi M, Hamamouchi J. Evaluation of anti-fungal and mollusuicidial activities of Moroccan Zizyphus lotus (L.) Desf. Ann Pharm Fr. 2002; 60: 410–4.
- Berrichi M, Benammar C, Murtaza B, Hichami A, Belarbi M, Khan NA. Zizyphus lotus fruit attenuates obesity-associated alterations: in vivo mechanisms. Arch Physiol Biochem. 2019; 1:1-8.
- Borgi W, Bouraoui A, Chouchane N. Anti-ulcerogenic activity of Zizyphus lotus (L.) extracts. J Ethnopharmacol. 2007; 112:228–31
CrossRef - Borgi W, Chouchane N. Antispasmodic effects of Zizyphus lotus (L.) Desf. extracts on isolated rat duodenum. J Ethnopharmacol. 2009; 126(3):571-3.
CrossRef - Bakhtaoui FZ, Lakmichi H, Megraud F, Chait A, Gadhi CEA. Gastro-protective, anti-Helicobacter pylori and antioxidant properties of Moroccan Zizyphus lotus Journal of Applied Pharmaceutical Science. 2014; 4(10):81–87.
CrossRef - Benammar C, Baghdad C, Belarbi M, Subramaniam S, Hichami A, Khan NA. Antidiabetic and antioxidant activities of Zizyphus lotus L aqueous extracts in Wistar rats. J Nutr Food Sci. 2014; S8 :004.
CrossRef - Benammar C, Hichami A, Yessoufou A, Simonin AM, Belarbi M, Allali H, Khan NA. Zizyphus lotus (Desf.) modulates antioxidant activity and human T-cell proliferation. BMC Complement. Altern. Med. 2010; 10:1–9.
CrossRef - Abdelhafidh K, Mhadhbi L, Mezni A, Badreddine S, Beyrem H, Mahmoudi E. Protective effect of Zizyphus lotus jujube fruits against cypermethrin-induced oxidative stress and neurotoxicity in mice. 2018; 23(2):167-173.
CrossRef - Devi MA, Das NP. In vitro effects of natural plant polyphenols on the proliferation of normal and abnormal human lymphocytes and their secretions of interleukin-2. Cancer Lett. 1993; 69:191–6.
CrossRef - Aziz NH, Farag SE, Mousa LAA, Abo-Zaid MA. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998; 93(374):43–54.
- Chan AS, Yip EC, Yung LY, Pang H, Luk SC, Pang SF, Wong YH: CKBM stimulates MAPKs but inhibits LPS-induced IFN-gamma in lymphocytes. Phytother Res. 2006; 20:725-731.
CrossRef - Roy MK, Koide M, Rao TP, Okubo T, Ogasawara Y, Juneja LRORAC and DPPH assay comparison to assess antioxidant capacity of tea infusions: relationship between total polyphenol and individual catechin content. Int J Food Sci Nutr. 2010 Mar;61(2):109-24. doi: 10.3109/09637480903292601.
CrossRef - Bin-Hafeez B, Ahmad I, Haque R, Raisuddin S. Protective effect of Cassia occidentalis L. on cyclophosphamide-induced suppression of humoral immunity in mice. J Ethnopharmacol. 2001 Apr;75(1):13-8.
CrossRef - Bulmus V, Woodward M, Lin L, Murthy N, Stayton P, Hoffman A. A new pH-responsive and glutathione-reactive, endosomal membrane-disruptive polymeric carrier for intracellular delivery of biomolecular drugs. J Control Release. 2003 Dec 5;93(2):105-20.
CrossRef - Böyum A. Isolation of mononuclear cells and granulocytes from human blood: Isolation of mononuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968; 97:77-89.
- Kobayashi SD, Voyich JM, Buhl C. L., Stahl RM, DeLeo FR. Global changes in gene expression by human polymorphonuclear leukocytes during receptor-mediated phagocytosis: cell fat is regulated at the level of gene expression. Natl. Acad. Sci. U.S.A. 2002; 99, 6901–6906.
CrossRef - Bendich A. Physiological role of antioxidants in the immune system. J Dairy Sci. 1993 Sep;76(9):2789-94.
CrossRef - Soobrattee M, Bahorun T, Aruomab O. Chemopreventive actions of polyphenolic compounds in cancer. BioFactors. 2006; 27: 19–35.
CrossRef - Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell signaling. 2012; 24(5): 981–990.
CrossRef - Kalt W, Forney CF, Martin A, Prior RL. Antioxidant capacity, vitamin C, phenolics and anthocyanins after fresh storage of small fruits. J Agric Food Chem. 1999; 47:4638–4464.
CrossRef - Mohandas N, Gallagher P. Red cell membrane: past, present and future. Blood. 2008; 112: 3939-3948.
CrossRef - Dahiru D, Obida O. Evaluation of the antioxidant effects of Ziziphus mauritania Leaf extracts against chronic ethanol-induced hepatotoxicity in rat liver. Afr J Tradit Complement Altern Med. 2008; 5(1): 39–45.
CrossRef - Siddiqui S, Patil M. Assessment of antioxidant and cytotoxic activities of extracts of some Ziziphus species with identification of bioactive components. European J Med Plants. 2016; 8(4): 202-213.
CrossRef - Ciz M, Denev P, Kratchanova M, Vasicek O, Ambrozova G, Lojek A. Flavonoids inhibit the respiratory burst of neutrophils in mammals. Oxid Med Cell Longev. 2012 ; 181295.
CrossRef - Chen Y, Junger WG. Measurement of Oxidative Burst in Neutrophils, Methods Mol Biol. 2012; 844: 115-124.
CrossRef - Teng TS, Ji AL, Ji XY, Li YZ. Neutrophils and Immunity: Form bactericidal action to being conquered, Journal of Immunology research. 2017; 9671604.
CrossRef - Costa D, Moutinho L, Costa Lima JLF, Fernandes E. Antioxidant Activity and Inhibition of Human Neutrophil Oxidative Burst Mediated by Arylpropionic Acid Non-steroidal Anti-inflammatory Drugs. Biol. Pharm. Bull. 2006; 29(8): 1659-1670.
CrossRef - Chaudhuri S, Banerjee A, Basu K, Sengupta B, Sengupta PK. Interaction of flavonoids with red blood cell membrane lipids and proteins: Antioxidant and antihemolytic effects. Int J Biol Macromol. 2007; 41: 42-48.
CrossRef - Singh N, Rajini PS. Antioxidant-mediated protective effect of potato peels extract in erythrocytes against oxidative damage. Chemico-Biological Interactions. 2008; 173: 97-104.
CrossRef - Adhvaryu MR, Reddy N, Parabia MH. Effects of four Indian medicinal herbs on Isoniazid-, Rifampicin and Pyrazinamide-induced hepatic injury and immunosuppression in guinea pigs. World J Gastroenterol. 2007 ; 13(23): 3199-205.
CrossRef