Devendra Kumar Bhopte1 , Rakesh Sagar2
, Rakesh Sagar2 and Mohan Lal Kori1*
and Mohan Lal Kori1*
1Vedica College of B. Pharmacy, RKDF University, Bhopal, M.P.
2Shri G. S. Institute of Technology and Science, Indore, M.P.
Corresponding Author Email Id: mlkori.research@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2539
Abstract
The majority of formulations are available as oral dosage forms. In spite of some pharmaceutical challenges, this route is considered as most suitable way of drug delivery. For that reason it is necessary to optimize dose and dosing frequency to reduce the toxic effects of drug. Therefore, in this study, we have conceptualized the fabrication of floating microspheres of Quinapril Hydrochloride by the solvent evaporation technique with varying ratios of HPMC, Carrageenan, and Poly methyl methacrylate with polyvinyl alcohol that will augment its gastric retention time in conjunction with the sustained pharmacological activity. The formulation process of floating microspheres was optimized for stirring speed (X1) and concentration of polymer ratio (X2) on dependent variables such as percentage entrapment efficiency (Y1), percentage yield (Y2), in vitro buoyancy (Y3), and percentage of drug release (Y4) by using the factorial design. The drug was characterized by Fourier transform infrared spectroscopy, Differential scanning calorimetry was used for the identification of drug polymer blend interaction. The prepared microspheres were characterized by number of parameters including; scanning electron microscopy, percentage yield, particle size, in vitro buoyancy, drug entrapment efficiency, in vitro drug release, and in vivo floating behavior in albino rabbits. The release profile of microspheres prepared with hydrophilic, pore forming material and ion exchange resin combinations was dependent on the layer of pores developed by the fluid present at the absorption site of stomach and the drug release rate was retarded at site of action i.e. GIT.
Keywords
Buoyancy Effect; Floating Microspheres; Polymeric Concentration; Quinapril Hydrochloride
Download this article as:| Copy the following to cite this article: Bhopte D. K, Sagar R, Kori M. L. Fabrication, Optimization and Characterization of Floating Microspheres of Quinapril Hydrochloride using Factorial Design Method. Biomed Pharmacol J 2022;15(4). |
| Copy the following to cite this URL: Bhopte D. K, Sagar R, Kori M. L. Fabrication, Optimization and Characterization of Floating Microspheres of Quinapril Hydrochloride using Factorial Design Method. Biomed Pharmacol J 2022;15(4). Available from: https://bit.ly/3rBfrSm |
Introduction
The oral route of drug administration is regarded as the best because of benefits such as maintaining drug concentrations within therapeutic windows, patient compliance, and ease of administration, but it also has drawbacks such as significant drug level fluctuation, low bioavailability, gastric retention time of the dosage form, surface area, and enzymatic activity. Floating systems have less bulk density than gastric fluid so stay buoyant in the stomach without affecting the gastric emptying rate for a prolonged period of time thus enhance gastro retention and consequently drugs are released in a controlled manner which manage the fluctuation in plasma drug concentration. The drug from floating drug delivery systems releases gradually at a precise rate. These systems also amplified bio- availability and solubility and reduced drug dumping. Floating dosage systems are gaining appreciation among other dosage system3-6. Gas generating floating microspheres perform for the extended release of drugs as a driving mechanism, while non-effervescent system are prone to shape balloon like structure inside microspheres because of volatile solvents which make it possible for them to float on the gastric fluid7. Floating systems are administered as single unit and multiple units dosage systems, later float for longer durations and provide reliable release of drugs. The multiple unit dosage form has a specific surface that is about thousand times greater than the single dosage form in equivalent dosage8.
