Aditya Sharma1* and Navneet Verma2
and Navneet Verma2
1School of Pharmaceutical Sciences, IFTM University, Moradabad-244102, Uttar Pradesh, India.
2Pharmacy Academy, IFTM University, Moradabad-244102, Uttar Pradesh, India,
Corresponding Author E-mail: aditya_iftm@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2534
Abstract
Diclofenac Diethylamine has been used generally in the management of pain and inflammation caused by musculoskeletal disorders. The current study revealed the preliminary investigation into the analgesic and anti-inflammatory activities of an optimized double-layered transdermal patch of Diclofenac Diethylamine. The patch was prepared by using the hydrophobic acrylic polymer Eudragit RL 100 and the hydrophilic polymer Polyvinyl pyrrolidone K-30 in combination as the first layer of matrix type and pressure-sensitive acrylic adhesive Duro Tak 387-2510 as the second layer of drug-in-adhesive type patch. The solvent casting method was employed to prepare the transdermal patch over the backing membrane. We optimized the patch in terms of its concentration based on results exhibited by ex-vivo and in-vitro studies using FDC and the rat’s skin. This study was designed to assess the analgesic and anti-inflammatory effects of an optimized patch with the respective models in laboratory animals. In a comparison of the developed transdermal patch with commercially available Diclofenac Diethylamine gel (Volini gel), the developed patch was found to be statistically significant (p < 0.01) and effective for decreasing pain and inflammation symptoms. The findings of the study suggest that the prepared double-layered transdermal patch of Diclofenac Diethylamine can serve as the best carrier to provide a sustained effect for the management of pain and inflammation.
Keywords
Analgesic; Anti-inflammatory; Diclofenac Diethylamine; Double-layered Transdermal Patch; Musculoskeletal disorders
Download this article as:| Copy the following to cite this article: Sharma A, Verma N. Assessment of Analgesic and Anti-inflammatory Activity of Double-layered Diclofenac Diethylamine Transdermal Patch. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Sharma A, Verma N. Assessment of Analgesic and Anti-inflammatory Activity of Double-layered Diclofenac Diethylamine Transdermal Patch. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3UEvj34 |
Introduction
Most diseases manifest themselves through inflammation and pain. An immune system is responsible for the destruction of injurious stimuli as well as for the initial healing of tissue after an injury 1. Pain and inflammation associated with musculoskeletal (MSK) disorders are usually reduced with nonsteroidal anti-inflammatory drugs (NSAIDs) 2. Diclofenac is a frequently used drug having additional pain-relieving and antipyretic action 3. It performs a crucial task in the management of MSK disorders caused by a variety of etiological illnesses, such as rheumatoid arthritis and osteoarthritis. The inhibition of an enzyme namely cyclooxygenase (COX), specifically COX 1 or COX 2, assists in the anti-inflammatory process which reduces prostaglandin synthesis at the inflammation site 2, 4. Diethylammonium salt of Diclofenac (Diclofenac Diethylamine) is said to be utilized for topical treatments. Diclofenac Diethylamine (DDA) is present in the formulations of Volini®, Voltaren®, and Emulgel®, and has been one of the most widely used topical treatments in Europe since 1985 for pain relief, inflammation, and muscle and joint problems. Diclofenac is usually preferred as an oral formulation (100 to 150 mg with 2 to 3 divided dosages) and is capable of achieving good absorption through the gastrointestinal system. However, it goes through a lot of first-pass metabolisms and causes stomach irritation which can lead to nausea, bleeding, abdominal pain, and ulcers, making it unsuitable for long-term use 3-6. Therefore, it is necessary to develop an alternative route of administration for diclofenac to avoid oral complications.
In comparison with conventional dosage forms, transdermal delivery is becoming increasingly popular for analgesics, NSAIDs, hormones, scopolamine, and nicotine 7-9. Transdermal delivery offers various advantages like avoiding first-pass hepatic metabolism which significantly improves the bioavailability of the drug 10. However, these are not without challenges when attempting to exploit this route for drug absorption and permeation. Transdermal delivery is hindered by the stratum corneum which works as a barrier to percutaneous penetration into the skin. But it can be improved by several approaches such as iontophoresis, electroporation, and microneedles 11, 12. Transdermal formulations must be pleasant to touch and feel or else the patient will reject them instantly 12. Thus, it is essential to develop transdermal formulations that are effective, safe, and economical.
