Renuka Munshi* , Miteshkumar Maurya
, Miteshkumar Maurya and Pranesh Pawaskar
and Pranesh Pawaskar
Department of Clinical Pharmacology, Topiwala National Medical College and BYL Nair Charitable Hospital, Mumbai, Maharashtra, India
Corresponding Author E-mail: renuka.munshi@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2532
Abstract
Drug safety is an important health concern for every individual on medications. Pharmacovigilance programme focuses on the reporting, evaluation and prevention of any adverse drug reactions and needs the equal support from every stakeholder that includes health care professionals, pharmacists and patients as well. Public participation for reporting Adverse Drug Reactions [ADR] is quite low in developing country like India. Therefore, this study was planned with objective to evaluate awareness and perception about drug safety practice and adverse drug reactions reporting system among the lay population. A validated and Ethics Committee questionnaire was distributed to consenting participants residing in the Mumbai region by reaching to the lay public through the community centers and the responses were collected from year 2019 to 2021 to assess awareness and understanding about drug safety and adverse drug reaction reporting among lay public. A total of 1876 questionnaires were collected with a response rate of 75%. 86.7% of the participants believed drugs can have both benefit and adverse effects. 62.41% participants were unaware of any common drug related side effects. Only 8.04% of the public were aware of adverse drug reactions and reporting same to Adverse Event Monitoring Centre and 98.3% participants never heard of Adverse Event Monitoring Center. 66.57% participants learnt about the Pharmacovigilance program through our survey. Also, it was the patient asking the doctor about potential drug related side effects [37.58%] rather than the other way round [24%]. Although the educational survey created awareness among all participants, a greater impact was seen among the younger generation [18-30 years’ age group] irrespective of their literacy status [p<0.001]. We concluded that the Pharmacovigilance program of any nation needs health care professionals to sensitize the lay public to participate in ADR reporting thereby promote patient safety.
Keywords
Adverse Drug Reaction; National Coordinating Centre; Patient Safety; Pharmacovigilance; Public Awareness; PvPI
Download this article as:| Copy the following to cite this article: Munshi R, Maurya M, Pawaskar P. Public Awareness and Perception About Drug Safety in the Population Residing in Western India - A Questionnaire Based Cross Sectional Survey. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Munshi R, Maurya M, Pawaskar P. Public Awareness and Perception About Drug Safety in the Population Residing in Western India - A Questionnaire Based Cross Sectional Survey. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3SVA3QN |
Introduction
The Pharmacovigilance Program of India [PvPI] was established in 2010. The main objective of the PvPI is to detect, assess and take measures to prevent adverse effects to ensure patient wellbeing and safety1. Lots of effort has been taken to train the health care professionals such as doctors, dentist, nurses, pharmacists to understand the importance of monitoring and reporting of adverse drug reactions. However, drug consumers or patients are the least focused groups for training on ADR reporting due to widespread population. Various confounding factors like educational status, knowledge about the medications and disease, languages and variable socio-economic status can impact the understanding and perception of the public towards the drug safety practices2. There are constant efforts invested by the Indian Pharmacopoeia Commission, Ghaziabad, Government of India, the National Coordinating Center for the Pharmacovigilance program of India to inculcate the practice of Adverse Drug Reaction [ADR] reporting through health advertisements, newsletters and training and awareness campaigns conducted by the various Adverse Drug Reactions Monitoring Centres [AMCs] all over the country3. Any reaction occurring after drug intake remains under-reported by patients and/or drug consumers in India which highlights the failure of the PvPI system in reaching out to the general population despite emphasis on mass educational efforts since 2010. In contrast, consumers in the United States submitted more ADR reports than health care professionals since 2007 according to United States Food and Drug Administration (U.S. FDA) report, 20154. In United Kingdom, most of the general public is aware of their ability to report ADRs at the individual level. Since the beginning of pharmacovigilance initiatives globally, the methods adopted by many countries have fell deficient to promote direct ADR reporting by patient drug consumers. Rational and safe use of drugs with early ADR detection remains the backbone of any pharmacovigilance program where the active role of public reporting will provide useful information as experienced by them in contrast to reports from health professionals5.