Quinapril hydrochloride is an angiotensin-converting enzyme inhibitor (ACE inhibitor) used in the treatment of hypertension and congestive heart failure. Quinapril HCl is a prodrug that belongs to the angiotensin-converting enzyme (ACE) inhibitor class of medications. It is metabolized to quinaprilat (quinapril diacid) following oral administration. A prodrug, it is converted to its active metabolite, quinaprilat, in the liver. Due to reduced angiotensin production, plasma concentrations of aldosterone are also reduced, resulting in increased excretion of sodium in the urine and increased concentrations of potassium in the blood. Quinapril HCl is indicated for the treatment of high blood pressure (hypertension) and as adjunctive therapy in the management of heart failure. It may be used for the treatment of hypertension by itself or in combination with thiazide diuretics, and with diuretics and digoxin for heart failure. Quinapril HCl has short half-life of 2 hrs. So the gastro retentive drug delivery systems are needed for Quinapril HCl to prolong its duration of action, to increase its oral bioavailability, to reduce the frequency of administration and to improve patient compliance 9-10. To reduce the frequency of administration and to improve patient compliance, gastro retentive floating system formulation is desirable. Quinapril HCl is a nonpeptide, non-sulfhydryl prodrug that is deesterified to quinaprilat (quinapril diacid), its major active metabolite following oral administration. Quinaprilat lowers blood pressure by antagonizing the effect of the Renin-Angiotensin-Aldosterone System (RAAS). The RAAS is a homeostatic mechanism for regulating hemodynamics, water and electrolyte balance. The most commonly method of modulating the drug release is to include it in a matrix system. Because of their flexibility, hydrophilic polymer matrix systems are widely used in oral controlled drug delivery to obtain a desirable drug release profile, cost effectiveness and broad regulatory acceptance. The objective of the present investigation is to develop a gastroretentive drug delivery system containing quinapril HCl as a drug candidate which would remain in the stomach or upper part of GIT for prolonged period of time thereby maximizing the drug release at the desired site within the stipulated time with a view to improve bioavailability to provide effective treatment to the patients suffering from hypertension. Hence in the present work an attempt has been made to develop prolong release floating microspheres of Quinapril HCl using hydrophilic matrix materials like HPMC. Caragennan used for promoted water uptake capacity of system inside the matrix system. The utility of PMMA (Methyl methacrylate polymer) as ion exchange resin will be able to develop incorporation of air bubbled and formation of large hollow structure inside the solid structure of floating microspheres. Thus the combination of all these polymers in various proportions was used for completion of objective of proposed work.
Materials and Methods
Materials
Quinapril HCl was gifted by Torrent Pharma, Gujarat, India. Di- chloromethane was purchased from Thomas Baker Chemicals, Mumbai. Hydroxypropyl methylcellulose, Carrageenan and Poly methyl methacrylate were purchased from Central Drug House, New Delhi. Ethylcellulose was purchased from High Purity Laboratory Chemicals, Mumbai. Other reagents used were of analytical grade. The animal study was approved by Institutional Animal Ethical Committee (Protocol No: IAEC/VCP/2019/001/5).
Preparation of Floating Microspheres
Floating microspheres containing Quinapril HCl was prepared by the solvent evaporation technique. The suspension of hydroxypropyl methylcellulose, carragennan and PMMA was mixed uniformly and then dissolved in a mixture of dichloromethane and ethanol (1:1). Drug was dispersed in PEG 400 (0.1% v/v) in a conical flask and stirred for 1 h using various speed (Table 2) of the mechanical stirrer (Remi Laboratory Stirrer, RQ-127A/D, India). Both the polymeric suspension and drug solution were poured into a separate conical flask with polyvinyl alcohol (1% w/v) and stirred for 1 h to ensure complete evaporation of the organic phase, leaving behind prepared floating microspheres11.
Factorial Design
In the present work, a 32 factorial design was employed to develop the optimized formulation and incorporating interactive and polynomial terms to evaluate the response (Equation 1):
Y = b0 + b1X1 + b2X2 + b12X1X2 + b11X1X1 + b22X2X2 (1)
where Y is the dependent variable, b0 is the arithmetic mean response of all runs, and bi (b1,b2, b12,b11, and b22) is the estimated coefficient for the factor Xi (X1,X2,X12,X11, and X22). X1 and X2 represent the average data of each factor at a time from low–medium–high values. The interaction terms, X1 and X2, show response changes when two factors are changed simultaneously, whereas polynomial terms (X12 and X22) are included to investigate nonlinearity. Where, b1 is the estimated coefficient for the factor X1, while Y1 is the measured response. The coefficients corresponding linear effects (b1 and b2), interaction (b12) and the quadratic effects (b11 and b22) were determined from the results of the experiments. The model, a comparison between the experimental and predicted values of the responses is also presented in terms of percent accuracy12-13.
Bias was calculated by the following equation:
% Accuracy= Predicted value – Experimental value * 100
Predicted value
Various variables were selected as follows:
Independent variables:
Stirring speed (X1)
Polymeric concentration (X2)
Dependent variables:
Percentage entrapment efficiency (Y1)
Percentage yield (Y2)
In vitro buoyancy (Y3)
Percentage of drug release (Y4) 14
Characterization of Quinapril HCl Floating Microspheres
Particle size calculation
The particle size of Quinapril HCl loaded prepared floating microspheres were examined by optical microscopic method. A small quantity of microspheres was dispersed in 10 mL of purified water. The dispersion was kept under sonication for about 5 mins. A small drop of resultant solution was further placed on a clean glass slide and diameters of particles were measured (n±100)15-16.
Flow properties Determination
Various micromeritic properties of microspheres have been characterized.
Bulk density (ƿb)
The bulk density of the powder was determined by adding the powder sample into a measuring cylinder. The resulting bulk volume and weight of the powder are used for calculating bulk density:
ƿb = M=Vb
where M is the weight of the powder (g) and Vb is the bulk volume (mL).
