Amritha C. A1 , K. Kranthi2
, K. Kranthi2 , S. Sundar3
, S. Sundar3 and K Punnagai2
and K Punnagai2
1Department of Pharmacology, Government Mohan Kumaramangalam Medical College, Salem, Tamilnadu.
2Department of Pharmacology, Sri Ramachandra Medical College and Research Institute, Porur, Chennai Tamil Nadu, India.
3Department of Neurology, Sri Ramachandra Medical College and Research Institute, Porur, Chennai, Tamil Nadu, India.
Corresponding Author E-mail:jayadev72@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2551
Abstract
Objective: To compare the safety and efficacy of two anti-migraine Venlafaxine and Amitriptyline in migraine prophylaxis. Background of the study: The efficacy of Venlafaxine and Amitriptyline on various headache disorders has been proved in previous studies. This Open-Label Parallel group study of 12 weeks compares the safety and efficacy of Venlafaxine and Amitriptyline in migraine prophylaxis. Methods: 100 migraine patients were randomized and they were allocated equally to Venlafaxine XR and Amitriptyline group. The frequency, duration, severity of headache attacks and safety profiles were monitored for 3 months. Patient satisfaction and global tolerance were also evaluated during this period. Results: The statistical difference between the treatment groups in the number of headache attacks (p = 0.012) the duration of attacks (p=0.046) and the severity of the attacks (p=0.032) was notable at the end of the study period with Venlafaxine better than Amitriptyline. Side effects like hypersomnia and constipation were reported in both the groups. Analgesic consumption was also significant reduced in Venlafaxine group when compared with Amitriptyline (p=0.021). Venlafaxine has higher patient satisfaction when compared to Amitriptyline. Global efficacy and global tolerance were also higher in the Venlafaxine group. Conclusions: The results support the safety and efficacy profile of Venlafaxine as preventive therapy for migraine.
Keywords
Amitriptyline; Antidepressant; Migraine; Noradrenaline; Serotonin; SSRIs; Venlafaxine XR
Download this article as:| Copy the following to cite this article: Amritha C. A, Kranthi K, Sundar S, Punnagai K. Efficacy and Safety of Venlafaxine Versus Amitriptyline in Decreasing Severity and Frequency of Migraine Attacks: A Randomized Controlled Trial. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Amritha C. A, Kranthi K, Sundar S, Punnagai K. Efficacy and Safety of Venlafaxine Versus Amitriptyline in Decreasing Severity and Frequency of Migraine Attacks: A Randomized Controlled Trial. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3fX9qgk |
Introduction
Migraine is a recurrent headache disorder for which various theories have been proposed. It is a neurovascular disease with a central nervous system (CNS) component that manifests itself with throbbing headaches and intense pain. This pain syndrome occurs unilaterally along with nausea, vomiting, photosensitivity and phono sensitivity .1 Nearly 10% of adolescents and children are affected. Conventional treatment of migraine includes symptomatic and prophylactic drugs. If not treated it might result in significant functional impairment.2
Drugs available for the management of migraine headache are NSAIDS, Ergotamine, 3 triptans, 4 antidepressants, 5 betablockers6 and calcium channel blockers.7
Antidepressants for migraine prophylaxis have become very common nowadays. This is because of their role in serotonergic mechanisms in the pathophysiology of migraine. Tricyclic antidepressants like Amitriptyline and SSRIs like citalopram, escitalopram and fluoxetine are commonly used in patients as prophylactic drugs.8 But they are very frequently associated with adverse effects like loss of appetite, weight gain, fatigue, dizziness, reduced concentration etc. that may lead patients to discontinue therapy. Amitriptyline is a tricyclic antidepressant. It acts by preventing the uptake of norepinephrine and serotonin in the brain.9 Venlafaxine, a selective serotonin reuptake inhibitor, prevents the reuptake of nor epinephrine, serotonin, and dopamine. It has a specific action on serotonin receptors and does not act on receptors that cause anticholinergic or cardiovascular adverse effects. Both drugs are proved to have good prophylactic efficacy to other anti-migraine drugs.10
The rationale of this study is to compare Venlafaxine and Amitriptyline, and to find out the better drug in controlling the frequency of migraine attacks and to have improved cure rates with minimal adverse effects. Some studies have previously compared the efficacy of Venlafaxine and Amitriptyline. In previous studies, patients who received extended release of Amitriptyline or Venlafaxine had a significant effect on pain parameters.11 since there is no evidence of studies conducted in India; this study was done to compare the two drugs in migraine prophylaxis with different end parameters in the Indian population.