With this background of suboptimal participation of the general public in PvPI activity, we decided to conduct a survey to evaluate the awareness and perception of general public residing in Western India towards pharmacovigilance and ADRs and tried to know the cause of inactivity at grass root level.
Methods
The Institutional Ethics Committee approval [ECARP/20l9/57] was taken for the conduct of cross-sectional study. The validated well-structured questionnaire was distributed among 1876 lay public willing to give written informed consent and residing in the Mumbai region in a phase wise manner for convenience from year 2019-2021. The way to reach the lay public was through word of mouth, targeting various community centers across Mumbai region. The validated questionnaire consisted of questions to evaluate the understanding about the pharmacovigilance activity among patients and healthy drug individuals. The participants were asked to fill the questionnaire according to their understanding and the same questionnaire was then used to educate them if they were not aware of the same so as to evaluate the change in their knowledge, attitude and practice towards reporting of adverse drug reactions.
Results
Demographics
There were significant differences across the distribution of the various age groups, gender and literacy status of the study participants [p<0.001]. [Table 1.]
Table 1: Age, gender and literacy status characteristics of the study participants.
| Demographic Characteristics | Number of participants [%]
[N=1876] |
p value |
| Age groups | ||
| 18 to 30 years
31 to 50 years 51 years and above |
632 [33.68]
1017 [54.21] 227 [12.1] |
<0.001* |
| Gender distribution | ||
| Males
Females |
1262 [67]
614 [33] |
<0.001* |
| Literacy status | ||
| Post-graduates
Graduates Undergraduates – HSC pass [12th std] Undergraduates – SSC pass [10th std] Undergraduates – below SSC [below 10th std] |
352 [19]
808 [43] 256 [14] 226 [12.04] 234 [12.47] |
<0.001* |
*p<0.05 indicates the statistically significant results
There was no significant association found between the correct responses made and educational/literacy status of the patients/consumers. When enquired if the survey had created awareness among them, 94.33% responded yes [p=0.09], when asked if they had reported any adverse drug reactions [ADR] previously, 7.24% responded yes [p=0.94], would they report ADRs in the future if they experience/come across any ADRs, 99.02% agreed to it [p=0.8]. This indicates that although lesser number of participants had reported ADRs in the past, after the awareness created through our survey, more participants were interested in reporting ADR in the future [p<0.0001]. Association was evaluated between the proportion of positive responses of the participants to the question asked and their literacy status. [Figure 1.]
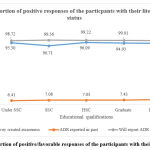 |
Figure 1: Proportion of positive/favorable responses of the participants with their literacy status. |
There was no significant association across the three different age groups i.e between age: 18-30 years, 31-50 years and those >51 years and correct responses made by the patients’ /drug consumers. [Figure 2.] The questions asked were whether the survey created awareness among them: 94.25% responded yes [p=0.66], if they had reported any adverse drug reactions [ADR] previously, 7.53% responded yes [p=0.64], would they report ADRs in the future if they experience/come across any for which 98.65% agreed [p=0.01] and this response was significant across the three age groups. This indicates that a larger number of study participants were willing to report adverse drug reactions, the response was overwhelming from the younger age group of 18 to 30 years. Again, the number of participants willing to report the adverse drug reactions in the future if they came across any increased significantly after the awareness created through the educational survey [p<0.0001].
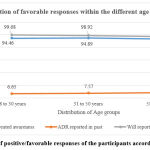 |
Figure 2: Proportion of positive/favorable responses of the participants according to the age groups. |
Following are the responses of the study participants based on the Knowledge on drug safety. [Table 2.]