Numerous studies have been conducted to enhance the transdermal delivery of diclofenac and other drugs 13-17, including EC-PVP-based patches that enhances glibenclamide permeation by different oils 15 which facilitates the transdermal delivery system. Additionally, bioavailability studies suggested that diclofenac is also proving feasible by achieving IVIVC when delivered via a topical route 16 which indicates transdermal delivery could be a noteworthy approach for future research and development.
As a result, compared to oral diclofenac, transdermal patches have been shown to improve patient compliance 6, 17.
Dimethyl sulphoxide (DMSO) acts as a most common penetration enhancer (PE). DMSO facilitates safe and effective transdermal delivery of hydrophilic and lipophilic drugs to provide localized delivery 18,19.
The present study is aimed at developing a sustained release DDA double-layered transdermal patch using hydrophobic acrylic polymer Eudragit RL100 (ERL 100) and non-acrylic hydrophilic polymer Polyvinyl pyrrolidone (PVP K-30) in combination as the first layer of a matrix type patch and pressure sensitive acrylic adhesive Duro Tak 387-2510 (DT-2510) as the second layer of a drug-in-adhesive type patch. The patch was assessed for anti-inflammatory and analgesic properties by acetic acid-induced writhings and carrageenan-induced paw edema models in laboratory animals, respectively.
Materials and Methods
Materials
DDA was gifted by SGS Pharmaceuticals Pvt. Ltd., Roorkee, U.K.; ERL 100, PVP K-30, DMSO, Cetyl alcohol, Chloroform, di-butyl phthalate (DBP), Ethyl acetate was obtained from Merck Ltd., India; DT-2510 was from Henkel, Belgium; and 3M Scotchpak TM 9723 backing polyester membrane from 3M Food Safety Division, Healthcare Business, India.
Preparation of double-layered transdermal patches of DDA
In the first layer (matrix type) of the optimized double-layered DDA transdermal patch, the solvent casting method was employed to prepare the patch 20 by using a 7:3 ratio of ERL 100 and PVP K-30 respectively, and drug DDA fixed at 100 mg. The forming solution was prepared by dissolving an accurately weighed quantity of polymers followed by DDA in 10 ml of chloroform (solvent) using a magnetic stirrer with Teflon-coated magnetic micro bead for 2 hr following the addition of PE i.e. 10 % (of the weight of the total polymer) DMSO and 40 % (of the polymers weight) plasticizer (DBP) under 50 rpm constant stirring at RT. Continuous stirring of the mixture was done in such a manner to prevent the solvent from pre-evaporating or to keep it at a minimum. The polymeric solution was then poured onto the 3M Scotchpak TM 9723 backing polyester membrane using an inverted funnel placed over it to acknowledge controlled evaporation of the solvent in an oven for 4 hr at 37 ± 5°C.
The spreading method was used to prepare the second layer (drug-in-adhesive) of the double-layered DDA transdermal patch 21. A polymeric solution was prepared by dissolving 78 % (of the total weight of polymeric solution) of adhesive DT-2510 (optimized concentration by preliminary trials) in 10 ml of ethyl acetate followed by the addition of 2 % cetyl alcohol, and 75 mg DDA with 10 % DMSO; and cast onto already prepared first layer (matrix type) of the patch. This polymeric solution was again permitted to assist the well-ordered evaporation of ethyl acetate in an oven for 2-3 hr at 37 ± 5°C to remove the residual organic solvents, then stored in a desiccator overnight. The prepared patch is presented in Fig. 1.
 |
Figure 1: Image of Prepared Double-layered DDA Transdermal Patch |
In vivo pharmacodynamics study
Animals: 18 mice (weighing between 22-30 g) and 18 albino Wister rats (weighing between 165-225 g) of either sex were used to perform the analgesic and anti-inflammatory activities of the prepared double-layered DDA transdermal patch in comparison to the marketed formulation i.e. Volini gel. All the animals were housed in polypropylene cages (6/cage) with distilled water and required food on a daily basis at standard conditions (temperature 25 ± 2°C and humidity) as per CPCSEA guidelines 22, 23. Animals were handled and used according to institutional and national guidelines. The ethical approval for animal experimental work was received by Institutional Animal Ethical Committee, IFTM University, Moradabad vide resolution no. 2021/837ac/Ph.D/15 following the OECD guidelines.