Material and Methods
Study design
This is a randomized, active controlled, open Label, parallel group, prospective, interventional Study, of 12 weeks duration. The study was carried out in the Department of General Medicine, Sri Ramachandra Hospital, SRMC & RI, Sri Ramachandra University, Porur, Chennai from February 2015 to February 2016. The feasibility of samples was 100 patients. Simple randomization was done using computer generated randomization software called as Random allocation software (Version 1.0, May 2004). Consequently, patients were administered study drugs without bias.
Study population
Patients with a history of intermittent recurrent headache coming to the OPD of the medicine department of SRMC
Inclusion criteria
Age 18 to 65 years; both genders (male and female); Patients with history of migraine for ≥ 2 months, and at least two attacks per month; Migraine with aura patients were included. No other form of long term anti-migraine drugs were permitted except for rescue medications like NSAIDs and triptans during acute attacks; Patients who came for follow up every 15 days and maintained headache diary.
Exclusion criteria
Patients who had headaches due to other causes such as sinusitis, infection, head injury, vascular disorders, tumors; Patients with depression and other psychiatric diseases; Patients allergic to Venlafaxine or Amitriptyline; Patients with liver or kidney failure, heart diseases; Pregnant and lactating women were excluded. Women in childbearing age were allowed to take oral contraceptives and who intend to get pregnant was not included in the study; Patients taking other prophylactic medication were asked to stop 2 weeks before the start of the study.
Patient monitoring
After a thorough physical examination each patient is entered in the case report form. Hamilton Rating Scale for Depression (HAM-D) was used to rule out the patients with depression and they were not included in the study. Eligible patients were informed of the trial in detail and informed consent was obtained.
Drug administration
Both genders were equally assigned to Groups A and B. The Group A patients were given Venlafaxine-XR, 37.5mg, single tablet per day and were instructed to take the tablet one hour after breakfast, and Group B patients were given Amitriptyline, 25 mg single tablet per day, and were instructed to take the tablet one hour after dinner every day. The patients were given instructions about the common adverse drug reactions like nausea, vomiting, dry mouth, hypersomnia that are possible to occur during the treatment course. The treatment continued for a period of 12 weeks in which there were 3 scheduled visits. The patients were assessed for the following endpoints at the beginning of the study, during treatment, and at the end.
Primary endpoints
Number of migraine attacks.
Duration of attacks in hours.
Severity of attacks.
Severity is assessed using a 1-3 scale [1-Mild – Able to work; 2-Moderate – Unable to work, but not staying in bed; 3-Severe – Staying in bed] and a Visual analog scale-Von Baker Faces (0 – 10). Patients are asked to choose the face that explains the pain (Figure 1).
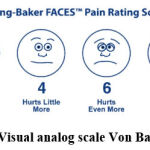 |
Figure 1: Visual analog scale Von Baker faces. |
The above parameters were recorded in a diary by the patients. The headache diary is given along with a questionnaire which consists of the above five questions pertaining to the symptoms of the patient.