Tapped density (ƿt)
Tapped density of the powder was determined by
ƿt = M=Vt
where M is the weight of the powder and Vt is the least volume occupied by the sample in a measuring cylinder (mL).
Carr’s index
This measures the compressibility of a powder. Carr’s index (IC) ‡25 indicates poor flowability and £15 good flowability:
IC = ƿt – ƿb / ƿt * 100
where rt represents the tapped density and rb the bulk density.
Angle of repose (Ɵ)
It was determined by the funnel method where powders were allowed to pass through the funnel, which is vertically raised to a maximum height (h) of the cone, and the radius (r) of the cone was measured.
h = tan-1 h / r
Surface Electron Microscopy
The surface texture of drug-loaded floating microspheres (FLMs) were inspected using scanning electron microscopy (SEM) technology (Jeol JSM- 1600, Japan) at RGPV, Bhopal, India. The samples were dried thoroughly in vacuum desiccators before mounting on brass specimen studies. A small quantity of drug-loaded microspheres was spread manually on a carbon tape and gold alloy of 120Aº knees was coated attached to an aluminum stub unit in Argon ambient of 8 – 10 Pascal with plasma voltage about 10mA. The sputtering was done for nearly 10 sec to obtain uniform coating on the sample to enable good quality SEM images. The SEM was operated at low accelerating voltage of about 10mA. The gold coated sample placed in chamber of SEM (Jeol, JSM 6390 LA) and secondary images were recorded. Samples were analyzed by SEM with direct data capture of the image on a computer screen17-18.
Drug–polymer compatibility study
FT-IR spectra for pure Quinapril HCl and drug with excipients combinations were acquired at room temperature using FTIR spectrophotometer (FTIR-8400S, Shimadzu, Japan) in transmittance mode. The samples were ground in a mortar, mixed with Nujol and placed between two plates of KBr and compressed to form a thin film. The sandwiched plates were placed in the infrared spectrometer and the spectra were obtained. Scanning was performed between wave numbers 3600-1200 cm-1 19.
Differential scanning calorimetric study
Differential scanning calorimetry (DSC) curves were measured for thermoanalytical examinations of samples (Perkin Elmer DSC7, Waltham, MA). The DSC curves of the pure drug, drug with excipients were analyzed and compared by its samples (10 mg) were crushed and kept inside a sealed aluminum pan. Purity check was performed at a heat- ing rate of 10°C min-1, with temperature variation from room temperature to 400°C (N2 at a flow rate of 30 mL/min), using an empty aluminum sample pan as the reference 20.
Percentage of drug entrapment efficiency
The prepared floating microspheres equivalent to 25 mg of pure drug were crushed in pestle mortar and transferred to a 50 mL volumetric flask. Microspheres were dissolved in 10 mL of ethanol and the final volume (50 mL) was made using 0.1N HCL (SGF). The mixture was then sonicated for about 1 h and the solution was further filtered through the whatman filter paper (#44) and appropriately diluted 21. The resultant solution was spectrophotometrically analyzed at 214 nm to determine the amount of drug entrapped. The percent drug entrapment was determined by following equation:
In vitro buoyancy
USP Type I dissolution test apparatus was used for such study. The prepared floating microspheres (equivalent to 25 mg drug) were spreading in 100 mL of 0.1N HCL, pH 1.2 containing surfactant, which was stirred at 100 rpm and at 37 ± 0.5°С to imitate gastric fluid. After specific interval of time, both the fraction of microspheres (floating and settled microspheres) was collected. After all experiment the layer of buoyant microspheres (Wf) was pipette out and separated by filtration. At the same time, the sink microspheres (Ws) were separated 22. Both microspheres were dried overnight (40°C) and the final weight was calculated separately, and buoyancy was checked by the following formula:
Percent Buoyancy = Weight of microspheres floated * 100
Total weight of microspheres (Floated +Settled)
In vitro drug release
The in vitro – in vitro dissolution studies were carried out using USP Type I dissolution apparatus. Dissolution studies of prepared floating microspheres (equivalent to 25 mg drug) were performed using the USP type I dissolution testing apparatus for 12 h. The dissolution medium used for the study was 0.1N HCL (900 mL, 37 ± 0.5°С), which was agitated (100 rpm). Perfect sink conditions were maintained during the study. 1 mL samples were withdrawn periodically at different time intervals and replaced with pre-warmed fresh medium, diluted appropriately, passed through a membrane filter (#5 mm), and analyzed spectrophotometrically using a UV spectrophotometer (Shimadzu UV-1700, Japan) at 214 nm. Each experiment was performed thrice, but average data were considered in the analysis 23.