Table 2: Knowledge on drug safety based questions and responses from the drug consumers’ /study participants.
| Serial Question no. | Knowledge based questions [N=1876] |
| K1 | Do you know that drugs have good as well as bad effects? |
| K2 | Which type of medicines cause side effects according to you? |
| K3 | Do you know some common side effects of drugs? |
| K4 | Are you aware of any drug that has been banned due to side effects? |
| K5 | Do you know that if you have ADR you can fill up an ADR form and send it to an ADR center? |
| K6 | Are you aware of existence of ADR reporting and monitoring system in your vicinity? |
| K7 | Do you aware about the PV helpline to report any suspected ADRs after the use of medicine? |
| K8 | Who can report ADRs? |
The participants were asked eight questions [K1 to K8] based on their knowledge about the drug safety. 86.7% of the participants believed that ingestion of drugs for treatment can have both good and as well as adverse effects, 1.1% were of the thought that drugs have good effects and cannot harm the patients or drug consumers while 12.2% were not sure of either of the options. When the participants were asked regarding which type of medicines can cause side effects, the responses were allopathy [49.95%], homeopathy [0.8%], Ayurveda [0.6%], others [4.4%] while only 4.2% of the participants were aware of and correctly attributed side effects can be due to any system of medicines. Only 26.9% of the participants were aware about the common side effects of the drug, 64.1% were not aware of any one of the side effects related to drug while 8.9% of the participants were not sure if the side effects occurred was due to drug. Only 12.53% of the participants were aware about at least one drug that was banned due to toxicity profile while 87.47% did not hear of any recalled drugs. Only 8.04% of the participants were aware about the filling of Adverse Drug Reaction [ADR] form and submitting the same to nearest ADR center, 90.4% participants were totally unaware of the same while 1.5% were not sure about either of the options. When asked about the existing ADR monitoring center in the vicinity, 1.7% participants could only answer it correctly while 98.3% never heard of such an existing center. 1.2% of the participants were aware about the existing toll-free helpline number to report the ADR to National coordinating center [NCC] i.e. Indian Pharmacopoeia Commission [IPC], Ghaziabad while the rest 98.72% of the participants have never heard of such contact number. 7.62% participants believed that reporting adverse drug reactions is the duty of the healthcare professional only, 13.54% believed its duty of non-healthcare professional to report ADRs while 78.84% participants responded that reporting adverse drug reaction details to NCC is the duty of all irrespective of their profession in healthcare.
Following are the responses from the drug consumers’/study participants based on the attitude towards drug safety. [Table 3.]
Table 3: Attitude towards drug safety based questions and responses from the drug consumers’ /study participants.
| Serial Question no. | Attitude based questions
[N=1876] |
Number of participants who Strongly agree
[%] |
Number of participants who Agree [%] | Number of participants who disagree
[%] |
Number of participants who Strongly disagree
[%] |
| A1 | Do you think side effects can happen when you start/stop/change dose of medication? | 585 [31.2] | 958 [51.06] | 304 [16.2] | 29 [1.54] |
| A2* | If you develop the side effects should you take that medicine in future? |
|
|||
| A3 | Do you think that serious ADR/event can result in death or life threatening? | 685 [36.5] | 1021 [54.4] | 122 [6.5] | 48 [2.56] |
| A4 | Do you think ADRs needed to be reported? | 720 [38.38] | 1086 [57.89] | 52 [2.77] | 18 [0.95] |
| A5* | How did you come to know about Adverse drug reactions and Pharmacovigilance? | ||||
| A6 | Do you think reporting of ADRs to be done by doctors only? | 914 [48.72] | 872 [46.48] | 50 [2.66] | 40 [2.1] |
| A7 | Do you think that reporting of ADRs by patients is helpful for doctors? | 820 [43.7] | 990 [52.7] | 36 [1.9] | 30 [1.6] |
| A8 | Do you think that reporting of ADRs by patients can have negative consequences on them? |
932 [49.68] |
245 [13.05] |
216 [11.51] |
483 [25.75] |
| A9* | Has this survey created awareness on ADR report in you? | ||||
| A10 | Do you think that existing ADR reporting system would benefit/ improve the patient/patient care? | 764 [40.72] | 1079 [57.51] | 29 [1.54] | 4 [0.21] |
| A11 | Do you think it’s a good idea that patients are now able to report side effects of medicine? | 874 [46.58] | 951 [50.61] | 24 [1.27] | 27 [1.44] |
*Responses to questions marked with asterisks are described below in details.