Assessment of Analgesic Activity
The acetic acid-induced writhings model was adopted to assess the analgesic effect of the DDA transdermal patch for the management of pain 24, 25. Mice (22-30 g) were gathered into 3 different groups (each containing 6 mice). Group details are as follows:
I (Control group): No administration
II (Treated Transdermal Patch): DDA patch (2 cm2)
III (Volini gel): Reference for comparison
The hairs of mice’s abdominal skin were trimmed 12 hours prior to start the activity. The abdominal writhing was introduced by giving an injection of acetic acid (0.6% in distilled water, 0.1 ml/g) intraperitoneally to all mice after 2 hours of the application of a 2 cm2 transdermal patch and Volini gel as per the respective group. To evaluate the analgesic effect of the patch, we counted the number of writhing sessions that took place after a latency period of 5 minutes, which involves abdominal muscle contraction and hind limbs stretching throughout the 20 minutes 22. The analgesic effect of the transdermal patch was demonstrated by the reduction in the number of writhes (contractions) as compared to the control group, and percent pain inhibition (PPI) was calculated according to the following formula 26:
W is the mean counted number of writhing.
Anti-Inflammatory Activity
The prepared transdermal patch was used for anti-inflammatory effects by the carrageenan-induced rat paw edema method using a plethysmometer 27, 28. The albino rats were divided into 3 different groups (each containing 6 animals), similarly as grouped for analgesic activity. Volini gel was used as a reference for comparison. Following the application of the patch for 2 hours, the subplantar region of the right hind paw of the rat was injected subcutaneously with 0.1 ml of freshly prepared carrageenan suspension in saline at a concentration of 1 % w/v to produce acute inflammation. After putting carrageenan 29 under the skin of rats, the volume of their paws was measured at 1, 2, 4, 6, 8, and 24 hours and the percent inhibition was calculated according to the following formula:
V (control) and V (treated) are the mean paw volumes of the control and treated groups of animals, respectively.
Result
The acetic acid writhing test and carrageenan-induced paw edema test allowed us to evaluate the Double-layered DDA Transdermal Patch, which are commonly used to investigate analgesic and anti-inflammatory activities.
Analgesic Activity
According to the result and test, an optimistic analgesic effect with a statistically reduced mean number of abdominal contractions (13.87) was observed when the mice with a prepared DDA transdermal patch as compared to the control group; and in comparison, with the marketed preparation of DDA (Volini Gel), statistically no significant variation (P > 0.05) was found between both the groups, as shown in Table 1 and Fig. 2. The percentage pain inhibition (PPI) was reduced by 72.05% with treated transdermal patch, and 73.50% with marketed product (Volini gel) as compared to control group.
Table 1: Results of analgesic effect of DDA transdermal patch and Volini gel on acetic acid-induced pain (n = 6 mice/group)
| Groups | Dose | Number of Writhing (Mean ± SEM) | PPI (%) |
| I (Control- D.W.) | 2 ml/ kg | 49.63 ± 0.08 | – |
| II (Treated Transdermal Patch) | Approx. 10 mg | 13.87 ± 0.06 | 72.05 |
| III (Volini Gel) | Medium of 1.16 % w/w | 13.15 ± 0.05 | 73.50 |
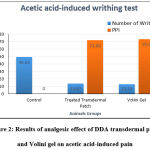 |
Figure 2: Results of analgesic effect of DDA transdermal patch and Volini gel on acetic acid-induced pain |
Anti-inflammatory Activity
DDA has been observed to exert its anti-inflammatory actions via controlling many inflammatory pathways in both acute and chronic inflammation 6, 16. Table 2 and Fig. 2 shows the anti-inflammatory effect of the DDA transdermal patch and Volini Gel in comparison. The results and graphs gives an important data that DDA transdermal patch exhibited a statistically significant reduction in rat paw edema volume in descending order with better % inhibition (63.81 %) at 24 h as compared to the control group. In comparison to the Volini Gel, it also exhibits a reduction in rat paw volume with higher % inhibition (65.57 %) but it was limited at 6 h and it was decreased thereafter upto 36.18 % at 24 with increased rat paw volume.