Secondary endpoints
Adverse events that occurred during the treatment course; Effect of drugs on the number of analgesic consumption; Global efficacy (1 – Excellent, 2 – Good, 3 – Moderate, 4 – Ineffective) and Global tolerance (1 – Very good, 2 – Good, 3 – Moderate, 4 – Poor) of treatment and Patient satisfaction (1 – Excellent, 2 – Good, 3 – Moderate, 4 – Poor) of treatment was also recorded using appropriate scores. Acute rescue medications like paracetamol, triptans and chlorpromazine were allowed during severe painful attacks. The patients were instructed to record the time they used rescue medications and to bring back the headache diary and empty tablet strip during each visit to verify compliance. They were also advised to report any adverse effect immediately. During the follow up patients gave information on the treatment progress and adverse effects of the drug. Patients had regular follow up visits and they were also enquired by phone calls every 15 days for the above five questions. The headache diary was collected after the study and was evaluated for all parameters.
Statistical Analysis
The statistical package for the Social Sciences (SPSS, Version 17) for Microsoft windows was used. The data was normally distributed. Descriptive statistics were presented as numbers and percentages. Mean and standard deviation were used for the and nonparametric tests were performed. A Chi-square test was applied for comparison between categorical variables. It was used to determine the significant difference between two groups. Fisher’s exact test was also used to determine the associations between categorical variables. The P value of < 0.05 was significant.
Results
Totally 125 patients were screened. 100 patients were taken for the study and the rest 25 patients were not eligible. The patients selected were randomized and allocated to both the treatment groups. There were no dropouts in the study (Figure 2).
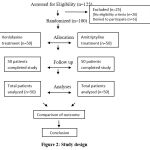 |
Figure 2: Study design. |
Age and Gender Distribution
With a sample size of 50, in group A the mean age of the patients was 36.87% and in group B it was 39.66%, the value of ‘P’is 0.260. There were no statistical differences for ages in both groups. In group A, 42% were men and 58% were women. In group B, 34 % were men and 66% were women. The ‘P’ value was found to be 0.409. Hence, there was no difference in the demographic features among the study population (Table 1).
Table 1: Baseline Patient Characteristics.
|
Parameters |
Venlafaxine group | Amitriptyline group | ‘P’ Value | |||
|
No. |
% | No. |
% |
|||
| Gender | Male
Female Total |
21
29 50 |
42.0
58.0 100.0 |
17
33 50 |
34.0
66.0 100.0 |
0.409 |
| Age (Mean ± SD) | 36.87 ± 12.55 | 39.66 ± 12.70 | 0.2601 | |||
| Baseline severity | Venlafaxine group | Amitriptyline group | 0.194
|
|||
| No. | % | No. | % | |||
| Mild
Moderate Severe |
17
7 26 |
34.0
14.0 52.0 |
12
14 24 |
24.0
28.0 48.0 |
||
Primary Analyses
Number of migraine attacks per month
In the pretreatment period, there was no variation between the two treatment groups in relation to the number of attacks. The “P” value is 0.853. During the treatment period the “P” Value was 0.349. At the end of study, in the Venlafaxine group, 48% of patients had < 5 attacks, 34% of patients had 5-15 attacks and only 18% of patients had > 15 attacks.
In the Amitriptyline group 20% of the patients had < 5 attacks, 54% of the patients had <5-15 attacks, and 28% of the patients had > 15 attacks. Hence at the end of the study period, there was a statistically significant difference between the two treatment groups with a “P” Value of 0.012. The group A patients responded well to the treatment. This shows that Venlafaxine has more efficacy than Amitriptyline in reducing the migraine attacks (Table 2).
Table 2: Number of attacks in both groups.