In vivo floating behaviour
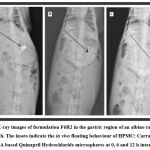
In vivo floating behaviour of the prepared microspheres was observed through X-ray images with modification. The barium sulphate was used as a diagnostic agent or as a core material for the justification of floating behaviour. The microspheres was prepared placebo without addition of drug with barium sulphate was kept in empty gelatine capsular shell. Microspheres in gelatine capsules were administered with 10 ml of water to a albino rabbit after a light meal. The source of the X-ray machine and the animal were kept uniform throughout the procedure, and finally, images of the gastric region were captured at 0, 6 and 12 h to observe the floatability of microspheres 24. This experimental design was conducted at the RKDF University, Bhopal and approved by the Institutional Animal Ethics Committee (Registration No. IAEC/VCP/2019/001/5).
Data and statistical analysis
All data are presented as mean standard deviation. Statistical analysis was performed using GraphPad Prism, Version 4, software (GraphPad Software, Inc., La Jolla, CA).
Results and Discussion
Preparation and Characterization of Floating Microspheres
From the various concentrations of HPMC, Carrageenan and PMMA, in the ratio of 1:2:3 (300 mg) with a suitable blend with drug (25 mg) provides a better spherical shape of microspheres. The presence of PEG 400 in the formulation enhances the porous surface of the microspheres in the polymeric solution and aids in conversion into network like aggregates. This were get solidified before droplet formation due to evaporation of ethanol as a result of the slow diffusion rate of the solvent. The utility of PMMA (Methyl methacrylate polymer) as ion exchange resin developed incorporation of air bubbled for the formation of large hollow structure inside the solid structure of floating microspheres. Solvent addition, polymeric nature, its quantity, and its proportion affect the floating nature, and the increasing amount of polymer enhances floatability. Increase in the polymeric blend combination (PMMA amount) level or its molecular weight was expected to affect the drug release rate from the microsphere surface environment 25.
Experimental Design
Quinapril HCl loaded floating microspheres were developed by the solvent evaporation method. The 32 factorial experimental designs were execute to check the effect of formulation variables on all set of floating microspheres. To demonstrate the effects of independent variables on the prepared floating microspheres, A 32 factorial design was performed with two factors (X1 and X2), each at three levels (low [-1], medium [0], and high [+1]) for identifying optimum levels, which are reported (Table 1). Total eighteen experimental protocols were suggested by the model, and each variable was varied to optimize for different responses. X1 and X2 factors indicate the stirring speed and concentration of the polymer (independent variables); while Percentage entrapment efficiency (Y1), percentage yield (Y2), in-vitro buoyancy (Y3), percentage of drug release (Y4).
Table 1: The different variables parameters for the 32 factorial study as independent Variables and Their Levels.
| Levels | Stirring speed (rpm) X1 | Polymer concentration (mg) X2 |
| -1 | 250 | 50 |
| 0 | 500 | 100 |
| 1 | 750 | 150 |
Table 2: Formulation of Various Batches of Floating Microspheres of Quinapril Hydrochloride.
| S. No. | Formulation Code | Drug (mg) | HPMC (mg) | Carrageenan (mg) | PMMA (mg) | DCM
ethanol |
PVA (% w/v) | Stirring speed (rpm) |
| 1 | F1R1 | 25 | 150 | 100 | 50 | 01:01 | 1 | 250 |
| 2 | F1R2 | 25 | 150 | 100 | 50 | 01:01 | 1 | 500 |
| 3 | F1R3 | 25 | 150 | 100 | 50 | 01:01 | 1 | 750 |
| 4 | F2R1 | 25 | 150 | 50 | 100 | 01:01 | 1 | 250 |
| 5 | F2R2 | 25 | 150 | 50 | 100 | 01:01 | 1 | 500 |
| 6 | F2R3 | 25 | 150 | 50 | 100 | 01:01 | 1 | 750 |
| 7 | F3R1 | 25 | 100 | 50 | 150 | 01:01 | 1 | 250 |
| 8 | F3R2 | 25 | 100 | 50 | 150 | 01:01 | 1 | 500 |
| 9 | F3R3 | 25 | 100 | 50 | 150 | 01:01 | 1 | 750 |
| 10 | F4R1 | 25 | 100 | 150 | 50 | 01:01 | 1 | 250 |
| 11 | F4R2 | 25 | 100 | 150 | 50 | 01:01 | 1 | 500 |
| 12 | F4R3 | 25 | 100 | 150 | 50 | 01:01 | 1 | 750 |
| 13 | F5R1 | 25 | 50 | 150 | 100 | 01:01 | 1 | 250 |
| 14 | F5R2 | 25 | 50 | 150 | 100 | 01:01 | 1 | 500 |
| 15 | F5R3 | 25 | 50 | 150 | 100 | 01:01 | 1 | 750 |
| 16 | F6R1 | 25 | 50 | 100 | 150 | 01:01 | 1 | 250 |
| 17 | F6R2 | 25 | 50 | 100 | 150 | 01:01 | 1 | 500 |
| 18 | F6R3 | 25 | 50 | 100 | 150 | 01:01 | 1 | 750 |
The outcomes of formulation variables on percent drug entrapment efficiency, percentage yield, in vitro buoyancy percentage, and cumulative percent drug release (dependent variables) were studied. The results for different dependent variables were significant with each other. The optimized formulation was preferred based on the highest values of percent drug entrapment efficiency and percent drug release 26. Among various formulations, F6R2 revealed the maximum values of percent drug entrapment efficiency (88.7±1.29%), percentage yield (97.7±2.64%), in vitro buoyancy (94.2±2.43%) and Cumulative % drug release (77.11±1.21%) with an optimized formula (HPMC: carragenan: PMMA ratio of 1:2:3 and stirring speed of 500 rpm) confirmed the optimized batch and for further evaluation studies (Table 3).