The participants were asked eleven questions [A1 to A11] based on their attitude towards the drug safety. Apart from the above tabulated questions and response, the questions marked as asterisk are elaborated here. For attitude-based question A2, 5.97% participants believed that it was fine to continue consuming the same medicines again even if they had developed side effects to those medicine/s before while 17.8% of the participants are ready to consume same medicine with some changes made to time, dose or frequency. However, 73.9% responded that they would avoid consuming the same drug/s again if they developed side effects to it before. For attitude-based question A5, the participants learned about the adverse drug reactions and pharmacovigilance from hospital/physician/chemist [15.51%], previous experiences [13.65%], family/friend [1.17%], magazines/newspapers [1.01%], Internet [2.07%] while 66.57% learnt about the same through our survey. From attitude-based question A9, we learnt that 99.3% [1774/1876] of the participants were educated or made aware of adverse drug reaction and reporting ADR through the conduct of our questionnaire-based survey.
Following are the responses from the drug consumers’/study participants based on drug safety practice/approach. [Table 4.]
Table 4: Drug safety practice-based questions and responses from the ‘drug consumers/’study participants.
| Serial Question no. | Practice based questions [N=1876] |
| P1 | What should be done if any side effects occur? |
| P2 | Have you ever experienced any side effects? |
| P3 | Have you discussed with your doctor about side effects of medicine? |
| P4 | Have you been ever told by your doctor about particular side effects of medicine? |
| P5 | Did your doctor cautioned you about probable side effects of medicine? |
| P6 | Do you think this is your responsibility to inform about medication side effect? |
| P7 | Have you ever reported drug induced adverse reaction? |
| P8 | Were you discouraged from reporting ADR? |
| P9 | Would you report if you have side effects of the drugs in future? |
There were nine practice-based questions [P1 to P9] to assess the awareness and understanding of the participants and their willingness to learn the correct approach if they had or observed any adverse drug reactions following drug administration. When asked about the step to be taken in case of occurrence of adverse drug reactions, 8.63% believe that the treatment medications should be stopped, 88.91% said that they would like to report the event to the health care physicians, 1.06% would wait for side effects or drug related reactions to resolve while 1.38% said that they would switch to other medicines for further treatment of disease. Only 24.04 % confirmed that they had experienced the drug related side effects at least once in their lifetime. 37.58% of the total participants discussed about the drug related side effects with their family physicians while 27.07% participants said that they were advised about the side effects of the drugs by their doctor while prescribing the medicines. 23.66% of the participants were cautioned by their doctor about the probable side effects of the prescribed medicine. When participants were asked whether the reporting of side effects of medicines is the doctor’s responsibility only, the responses were strongly agreed [42.48%], agreed [53.99%], disagreed [1.9%], strongly disagreed [1.6%]. Only 7.3% of the participants have reported a drug induced adverse reaction at least once in their lifetime. No one agreed that they were discouraged from reporting adverse drug reactions by anyone. Also, when asked if they would voluntarily report side effects of the drug in future if it occurred to them, the responses were strongly agreed [52.45%], agreed [46.53%], disagreed [1.01%] while none of them strongly disagreed.
The current study has certain limitations as the participants were not trained or educated about the drug safety program or the pharmacovigilance system before exposing them to the drug safety survey. The study was cross-sectional in nature to evaluate the baseline awareness and understanding about the drug safety program run by the Government of India.