|
Period of study |
Pretreatment group % | During treatment % | End of study group % | |||
| Number of attacks | Venlafaxine | Amitriptyline | Venlafaxine | Amitriptyline | Venlafaxine |
Amitriptyline |
| < 5 attacks
5 – 15 attacks > 15 attacks Total |
18.0
36.0 46.0 100.0 |
22.0
32.0 46.0 100.0 |
24.0
48.0 28.0 100.0 |
28.0
34.0 38.0 100.0 |
48.0
34.0 18.0 100.0 |
20.0
54.0 26.0 100.0 |
| Mean ± S.D | 2.28 ± 0.757 | 2.24 ± 0.769 | 2.04 ± 0.727 | 2.1 ± 0.814 | 1.7 ± 0.762 | 2.06 ± 0.682 |
| Standard error | 0.107 | 0.112 | 0.102 | 0.115 | 0.107 | 0.096 |
| 95% Confidence interval | 0.796 – 3.763 | 0.678 – 3.801 | 0.614 – 3.465 | 0.503 – 3.686 | 0.205 – 3.194 | 0.722 – 3.397 |
| “P” Value | 0.853 | 0.349 | 0.012 | |||
Duration of Migraine attacks
In the pretreatment period and during the study period, there was no statistical difference between the two groups. Chi-Square test “P” Value was 0.663 and Fisher’s exact test “P” Value was 0.828. During the treatment period the Chi-Square test “P” Value was 0.488 and Fisher’s exact test “P” Value was 0.645. At the end of the study, in the Venlafaxine group, 88% of the patients had a migraine attack of < 6hour duration, 12% of the patients had a migraine attack of > 6 hours. In the Amitriptyline group 72% of patients had an attack of < 6 hour duration, 28% of patients had an attack of > 6 hour duration. Hence at the end of the study period, there was a statistically significant difference between the two treatment groups. Chi-Square tests showed a “P” Value of 0.046 and Fisher’s exact test showed a one sided significant “P” Value of 0.078 (Table 3).
Table 3: Duration of attacks in both the groups.
| Period of study | Pre treatment group % | During treatment % | End of study group % | |||
| Duration (hrs) | Venlafaxine | Amitriptyline | Venlafaxine | Amitriptyline | Venlafaxine | Amitriptyline |
| < 6 hrs
> 6 hrs Total |
72.0
28.0 100.0 |
68.0
32.0 100.0 |
78.0
22.0 100.0 |
72.0
28.0 100.0 |
88.0
12.0 100.0 |
72.0
28.0 100.0 |
| Mean ± S.D | 1.28 ± 0.453 | 1.32 ± 0.471 | 1.22 ± 0.4184 | 1.28 ± 0.453 | 1.2 ± 0.404 | 1.28 ± 0.453 |
| Standard error | 0.064 | 0.066 | 0.059 | 0.641 | 0.057 | 0.641 |
| 95% Confidence interval | 0.391 – 2.168 | 0.396 – 2.243 | 0.399 – 2.040 | 0.391 – 2.168 | 0.408 – 1.991 | 0.391 – 2.168 |
| “P” Value | 0.663 | 0.488 | 0.046 | |||
| Fisher’s exact test | 0.828 | 0.645 | 0.078 | |||
Severity of Migraine attacks with 1 – 3 Severity scale
The efficacy of the drugs was evaluated with respect to the severity of migraine attacks (1 – 3 severity scale). In the pre-treatment period, no difference was found. The ‘P’ value is 0.194. In the Venlafaxine group, during treatment, 50% of patients were able to work normally, 14% of patients were unable to work but did not stay in bed and 36% of patients were staying in bed. In the Amitriptyline group 22% of patients were able to work normally, 34% of patients were unable to work but not staying in bed and 44% of patients were staying in bed. During the treatment period and at the end of the study, there was a statistical difference between the groups with a ‘P’ value of 0.007 and 0.032, respectively (Table 4).