Table 3: 32 Factorial Design for Floating Microspheres of Quinapril Hydrochloride
| Formulation code | Independent variables | Dependent variables | ||||||
| X1 | X2 | Y1 | Y2 | Y3 | Y4 | |||
| HPMC (mg) | Carrageenan (mg) | PMMA (mg) | Percent entrapment | Percentage yield | in vitro buoyancy | Percentage of drug release | ||
| F1R1 | -1 | 1 | 0 | -1 | 82.2 | 79.5 | 71.5 | 97.84 |
| F1R2 | 0 | 1 | 0 | -1 | 84.7 | 82.3 | 74.9 | 94.26 |
| F1R3 | 1 | 1 | 0 | -1 | 78.38 | 78.24 | 79.54 | 99.34 |
| F2R1 | -1 | 1 | -1 | 0 | 84.25 | 79.21 | 78.56 | 92.01 |
| F2R2 | 0 | 1 | -1 | 0 | 83.3 | 83.8 | 84.11 | 89.54 |
| F2R3 | 1 | 1 | -1 | 0 | 83.87 | 81.56 | 81.85 | 91.65 |
| F3R1 | -1 | 0 | -1 | 1 | 86.24 | 82.54 | 84.35 | 89.24 |
| F3R2 | 0 | 0 | -1 | 1 | 84.1 | 85.1 | 91.3 | 83.1 |
| F3R3 | 1 | 0 | -1 | 1 | 81.25 | 86.12 | 82.98 | 86.21 |
| F4R1 | -1 | 0 | 1 | -1 | 86.21 | 88.87 | 81.89 | 96.25 |
| F4R2 | 0 | 0 | 1 | -1 | 83.4 | 90.1 | 82.3 | 92.35 |
| F4R3 | 1 | 0 | 1 | -1 | 81.56 | 87.95 | 83.78 | 94.87 |
| F5R1 | -1 | -1 | 1 | 0 | 82.78 | 87.56 | 79.56 | 91.54 |
| F5R2 | 0 | -1 | 1 | 0 | 81.3 | 95.2 | 88.3 | 88.6 |
| F5R3 | 1 | -1 | 1 | 0 | 83.45 | 92.85 | 81.78 | 90.87 |
| F6R1 | -1 | -1 | 0 | 1 | 81.85 | 92.87 | 89.45 | 89.78 |
| F6R2 | 0 | -1 | 0 | 1 | 88.7 | 97.7 | 94.2 | 77.11 |
| F6R3 | 1 | -1 | 0 | 1 | 84.89 | 91.85 | 90.56 | 85.45 |
|
X1 = stirring speed; X2 = concentration of HPMC: Carrageenan: PMMA; Y1 = percentage entrapment efficiency; Y2 = percentage yield; Y3 = in vitro buoyancy; Y4 = percentage of drug release. |
||||||||
Characterization of Quinapril HCl Floating Microspheres
Micromeritic properties
The result of various properties of micromeritics was showed in Table 4. The bulk density values vary between 0.32±0.018 to 0.41± 0.016 g/cm3 depending upon the amount of HPMC: Carrageenan: PMMA concentration blended in the floating microsphere. The formulations, F5R3, F5R2, and F4R2, showed higher bulk densities compared with F1R1, F1R3, F2R1, F2R2 due to HPMC content within the formulations have a major impact on the bulk density value. The values of tapped density ranged from 0.42± 0.017 to 0.55±0.019 g/cm3, indicating variability in HPMC and PMMA concentration in the formulation. The values of carr’s index ranged between 11.14± 0.15% and 17.28±0.15%, suggesting free-flowing microspheres. The angle of repose ranged between 25.80±1.12° and 30.58±2.58°, indicating an excellent flow of prepared floating microspheres.