Discussion
There results of our study indicates that, lack of awareness or insufficient information that the drugs consumed have comes with its own benefits and health related risks also. Many were not aware or could not recollect any drug related side effects nor were aware of any drugs withdrawn due to safety concerns. Very few public participants were aware of the concept of ADR and pharmacovigilance. Though the majority believed that ADRs should be reported by all including heath care and non-health care professionals. More than half of the participants [52.45%] agreed to report ADRs if they experienced them in the future depicting the good sense of responsibility and interests among the common public towards patient safety. The cause of their stubbornness to accept the change in public perception remains unaddressed by this survey which could be explained by various factors like lack of time, no financial incentive, legal implications of ADR reporting or no benefits at the individual level. This survey acted as an educational tool for many to gain awareness regarding the pharmacovigilance activity run by the Government of India. The current educational survey created awareness among all those who participated however the larger impact was on the younger generation [18-30 years’ age group] who agreed to report ADRs in the future irrespective of their literacy status [p<0.001]. The survey also concluded that very few doctors educate their patients about the adverse drug reactions when prescribing drugs. Alamari and colleagues [2010] assessed the literacy of patients coming to heath care clinic in Jeddah, Saudi Arabia, and observed that only 83.9% of the study participants had adequate literacy but patients reporting ADR still remains low6. A similar cross-sectional survey by Sales et al [2017] concluded that the lay public in Saudi Arabia are not aware of the existing pharmacovigilance system at all. Only 13(15.7%) of responders had heard of the term “Pharmacovigilance” and only 78.6% were aware about their ADR monitoring Center. 67% participants strongly believed that their physicians or pharmacists did not actively encourage them to report ADRs. Majority of the responders (73.2%) believed that it is the duty of the healthcare professionals only to report ADRs. The reason for not reporting ADRs was quoted by patients that they do not know whether the reaction is related to medication or not, some do not know about the Pharmacovigilance Center, lack of importance of ADRs reporting and few do not even know how to report ADRs7. A recent online survery was conducted by Bulcock et al. 8 [2021] to assess public perspective [N=1359] on the use of online social media digital tools for ADR reporting. The study revealed a lack of awareness about pharmacovigilance and ADR reporting however they were willing to share ADRs data on health social media with researchers and regulators, though they were cautious about automated text mining for detecting and reporting ADRs. Van Hunsel et al.9 conducted a survey in 11 countries on methods of patients reporting ADR and concluded that giving the public the opportunity to report will eventually give additional scientific value to the collected data. Fortnum H. et al.10 conducted a first of its kind survey through telephonic interviews to assess awareness about the yellow card scheme in general population staying at UK which showed low level of the population awareness about the reporting of adverse drug reactions. A study by Ahmad et al compared the efficient drug safety monitoring systems between developed countries and India to shed light on many drugs still being approved and marketed in India though they have been banned by drug regulatory agencies of developed nations. Also, there is time lag in the withdrawal of medicines from Indian market once the drug has been banned by the Indian drug regulatory agency ncreasing the risk of patients/drug consumers to adverse drug reactionsto adverse drug reactions11. A survey conducted at United States [2006] on 1726 adult public consumers who believed that there is a need for reforms in drug safety reporting system by Pharmaceutical industry and U.S. FDA. The same study has insisted on reporting of safety-related events following the use of drugs for approved and unapproved indications and medication errors12. A study by Qato et al. 13 [2015] who conducted cross sectional survey in Arab and eastern Mediterranean (20 countries) highlighted about the disparities in the pharmacovigilance system and need to collaborate regionally and internationally to improve awareness about the existing pharmacovigilance programme among the public and healthcare provider. Similar study by Wang et al. 14 in eastern and western china cities revealed poor understanding and knowledge among the general public about ADR monitoring/ reporting but was not associated with regional economic development. Social media platforms may be involved to facilitate and promote about the direct public reporting of adverse drug reactions. Chen et al. 15 study from western china also mentioned about the poor awareness but positive attitude of public towards pharmacovigilance. Lack of understanding about the seriousness of ADRs was attributed to patients not reporting ADRs, also with difficulty in filling the part of suspected ADR form. Kitabayashi et al.16 descriptive observational study [845 citizens and 300 physicians] in Japan found 83.7% of the patient unaware of direct patient reporting channel for adverse drug reaction. And the traditional physician–patient relationship may be one of the important causative factor attributed for poor awareness and utilization of the reporting system which need to be improved.
Conclusion
The general population residing in Western India are not fully aware about ADR reporting and the Pharmacovigilance system. The PvPI needs to focus more on sensitizing public for ADR reporting and pharmacovigilance system in promoting patient safety.
Ethical Approval: The study was approved by the Ethics Committee for Academic Research Projects of our institute [ECARP Protocol Reference No: ECARP / 20l9/57].