Table 4: Severity of attacks in both the groups before treatment (1-3 scale and Visual analog scale).
| Period of study | Pre treatment group % | During treatment % | End of study group % | |||
| 1 – 3 scale | Venlafaxine | Amitriptyline | Venlafaxine | Amitriptyline | Venlafaxine | Amitriptyline |
| Mild
Moderate Severe Total |
34.0
14.0 52.0 100.0 |
24.0
28.0 48.0 100.0 |
50.0
14.0 36.0 100.0 |
22.0
34.0 44.0 100.0 |
74.0
10.0 16.0 100.0 |
50.0
12.0 38.0 100.0 |
| Mean ± S.D | 2.18 ± 0.918 | 2.24 ± 0.822 | 1.86 ± 0.926 | 2.22 ± 0.789 | 1.66 ± 0.894 | 1.88 ± 0.939 |
| Standard error | 0.129 | 0.116 | 0.130 | 0.111 | 0.126 | 0.132 |
| 95% Confidence interval | 0.378 – 3.981 | 0.628 – 3.851 | 0.044 – 3.675 | 0.671 – 3.768 | -0.093 – 3.413 | 0.037 – 3.722 |
| “P” Value | 0.194 | 0.007 | 0.032 | |||
| Visual analog scale | Venlafaxine | Amitriptyline | Venlafaxine | Amitriptyline | Venlafaxine | Amitriptyline |
| 0 – No hurt | 0.0
6.0 12.0 16.0 24.0 42.0 100.0 |
0.0
8.0 14.0 22.0 26.0 30.0 100.0 |
8.0
8.0 12.0 14.0 26.0 32.0 100.0 |
2.0
6.0 20.0 24.0 12.0 36.0 100.0 |
12.0
16.0 14.0 18.0 18.0 22.0 100.0 |
6.0
18.0 22.0 16.0 10.0 28.0 100.0 |
| 2 – Hurts little bit | ||||||
| 4 – Hurts little more | ||||||
| 6 – Hurts even more | ||||||
| 8 – Hurts whole lot | ||||||
| 10 – Hurts worst | ||||||
| Total | ||||||
| Mean ± S.D | 7.68 ± 2.534 | 7.12 ± 2.560 | 6.76 ± 3.229 | 6.92 ± 2.834 | 5.6 ± 3.428 | 5.8 ± 3.313 |
| Standard error | 0.358 | 0.362 | 0.456 | 0.400 | 0.484 | 0.4682 |
| 95% Confidence interval | 2.711 – 12.648 | 2.101 – 12.138 | 0.429 – 13.090 | 1.364 – 12.475 | -1.12 – 12.32 | -0.694 – 12.294 |
| “P” Value | 0.785 | 0.224 | 0.622 | |||
Severity of Migraine attacks with Visual analog scale (Von-Baker faces)
In the pretreatment and during the study period there was no statistical difference. The “P” value is 0.785 and 0.224, respectively. At the end of the treatment period also there was no statistical difference. The ‘P’ value is 0.622 (Table 4).
Secondary analyses
Adverse events
In general, adverse effects were found to be more or less the same in both groups. The most frequent adverse effects were hypersomnia and constipation in the Venlafaxine group. There was a statistical difference in the occurrence of constipation. “P” Value = 0.032 by Chi-Square test and “P” Value = 0.056 by Fisher’s exact test. The Venlafaxine group had more patients with constipation compared to the Amitriptyline group. There was also a variation between the groups in patients who reported no adverse events. The value of ‘P’ = 0.007 by the chi-square test and the value of ‘P’ = 0.013 by Fisher’s exact test. The occurrences of other adverse events were not found to be statistically significant (Figure 3).
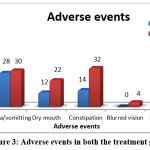 |
Figure 3: Adverse events in both the treatment groups. |
Analgesic consumption
In the Venlafaxine group, 24% of patients consumed analgesics as rescue medication while 46% of patients consumed analgesics in the Amitriptyline group. There was a decrease in the values for the Venlafaxine group compared to the values for the Amitriptyline group, the value ‘P’ = 0.021 by the chi-square test (Figure 4).
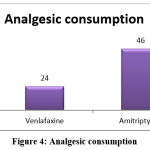 |
Figure 4: Analgesic consumption. |
Patient satisfaction
Patient satisfaction was higher in the Venlafaxine group. “P” Value is 0.191 by Chi-Square test (Figure 5).