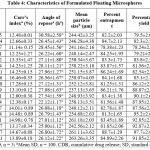 |
Table 4: Characteristics of Formulated Floating Microspheres. |
Particle size determination
The mean particle size of floating microspheres was found to be between 237.13±3.65 and 263.26±3.35 μm. As the polymer concentration of methacrylate polymer and its combination of HPMC polymer concentration with rises in stirring speed were enhanced (F6R3), the viscous level rises and sudden bulkiness in the particle size appeared during study.
Surface electron microscopy
The surface morphological structure of floating microspheres was examined by surface electron microscopic study (Figure 3). The result of SEM confirmed was observed that the fabricated microspheres were spherical with a smooth surface. The presence of pores was detected on the microspheric surface, which indicated leaching of the drug during the dissolution formation of pores on the polymeric surface. The floating microspheres have a porous structure due to evaporation of the trapped solvent during fabrication from microspheres 27. An optimized floating microsphere, F6R2, at different magnification is represented in Figure 1.
 |
Figure 1: (a) Single spherical microsphere, F6R2. (b) SEM image of the optimized floating microsphere formulation, F6R2. |
Drug–polymer compatibility study
The fourier transform infrared (FTIR) spectroscopy study was conducted for pure drug, polymer, and optimized formulation to investigate the physicochemical characterization. The compatibility study of drug with polymeric blend was performed (Figure 2). FTIR spectra of pure drug were observed principal peaks at 1700 cm-1 (C=O (Carbonyl)), 1645 cm-1 (C=C stretching (Alkenyl)), 1590 cm-1 (N-H bending (Primary amine)), 1355 cm-1 (C-N stretching (Aromatic tertiary amine)), 1277 cm-1 (O-H in plane bending), and 650 cm-1 (C-H bending (Alkyne)), which are as per established standards (Figure 2). These prominent characteristic peaks are no change with drug polymeric blend combination no changes the intensity of principal peaks. The result was there was no interaction between these excipients and drug for floating microspheres.
 |
Figure 2: FTIR spectra of (a) Quinapril Hydrochloride, (b) Drug and polymer blend. |
The DSC thermogram of the drug was observed in the DSC study, an important technique used to characterize the solubility and physical state of the drug and its combination with polymeric blend (Figure 3). A peak at 111.92°C exhibits the melting point of drug. No alteration in the melting point of drug (113.12°C) was observed in the DSC thermogram curve of drug and its polymeric blend combination 28.
 |
Figure 3: DSC thermogram of (a) Quinapril Hydrochloride, (b) Drug and polymer blend. |
Drug entrapment efficiency
The drug entrapment percentage was found to be ranging from 78.38±1.23 to 88.7±1.29%. An decrease the polymer concentration (HPMC) but increases the PMMA polymer increases deceases the drug loading The important parameter of drug loading was based on the but stirring rate (250– 750 rpm), The result of drug loading is reduced due to formation of smaller microspheres, which occurred due to increased drug loss from their surface while washing during the collection process 29.
In vitro buoyancy
The in vitro buoyancy rate was found to be in the range of 71.5±2.15% to 94.2±2.43%. The in vitro buoyancy of prepared microspheres was found to be depends on types of polymers combination, quantity, ratio, and solubility of polymers in the solvent30. The buoyancy percentage was low, having higher polymer concentrations (F1R1 and F1R3). F6R2, F5R2, F6R1, and F5R3 were able to float with good percent buoyancy, hinting at the presence of pores and cavities. F6R3 (larger mean particle size) significantly showed the highest percent buoyancy, which provides a clear suggestion that the air bubbled and hollow structure was developed inside the particles and particle size could be with buoyancy in microspheres.
In vitro drug release
The cumulative percentage release of Quinapril HCl from prepared floating microspheres was measured using the USP type I dissolution apparatus after 12 h. The result was found to be within 77.11±1.21% and 97.84±2.41% (Figure 4). Drug release from the HPMC, Carrageenan and PMMA combinations diffuses through the matrix of surface of microspheres into 0.1N HCL. The solubility of drug at acidic pH affects the dissolution rate and bioavailability of drug. The burst effect around 75-80% of drug released within 12 h results in an increasing HPMC level and non significant microsphere size produced by PMMA. A higher HPMC concentration and moderate stirring speed (500 rpm) were exhibiting the highest release rate (97.7±2.64%). This retardation may happen due to a higher concentration of polymer PMMA used as a resinous material, which reduces the porous structure on surface. The presence of PVA has increased solubilisation, which aids in release of the drug from microspheres. F6R2 retarded the release of drug at the desired site (77.11±1.21%), whereas other formulations were having a higher drug release rate 31.