Acknowledgement
None
Conflict of Interests
There is no conflict of interest
Funding Sources
There is no funding source.
References
- Kalaiselvan V, Thota P, Singh GN. Pharmacovigilance Programme of India: Recent developments and future perspectives. Indian J Pharmacol. 2016 Nov-Dec; 48(6):624-628.
- Braveman P, Gottlieb L. The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep. 2014; 129 Suppl 2(Suppl 2):19-31.
- Agrawal V, Shrivastava TP, Adusumilli PK, Vivekanandan K, Thota P, Bhushan S. Pivotal role of Pharmacovigilance Programme of India in containment of antimicrobial resistance in India. Perspect Clin Res 2019; 10:140-4
- FAERS Reporting by Healthcare Providers and Consumers by Year [Internet]. U.S. Food and Drug Administration. Nov-2015. Available from: https://www.fda.gov/drugs/questions-and-answers-fdas-adverse-event-reporting-system-faers/faers-reporting-healthcare-providers-and-consumers-year, last accessed on July 18, 2022.
- Suke SG, Kosta P, Negi H. Role of Pharmacovigilance in India: An overview. Online J Public Health Inform. 2015 1;7(2): e223.
- Alamari B, Aljasir B, Habadi M. Assessing the Level of Health Literacy among Adult Visitors in the Primary Health Care Sitting of National Guard Health Affair, Jeddah, Saudi Arabia. Imperial journal of interdisciplinary research [Internet]. 2017, (3): n. pag. Available from: https://www.semanticscholar.org/paper/Assessing-the-Level-of-Health-Literacy-among-Adult-Alamari-Aljasir/cbe4355e5fbd726f54c05399df201cbaacc4db3b , last accessed on July 18, 2022.
- Sales I, Aljadhey H, Albogami Y, Mahmoud MA. Public awareness and perception toward Adverse Drug Reactions reporting in Riyadh, Saudi Arabia. Saudi Pharm J. 2017;25(6):868-872.
- Bulcock A, Hassan L, Giles S, Sanders C, Nenadic G, Campbell S, Dixon W. Public Perspectives of Using Social Media Data to Improve Adverse Drug Reaction Reporting: A Mixed-Methods Study. Drug Saf. 2021 May; 44(5):553-564.
- Van Hunsel F., Harmark L. Experiences with adverse drug reaction reporting by patients: an 11-country survey. Drug Saf. 2012; 35(1):45–60.
- Fortnum H, Lee AJ, Rupnik B, Avery A; Yellow Card Study Collaboration. Survey to assess public awareness of patient reporting of adverse drug reactions in Great Britain. J Clin Pharm Ther. 2012; 37(2):161-5.
- Ahmad A, Patel I, Sanyal S, Balkrishnan R, Mohanta GP. A study on drug safety monitoring program in India. Indian J Pharm Sci. 2014; 76(5):379-86.
- Olsen AK, Whalen MD. Public perceptions of the pharmaceutical industry and drug safety: implications for the pharmacovigilance professional and the culture of safety. Drug Saf. 2009; 32(10):805-10.
- Qato DM. Current state of pharmacovigilance in the Arab and Eastern Mediterranean region: results of a 2015 survey. Int J Pharm Pract. 2018 Jun; 26(3):210-221.
- Wang N, Chen Y, Ren B, Xiang Y, Zhao N, Zhan X, Feng B. A cross-sectional study: comparison of public perceptions of adverse drug reaction reporting and monitoring in eastern and western China. BMC Health Serv Res. 2022 Mar 8; 22(1):318.
- Chen Y, Wang Y, Wang N, Xiang Y, Zhang R, Xiao J, Liu H, Feng B. Knowledge, attitude, and practice regarding pharmacovigilance among the general public in Western China: a cross-sectional study. Curr Med Res Opin. 2021 Jan; 37(1):101-108.
- Kitabayashi A, Inoue Y. Factors that Lead to Stagnation in Direct Patient Reporting of Adverse Drug Reactions: An Opinion Survey of the General Public and Physicians in Japan. Ther Innov Regul Sci. 2022 Jul; 56(4):616-624.