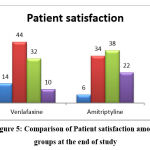 |
Figure 5: Comparison of Patient satisfaction among groups at the end of study. |
Global Efficacy
The global efficacy of migraine treatment was higher in the Venlafaxine group. 22% of the patients said excellent, 44% of the patients said good, 22% of patients said moderate and 12% of the patients said ineffective in the Venlafaxine group. 12% said it was excellent, 36% said good, 30% said moderate and 22% said it was ineffective in the Amitriptyline group. The value of ‘P’was 0.266 (Figure 6).
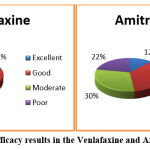 |
Figure 6: Global efficacy results in the Venlafaxine and Amitriptyline group. |
Global Tolerance
The global tolerance to migraine treatment was higher in the Venlafaxine group. 13% of patients said very good, 24% of patients said good, 16% of patients said moderate and 10% of patients said poor in the Venlafaxine group. 16% said very good, 32% said good, 26% said moderate, and 26% said poor in the Amitriptyline group. “P” Value is 0.566 by Chi-Square test (Figure 7).
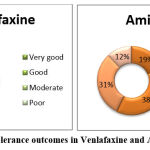 |
Figure 7: Global tolerance outcomes in Venlafaxine and Amitriptyline group. |
Discussion
This open label, randomized study was carried out to compare the prophylactic analgesic efficacy, safety and tolerability of Venlafaxine, a newer serotonin nor epinephrine reuptake inhibitor with Amitriptyline, a tricyclic antidepressant in patients with moderate to severe migraine for duration of 12 weeks in Indian population.12 Number, duration, severity of migraine attacks, analgesic consumption, patient satisfaction, adverse effects of drugs, Global efficacy and tolerance were assessed.
Headache is the fundamental reason for patient consultation. The most well-known finding for these patients is migraine headache. The scope of prophylactic drugs used in migraine is expanding.13 Amitriptyline as a notable medication for the treatment of depression, in lower doses might be useful for migraine sufferers who react unsatisfactorily to different treatments. Choices about treatment in a specific patient should clearly contemplate the nature of headache, reaction to different medications, and the long-term effects of newer prophylactic agents. Amitriptyline was powerful in migraine prophylaxis in older trials, and has a demonstrated antimigraine effect in animal’s also.12 AMT causes significant reduction of headaches but it has severe side effects. It is considered as an alternative drug for prophylaxis of migraine. 13
Various animal studies supported the chronic effect of Amitriptyline in low dose on the serotonin synthesis .14 Besides, the efficacy of Amitriptyline in combination with many antidepressant drugs was also compared. Common side effects of Amitriptyline like nausea, constipation, sedation, orthostatic Hypotension and skin reactions have made the clinicians to seek out newer antidepressants with lesser adverse events.15
VLF is a 5-HT and NE reuptake inhibitor. It prevents migraine attacks with much more tolerable side effects.16 An open examination was performed to estimate the effective use of Venlafaxine in migraine patients. The effective dose of VLF was 37.5 mg for each day.17 Hence, the same dose was used in this study. Since extended release form of Venlafaxine was reported to be more effective in migraine prophylaxis, it was given to both the study groups. Venlafaxine was also compared with other drugs such as propranolol, for vestibular migraine prophylaxis that showed positive results.18
Since the use of Amitriptyline in migraine prophylaxis has been shown to produce various adverse effects, another group of drugs were used in migraine. And there was a lack of data on migraine frequency in few studies and patients with co-morbid depression were not excluded. Few studies were limited by their use of global ratings and in certain studies patients who took Amitriptyline had a rebound effect in the control group.19 For Venlafaxine, previous studies had drawbacks like short length of follow up, and patients were allowed to take concomitant prophylactics that was likely to hamper the study results. Therefore, our study serves the purpose of a head-to-head comparison between Venlafaxine and Amitriptyline in the Indian population that overcomes the limitations of other studies.