 |
Figure 4: In vitro drug release of all formulations. (a) Floating microspheres. (F1R1–F3R3) (b) Floating microspheres (F4R1–F6R3). |
In vivo floating behaviour
For increased residency in the stomach, the floating capacity of dried floating microspheres was evaluated in simulated gastric fluid. F6R2 was prepared with HPMC: Carrageenan: PMMA (1:2:3) exhibiting good in vitro buoyancy percentage was further selected for study by the radiological technique. X-ray images taken at different time intervals for the buoyancy study (Figure 5) indicate the gastro- retentive property of microspheres, emphasizing a significant gastric residence time for optimum release and absorption of the drug due to the porous nature of floating microspheres 32.
Conclusion
Drug absorption through gastrointestinal tract is a highly variable procedure and prolonging gastric retention of the dosage form extends the time for drug absorption. Floating controlled drug delivery systems are engaged to resolve this trouble. Floating microspheres have been screening elevated prospective for gastroretention and offer a competent delivery system for enhancing bioavailability and controlling the release of many drugs. The present study of floating microspheres of drug such as Quinapril HCl. The drug was estimated with various parameters i.e. FTIR, DSC etc. Various parameters such as buoyancy percentage, in vitro drug release and in vivo floating behavior studies were characterized and evaluated. The floating microspherical particles with a wide size range have greater flow ability during finishing of capsules. SEM studies prove that porous-structured microspheres exhibited excellent buoyancy percentage in simulated gastric fluid. The F6R2 formulation showed the minimum cumulative percent drug release and could be regarded as the optimized formulation. X-ray images revealed that floating microspheres remained buoyant in the albino rabbit stomach for a longer duration. This proposed formulation was administered in oral dosage regimen, which in the long term will remain as the safest and alternative management to improve bioavailability and provide the effective treatment to the patients suffering from hypertension. The gastroretentive floating microspheres will beneficially alter the absorption profile of the active agent, thus enhancing its bioavailability.
Acknowledgement
The authors wish to thank the RKDF University, Bhopal for its financial support.
Conflict of Interest
All authors disclose that there is no institutional or commercial conflict of interest regarding the publication of a manuscript.
Funding Sources
There is no funding sources.
References
- Fini A, Bergamante V, Ceschel GC, Ronchi C, Moraes CA. Fast dispersible slow releasing ibuprofen tablets. Eur J Pharm Biopharm. 69 (1): 335–341 (2008).
CrossRef - Jain N, Kori ML. Anti Microbial Potential of Plumbagin β-Cyclodextrin Complex against Human Colonic Microflora, Inventi Rapid: Ethnopharmacology 1, 1-8 (2018).
- Jain N, Kori ML. Enhancement of solubility profile of plumbagin containing tablet at different colonic region for colon targeting drug delivery system, Indian drugs, 55(12): 78-82 (2018).
CrossRef - Jain AK, Jain N, Kori, ML, Jain UK. Formulation and Evaluation of Microspheres of Isoniazid for Treatment of Tuberculosis, Global Journal of Pharmacology, 14(2): 28-33 (2020).
- Kumaraswamy S, Thangasundaralingam SR, Sekar R, Jayakrishnan A. A floating-type dosage form of repaglinide in polycarbonate microspheres, Journal of Drug Delivery Science and Technology, 41: 99-105 (2017).
CrossRef - El Nashar NF, Donia AA, Mady OY, El Maghraby GM. Formulation of clarithromycin floating microspheres for eradication of Helicobacter pylori, Journal of Drug Delivery Science and Technology, 41: 213-221 (2017).
CrossRef - Stefani RM, Lee AJ, Tan AR, Halder SS, Hu Y, Guo XE, Stoker AM,. Ateshian GA, Marra KG, Cook JL, Hung CT. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair, Acta Biomaterialia, 102: 326-340 (2020).
CrossRef - Jain N, Kori ML, Jain AK. Pectin and Its Combination with Different Polymers for Colon-Targeted Drug Delivery: A Review, Inventi Rapid: NDDS, 1: 1-8 (2018).
- Fischer J, Ganellin CR. Analogue-based Drug Discovery. John Wiley & Sons. p. 468. (2006).
CrossRef - Fentie M, Belete A, Mariam TG. Formulation of Sustained Release Floating Microspheres of Furosemide from Ethylcellulose and Hydroxypropyl Methylcellulose Polymer Blends. J Nanomed Nanotechnol, 6: 262 (2015).
CrossRef - Panwar MS, Tanwar YS. Factorial design approach for optimization of floating microspheres of diltiazem hydrochloride. Asian J Pharm 9: 206-212 (2015).