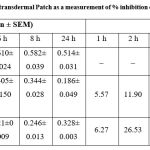 |
Table 2: Results of the anti-inflammatory effect of DDA transdermal Patch as a measurement of % inhibition on carrageenan-induced rat paw edema (n=6 rats/group). |
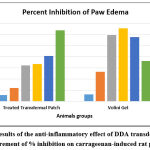 |
Figure 3: Results of the anti-inflammatory effect of DDA transdermal Patch as a measurement of % inhibition on carrageenan-induced rat paw edema. |
Discussion
The administration of DDA patch demonstrated a dose-dependent inhibition for the majority of tests performed in the current study. The acetic acid test is a valid model for testing peripheral and central pain. Additionally, acetic acid is a strong irritant of the epithelial wall, inducing an inflammatory and painful process that may result in the release of substance P 30. Because bradykinin stimulates the formation and release of prostanoids (PGE1, PGI2) from C fibres 31, it is possible that bradykinin, as an endogenous pain transmitter, is also an irritant. On the other hand, Diclofenac is widely used in the treatment and/or management of rheumatoid arthritis, osteoarthritis, and ankylosing spondylitis due to its anti-inflammatory and analgesic effects 32.
The acetic acid-induced writhing test was an effective, useful, and highly sensitive model to assess the analgesic effect of any drug substance. DDA possesses antipyretic and analgesic actions, which are characteristics of other NSAIDs. It works by preventing the formation of prostaglandins, prostacyclins, and thromboxanes, which are crucial elements of the inflammatory and nociceptive responses. An essential mechanism of action for NSAIDs is thought to be the reduction of prostaglandin biosynthesis caused by inhibition of the COX enzyme system 33.
The process of drug permeation was subjected to diffusion and concentration of polymers had a robust impact on the diffusivity. Thus, it was proved that this DDA transdermal patch had sufficient analgesic properties as compared to the control group but had a very little difference when compared with Volini gel. This very small difference seems due to the presence of polymers and different PE. NSAIDs are commonly prescribed for pain management, but antirheumatic drugs could be used to prevent joint destruction and also reduce the risk of joint damage and disability 34, 35.
The most common paradigm for assessing the anti-inflammatory properties of novel drugs is carrageenan-induced rat paw edema 4, 26. When carrageenan is administered, cyclooxygenases, histamine, leukotrienes, 5-hydroxytryptamine, and kinins, are released. The delayed phase of this form of inflammation has been connected to the generation of prostaglandins, neutrophil infiltration, bradykinin, etc. Several mediators and cellular actors are involved in the intricate process of inflammation. In comparison, the anti-inflammatory effects of the DDA transdermal patch and Volini Gel. The results and graphs clearly showed that the DDA transdermal patch reduced rat paw edema volume in descending order with a higher percentage inhibition (63.81%) at 24 h when compared to the control group. In comparison to the Volini Gel, it also shows a reduction in rat paw volume with a higher percentage inhibition (65.57%), but it was limited at 6 h and then decreased to 36.18% at 24 with increased rat paw volume. Consequently, it is a positive sign for the objective of the work because of the aim to provide sustained drug delivery action which was proved by the reduction of paw volume and better % inhibition of the anti-inflammatory activity of prepared transdermal patch as compared to the control group. Also, it has been demonstrated that DDA, a non-selective cyclooxygenase-1/2 inhibitor, can lower the joint inflammation 35, 36. Thus, the prepared system may be considered a more effective anti-inflammatory formulation to offer sustained drug delivery.
Conclusion
The outcomes of this study demonstrated that this optimized, safe, and effective double-layered transdermal patch of DDA presented better analgesic and anti-inflammatory effects as compared to the marketed DDA formulation (Volini Gel) specifically in a sustainable manner. Also, the DDA transdermal patch does not cause any kind of irritation to the animal’s skin (data not shown). Based on the foregoing, DDA patches are indistinguishable from other NSAIDs formulations. So, on behalf of the study, we can say that the possible mechanism is similar to NSAIDs. To conclude, the double-layered technique is viable and promising in the management of MSK disorders such as osteoarthritis and rheumatoid arthritis. Even so, there are some limitations to the study, since it does not include clinical studies because it is necessary to evaluate a drug’s safety for use in humans for general use. Clinical trials may be done in the future to ensure the prepared system’s safety.
Acknowledgement
The authors would like to express their gratitude to the honorable Vice-Chancellor of IFTM University, Moradabad, and the Faculty of Pharmacy, IFTM University, Moradabad, for providing unstinted affectionate support and research facilities. This work is a part of the work done for Ph.D. degree of IFTM University, Moradabad.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
There is no funding sources.