The treatment efficacy was assessed after 3 months, while the patient maintains a headache diary. In spite of the smaller sample size, both drugs showed good improvement in the treatment. In the present study, VAS is used to record pain throughout the study and increase the power of the study and overcome bias. The study groups have shown significant improvement in pain scores but to a lesser extent compared to previous studies. This difference could be attributed to less frequent administration of the study drug and also a lower dosage of the drugs. The results of our support the efficacy and tolerability of Venlafaxine as a preventive therapy for migraine. Venlafaxine was more effective than amitriptyline in most parameters.
The Venlafaxine group had better global efficacy than the Amitriptyline group. Global tolerance was moderately higher in the Venlafaxine group. There was a reduction in the number of headache attacks in the Venlafaxine group versus Amitriptyline. Patient satisfaction was also higher in the Venlafaxine XR groups. Adverse events hypersomnia and constipation were much more frequent in both. However, global tolerance was above 50% in all groups.
Limitations of the study
Regardless of the positive findings, this study also showed some limitations. This study includes include a smaller sample size since the feasibility of the samples was only 100 patients. Blinding was not done among the treatment groups. Lack of time for conducting the study was another major drawback. And few patients lost follow-up, who were replaced by new study subjects. The strengths of the study are randomization and patients were administered the study drugs without bias. Further plans can be extended to conduct the study in a multicenter level, in a larger population with additional end parameters and more frequent administration of drugs at different dose.
Summary
This study was a prospective, open labeled, randomized, parallel group study to compare prophylactic safety, efficacy and tolerability of Venlafaxine and Amitriptyline in 100 patients in a 1:1 ratio to receive Venlafaxine 37.5 mg OD or Amitriptyline 25 mg OD with moderate to severe migraine conducted at Sri Ramachandra Medical College and Research Institute from February 2015 – February 2016 for a duration of 12 weeks. Reduction in number, duration and severity, adverse events of migraine attacks were compared. Results were tabulated, efficacy and safety analysis were done statistically. The reduction of the number, duration, and severity of migraine was observed more with the Venlafaxine group. The occurrence of adverse events such as hypersomnia, constipation, and nausea / vomiting was higher in the Venlafaxine group.
Conclusion
Venlafaxine has a ‘three-drugs in one’ effect. At low doses, it reuptakes serotonin, at medium doses, Noradrenaline, and at higher doses, Dopamine. It has a similar mechanism to TCAs in conditions like migraine. In addition, it has lesser drug interactions compared to other drugs, and it can be used safely.
As a result of this study, we find that Venlafaxine could be preferred for migraine prophylaxis since it reduces pain intensity (from VAS scores) and also reduces the number and duration of migraine attacks. But, incidences of adverse events like nausea / vomiting, hypersomnia, constipation are common. However, this was a short-duration study with small sample size and minimum doses of both drugs. But for a more definite statement there is a requirement for further long-term studies with larger population and ideal drug. To conclude, Venlafaxine, a novel antidepressant drug with dual mechanism of action (serotonin and nor epinephrine reuptake inhibitor), at a dose of 37.5 mg OD provided significant pain relief and reduced migraine headache in patients when compared to Amitriptyline 25 mg OD.
Acknowledgement
None
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Ghadiri M, Motaghi M. Homeopathy Impact on Physical Health Aspects of Quality of Life of Patients with Chronic Headache in Isfahan . Biomed Pharmacol J 2016;9(1)
CrossRef - R, Pradeep et al. “Migraine Disability, Quality of Life, and Its Predictors.Annals of neurosciences:27(1) 18-23;2020.
CrossRef - Ngo M, Tadi P. Ergotamine/Caffeine. [Updated 2021 Sep 29] : StatPearls Publishing; 2022 Jan
- Nicolas S, Nicolas D. Triptans. [Updated 2021 Oct 30] : StatPearls Publishing; 2022 Jan
- Xu, Xiao-min, Liu, Yang , Dong, Mei-xue, Zou, De-zhi , Wei, You-dong . Tricyclic antidepressants for preventing migraine in adults, Medicine: June 2017; 96(22).