CrossRef - Pande AV, Nimbalkar UA, Dhoka MV, Sonawane PA. Floating microspheres of cefpodoxime proxetil: Formulation and optimization by factorial design. Int J Res Pharm Chem, 1:448‑457 (2011).
- Nila MV, Sudhir, MR, Cinu TA, Aleykutty, NA., Jose S. Floating microspheres of carvedilol as gastro retentive drug delivery system: 32 full factorial design and in vitro Drug Delivery; 21(2), 110–117 (2013).
CrossRef - Gonnabathula PK, Velmurugan S, Venkateswara Rao CH. Design and optimization of effervescent floating matrix tablet of quetiapine fumarate using 32factorial designs. J Biochem Pharmacol Res, 6:810-22 (2014).
- Jain AK, Jain CP, Tanwar YS, Naruka PS. Formulation, characterization and in vitro evaluation of floating microspheres of famotidine as a gastro retentive dosage form, Asi J of Pharmac, 3(3): 222-226 (2009).
CrossRef - Kiyoyama S, Shiomori K, Kawano Y, Hatate Y. Preparation of microcapsules and control of their morphology. J Microencapsul, 20:497‑508 (2003).
CrossRef - Yang Z, Song B, Li Q, Fan H, Ouyang F. Preparation of microspheres with microballoons inside for floating drug delivery systems. J Appl Polym Sci, 94:197‑202 (2004).
CrossRef - Jain A K, Jain C P. Buoyant microspheres of famotidine: An approachable dosage form for gastric treatment. J Young Pharmacists, 1:20-23 (2009).
CrossRef - Yushen G, Stephen R Byrn, George Z. Physical characteristics and chemical degradation of amorphous quinapril hydrochloride. , 89(1), 128–143 (2000).
CrossRef - Durowoju IB, Bhandal KS, Hu J, Carpick B, Kirkitadze M. Differential Scanning Calorimetry – A Method for Assessing the Thermal Stability and Conformation of Protein Antigen. J Vis Exp. 121:55262 (2017).
CrossRef - Dey S, Pramanik S, Malgope A. Formulation and Optimization of Sustained Release Stavudine Microspheres Using Response Surface Methodology. ISRN Pharmaceutics, 1–7 (2011).
CrossRef - Sharma M, Kohli S, Dinda A, In-vitro and in-vivo evaluation of repaglinide loaded floating microspheres prepared from different viscosity grades of HPMC polymer, Saudi Pharmaceutical Journal, 23, 6, 675-682 (2015).
CrossRef - Gharti K, Thapa P, Budhathoki U, Bhargava A. Formulation and in vitro evaluation of floating tablets of hydroxypropyl methylcellulose and polyethylene oxide using ranitidine hydrochloride as a model drug. J Young Pharm. 4(4):201-208 (2012).
CrossRef - Patel A, Modasiya M, Shah D, Patel V. Development and in vivo floating behavior of verapamil HCl intragastric floating tablets. AAPS PharmSciTech. 10(1):310-5 (2009).
CrossRef - Goswami S, Naik S. Natural gums and its pharmaceutical application, Journal of Scientific and Innovative Research 3 (1): 112-121 (2014).
CrossRef - Muhammad A, Nasir A, Hussain A, Saeed H, Shah A, Mohammad S. Formulation Optimization and in-vitro Evaluation of Oral Floating Captopril Matrix Tablets using Factorial Design. Tropical Journal of Pharmaceutical Research, 14 (10), 1737–1748 (2015).
CrossRef - Tanwar YS, Naruka PS, Ojha GR. Development and evaluation of floating microspheres of verapamil hydrochloride. Revista Brasileira de Ciências Farmacêuticas, 43(4), 529–534 (2007).
CrossRef - Mali A D, Bathe RS. Development and Evaluation of Gastroretentive Floating Tablets of A Quinapril Hcl by Direct Compression Technique. International Journal of Pharmacy and Pharmaceutical Sciences, 9(8), 35–46 (2017).
CrossRef - Mitra A, Dey B. Chitosan microspheres in novel drug delivery systems. Indian J Pharm Sci. 73(4):355-366 (2011).
- Streubel A, Siepmann J, Bodmeier R. Floating microparticles based on low density foam powder. , 241(2), 279–292 (2002).
CrossRef - Simoes MCR, Cragg SM, Barbu E, Frederico B. The potential of electrospun poly(methyl methacrylate)/polycaprolactone core–sheath fibers for drug delivery applications. Journal of Materials Science, (2018).
CrossRef - Yasunori S, Yoshiaki K, Hirofumi T, Hiromitsu Y. In vitro and in vivo evaluation of riboflavin-containing microballoons for a floating controlled drug delivery system in healthy humans., 275(1-2), 97–107 (2004).
CrossRef