References
- Maldini M, Sosa S, Montoro P, et al. Screening of the topical anti-inflammatory activity of the bark of Acacia cornigera Willdenow, Byrsonima crassifolia Kunth, Sweetia panamensis Yakovlev and the leaves of Sphagneticola trilobata Journal of Ethnopharmacology. 2009; 122(3):430-433.
CrossRef - Ju Z, Li M, Xu J, et al. Recent development on COX-2 inhibitors as promising anti-inflammatory agents: The past 10 years. Acta Pharmaceutica Sinica B. 2022; 12(6):2790-2807.
CrossRef - Nair B, Taylor-Gjevre R. A Review of topical diclofenac use in musculoskeletal disease. 2010; 3(6):1892-1908.
CrossRef - Sugimoto M, Toda Y, Hori M, et al. Topical Anti-Inflammatory and Analgesic Effects of Multiple Applications of S(1)-Flurbiprofen Plaster (SFPP) in a Rat Adjuvant-Induced Arthritis Model. Drug Development Research. 2016; 77:206-211.
CrossRef - Hinz B, Chevts J, Renner B, et al. Bioavailability of diclofenac potassium at low doses. British Journal of Clinical Pharmacology. 2005; 59:80-84.
CrossRef - Fritz UN, Morris SG, Gail SS, et al. Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. The Journal of Rheumatology. 2005; 32:2384-92.
- Yang M, Feng X, Ding J, Chang F, Chen X. Nanotherapeutics relieve rheumatoid arthritis. Journal of Controlled Release. 2017; 252:108-124.
CrossRef - Sharma A, Verma N Chaturvedi S, Prasad N, Rastogi V. A Review on Polymeric Invasive and Non-Invasive Nanocarriers Assisted Transdermal Drug Delivery for Improved Penetration and Bioavailability. Drug Delivery Letters. 2022; 12(1):19-34.
CrossRef - Jeengar MK, Rompicharla SVK, Shrivastava S, et al. Emu oil based nano-emulgel for topical delivery of curcumin. International Journal of Pharmaceutics. 2016; 506(1-2):222-236.
CrossRef - Kandil LS, Hanafy AS, Abdelhady SA. Galantamine transdermal patch shows higher tolerability over oral galantamine in rheumatoid arthritis rat model. Drug Development and Industrial Pharmacy. 2020; 46(6):996-1004.
CrossRef - Okur NU, Yozgatli V, Okur ME, Yoltas A, Siafaka PI. Improving therapeutic efficacy of voriconazole against fungal keratitis: thermo-sensitive in situ gels as ophthalmic drug carriers. Journal of Drug Delivery Science and Technology. 2019; 49:323-333.
CrossRef - Okur NU, Apaydin S, Yavasoglu NUK, Yavasoglu A, Karasulu HY. Evaluation of skin permeation and antiinflammatory and analgesic effects ofnew naproxen microemulsion formulations. International Journal of Pharmaceutics. 2011; 416:136-144.
- Boinpally RR, Zhou SL, Poondru S, Devraj G, Jasti BR. Lecithin vesicles for topical delivery of diclofenac. European Journal of Pharmaceutics and Biopharmaceutics. 2003; 56(3):389-392.
CrossRef - Tavano L, Mazzotta E, Muzzalupo R. Innovative topical formulations from diclofenac sodium used as surfadrug: the birth of Diclosomes. Colloids and Surfaces B: Biointerfaces. 2018; 164:177-184.
CrossRef - Rastogi V, Kumar A, Porwal M, Mishra AK, Rastogi V, Verma N, Verma A. Enhancement of skin permeation of glibenclamide from ethyl cellulose- polyvinyl pyrollidone based transdermal patches using olive oil and mustard oil as penetration enhancer: In-vitro, ex-vivo and in-vivo Drug Delivery Letters. 2015; 5(2):109-121.
CrossRef - Cordery SF, Pensado A, Chiu WS, et al. Topical bioavailability of diclofenac from locally-acting, dermatological formulations. International Journal of Pharmaceutics. 2017; 529(1-2):55-64.
CrossRef - Riecke BF, Bartels EM, Torp-Pedersen S, et al. A microdialysis study of topically applied diclofenac to healthy humans: Passive versus iontophoretic delivery. Results in Pharma Sciences. 2011; 4(1):76-79.