CrossRef - Gerwig, M., Niehaus, L., Stude, P. et al.Beta-blocker migraine prophylaxis affects the excitability of the visual cortex as revealed by transcranial magnetic stimulation. J Headache Pain: 2012; 83–89.
CrossRef - Ran, C., Fourier, C., Arafa, D., Liesecke, F., Sjöstrand, C., Waldenlind, E., Steinberg, A., & Belin, A. C. Anoctamin 3: A Possible Link between Cluster Headache and Ca2+Signaling. Brain sciences : 2019;9(184).
CrossRef - Martin VT, Feoktistov A, Solomon GD. A rational approach to migraine diagnosis and management in primary care. Annals of Medicine. 2021 Dec;53(1):1979-1990.
CrossRef - Gonçalves AL, Martini Ferreira A, Ribeiro RT, et al .Randomised clinical trial comparing melatonin 3 mg, amitriptyline 25 mg and placebo for migraine prevention: Journal of Neurology, Neurosurgery & Psychiatry ;87:1127-1132; 2016.
CrossRef - Thomson, Physicians‟ desk reference, Thomsdon PDR, Motvale, NJ, 58th edition; 3413-3424; 2004.
- Bulut S, Berilgen MS, Baran A, Tekatas A, Atmaca M, Mungen B.Venlafaxine versus Amitriptyline in the prophylactic treatment of migraine: randomized, double-blind, crossover study. Clin NeurolNeurosurg.; 107(1):44–48; 2004.
CrossRef - Sekar P, Punnagai K, David D. C. Comparative Study of Safety and Efficacy of Gabapentin Versus Amitriptyline in Patients With Painful Diabetic Peripheral Neuropathy, A Randomized open Label Parallel Group Study. Biomed Pharmacol J;10(3); 2017.
CrossRef - Shanmugam S, Karunaikadal K, Varadarajan S, Krishnan M. Memantine ameliorates migraine headache. Ann Indian Acad Neurol;22:286-90; 2019.
CrossRef - Meshalkina, D. A., Kysil, E. V., Antonova, K. A., Demin, K. A., Kolesnikova, T. O., Khatsko, S. L., Gainetdinov, R. R., Alekseeva, P. A., & Kalueff, A. V. The Effects of Chronic Amitriptyline on Zebrafish Behavior and Monoamine Neurochemistry. Neurochemical research, 43(6):1191–1199 ; 2018.
CrossRef - Berilgen MS, Bulut S, Gonen M, et al. Comparison of the effects of Amitriptyline and flunarizine on weight gain and serum leptin, C peptide and insulin levels when used as migraine preventive treatment. Cephalalgia; 25(11):1048–1053;2005.
CrossRef - Lempert T, Olesen J, Furman J, Waterston J, et al. Vestibular migraine: diagnostic criteria: consensus document of the Bárány Society and the International Headache Society. Nervenarzt : 84:511–6; 2013.
CrossRef - Mohaddeseh Hedayat, Surena Nazarbaghi, Mohammad Heidari, Hamdollah Sharifi.Venlafaxine can reduce the migraine attacks as well as amitriptyline: A noninferiority randomized trial,:Clinical Neurology and Neurosurgery,214 :2022.
CrossRef - Miyahara Y, Funahashi H, Haruta-Tsukamoto A, et al. Time course of effects of venlafaxine on migraine and generalized pruritus in a patient with depression. Clinical Case Report: Nov 16 ;9(11): 2021
CrossRef - Doyle Strauss, L., Weizenbaum, E., Loder, E. W., & Rizzoli, P. B. Amitriptyline Dose and Treatment Outcomes in Specialty Headache Practice: A Retrospective Cohort Study. Headache, 56(10), 1626–1634: 2016.
CrossRef