CrossRef - Marwah H, Garg T, Goyal AK, Rath G. Permeation enhancer strategies in transdermal drug delivery. Drug Delivery. 2016; 23(2):564-578.
CrossRef - Otterbach A, Lamprecht A. Enhanced Skin Permeation of Estradiol by Dimethyl Sulfoxide Containing Transdermal Patches. 2021; 13(3):320.
CrossRef - Shinde AJ, Garala KC, More HN. Development and characterization of transdermal therapeutics system of tramadol hydrochloride. Asian Journal of Pharmaceutics. 2008; 2:265-269.
CrossRef - Ganti SS, Bhattaccharjee SA, Murnane KS, Blough BE, Banga AK. Formulation and evaluation of 4-benzylpiperidine drug-in-adhesive matrix type transdermal patch. International Journal of Pharmaceutics. 2018; 550(1-2):71-78.
CrossRef - Tatiya AU, Saluja AK, Kalaskar MG, Surana SJ, Patil PH. Evaluation of analgesic and anti-inflammatory activity of Bridelia retusa (Spreng) bark. Journal of Traditional and Complementary Medicine. 2017; 7(4):441-451.
CrossRef - Santos TN, Costa G, Ferreira JP, et al. Antioxidant, anti-inflammatory, and analgesic activities of Agrimonia eupatoria L. Infusion. Evidence-Based Complementary and Alternative Medicine. 2017; 8309894.
CrossRef - Dannerman PJ. Monitoring of analgesia. In: Kohn D, Wixson S, White W, Benson G. (Eds.). Anesthesia and Analgesia in Laboratory Animals, 1; Academic Press, USA, 1977; pp. 1-133.
- Fyad K, Belboukhari N, Ould El Hadj-Khelil A, Sekkoum K. Analgesic and anti-inflammatory activity of aqueous extract of Bubonium graveolens. Biomedical Research and Therapy. 2020; 7(9):4002-4009.
CrossRef - Zhang Y, Cun D, Kong X, Fang L. Design and evaluation of a novel transdermal patch containing diclofenac and teriflunomide for rheumatoid arthritis therapy. Asian Journal of Pharmaceutical Sciences. 2014; 9(5):251-259.
CrossRef - Winter CA, Risley EA, Nuss GW. Carrageenin-induced Edema in Hind Paw of the Rat as an Assay for Antiinflammatory Drugs. Proceedings of the Society for Experimental Biology and Medicine. 1962; 111(3):544-547.
CrossRef - Shahana Wahid, Ali Alqahtani, Rafeeq Alam Khan. Analgesic and anti-inflammatory effects and safety profile of Cucurbita maxima and Cucumis sativus seeds. Saudi Journal of Biological Sciences. 2021; 28(8):4334-4341.
CrossRef - Vasudevan M, Gunnam KK, Parle M. Antinociceptive and anti-inflammatory effects of Thespesia populnea bark extract. Journal of Ethnopharmacology. 2007; 109(2):264-270.
CrossRef - Laduron P. l’inflammation Neurogenique, livre l’inflammatio. John Libbey eurotext. 2013; pp. 172-185.
- Garret CA, Carruette Fardin V, Moussaoui SM. Pharmacological proprieties of potent and selective nonpeptide substance P antagonist. Proceedings of the National Academy of Sciences. 1991; 88(22):10208-10212.
CrossRef - Small RE. Diclofenac sodium. Clinical Pharmacy. 1989; 8(8):545-558.
- Thenarasu V, Gurunathan D, Selvarasu K. Comparison of Efficacy of Diclofenac and Paracetamol as Preemptive Analgesic Agent. Biomedical & Pharmacology Journal. 2018; 11(3):1699-1706.
CrossRef - Takaaki K, Tsukasa S. Comparison of the efficacy and skin permeability of topical NSAID preparations used in Europe. European Journal of Pharmaceutical Sciences. 2012; 47:890-895.
CrossRef - Fries JF. Current treatment paradigms in rheumatoid arthritis. 2000; 1:30-35.
CrossRef - Elvira E, Calpena AC, Queralt J, Obach R, Domenech J. Assessment of diclofenac permeation with different formulations: anti-inflammatory study of a selected formula. European Journal of Pharmaceutical Sciences. 2003; 19(4):203-210.
CrossRef