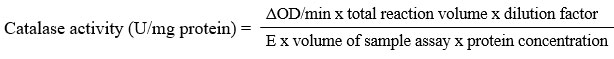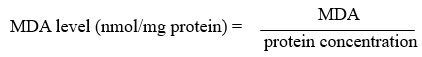Ahmad Rohi Ghazali1 , Chan Kam Soon1
, Chan Kam Soon1 , Noraisah Akbar Ali 1
, Noraisah Akbar Ali 1 and Dayang Fredalina Basri2*
and Dayang Fredalina Basri2*
1Center for Toxicology and Health Risk (CORE), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, 50300, Malaysia.
2Centre for Diagnostic, Therapeutic and Investigative Studies (CODTIS), Faculty of Health Sciences, Universiti Kebangsaan Malaysia, Kuala Lumpur, 50300, Malaysia.
Corresponding Author E-mail: dayang@ukm.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2475
Abstract
The high incidence and mortality of skin cancer along with the development of chemotherapy resistance have urged researchers to look into alternative strategies to combat cancer. Chemoprevention is one of the well-researched strategies using natural products. Canarium odontophyllum Miq. (dabai) is a local seasonal fruit that is mainly found in Sarawak, Malaysia. The leaves of the fruit harbor many medicinal useful phytochemicals that provide a new insight for chemoprevention. The present research has been planned to study the chemopreventive activity of methanolic extract of C. odontophyllum Miq. leaves on UVB induced B164A5 melanoma cells through its antioxidant profiles. The extract displayed statistically significant (p<0.05) antioxidant activity by enhancing superoxide dismutase, SOD (1023.02 ± 106.74 U/mg protein) and catalase (0.12 ± 0.003 U/mg protein) activities compared to negative control. Low oxidative damage was also observed whereby the protein carbonyl and malondialdehyde levels were significantly reduced (p<0.05), 1.69 ± 0.296 nmol/mg protein and 1.181 ± 0.03 nmol/mg protein respectively. In conclusion, the extract exhibited a promising skin cancer chemoprevention activity through its significant antioxidant activities. These findings evidently pave the path for further investigations in chemoprevention strategy.
Keywords
Antioxidant; B164A5 melanoma cells; Canarium odontophyllum Miq.; Chemoprevention
Download this article as:| Copy the following to cite this article: Ghazali A. R, Soon C. K, Ali N. A, Basri D. F. Promising Phyto-Antioxidant Methanolic Extract from Canarium Odontophyllum Miq. (Dabai) Leaves against UVB Induced B164A5 Melanoma Cells as a Potential Skin Chemoprevention Agent. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Ghazali A. R, Soon C. K, Ali N. A, Basri D. F. Promising Phyto-Antioxidant Methanolic Extract from Canarium Odontophyllum Miq. (Dabai) Leaves against UVB Induced B164A5 Melanoma Cells as a Potential Skin Chemoprevention Agent. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3UHWBq7 |
Introduction
Skin cancer is considered as one of the most widely recognised types of abnormal growth in humans1. Skin cancer can be categorized into melanoma and non-melanoma skin cancer. Non-melanoma skin cancer consists of squamous cell carcinoma and basal cell carcinoma2. According to World Cancer Research Fund International 20183, melanoma skin cancer has ranked as the 19th most commonly occur in men and women while non-melanoma skin cancer is ranked at 5th. Based on the Globocan 2020, International Agency for Research on Cancer (IARC)4, there is about 0.33 % of new melanoma skin cancers have been reported in Malaysia and ranked at 26th among the newly reported cancers.
Many skin cancers contributing factors have been identified such as ultraviolet (UV) radiation, genetic factors, sunburn, colour of skin, family history and present of melanocytic naevi5 & 6. However, UV radiation has been identified as a major contributing factor to skin cancer7. UV radiation can be subdivided into three different radiations which are UVA (315 – 400 nm), UVB (280 – 315 nm) and UVC (100 – 280 nm). UVB is substantial enough to cause cellular damages and leads to skin cancer8.
Chronic UV radiation exposure can result in lots of damages such as cell cycle alteration, DNA chain breaks, gene mutation, oxidative damage, base modification and photoproducts such as cyclopyrimidine dimers and 6-4 photoproducts9. Under normal conditions, the pro-oxidant and antioxidant system is in its best state of balance. However, the balanced state can be disrupted when there is overwhelmed production of free radicals due to repeated UV radiation exposure. It can be resulted in a tremendous production of cellular oxidative damages products such as protein carbonyl (PC) and malondialdehyde (MDA) and followed by DNA mutation and carcinogenesis as the ultimate effects 9 & 10.
Chemoprevention is a strategy that using natural or synthetic compounds to prevent, slow or reverse the carcinogenesis11. In cancer research, many scientists have eyeing on chemoprevention strategy due to its benefits overweigh the effects of the current anticancer therapy and its chemotherapy resistance. Chemoprevention primarily reduces or prevents cancer development and hence, avoiding anticancer therapy and its side effects12. On top of that, numerous significant studies have shown that crude extract or plant-based isolated phytochemicals are considerably active chemoprevention agents as they provide anti-inflammatory, antioxidant and anti-oncogenic activities towards different types of cancer13.
As for the present study, an underutilized local seasonal fruit, Canarium odontophyllum Miq. was investigated. C. odontophyllum Miq. locally known as “dabai” and mainly found in Sarawak, Malaysia. The fruits usually blossom during the end of the year from October to December14. The fruits were extensively studied for their phytochemicals content and biological activities previously15, 16 & 17. The fruits consist of phenolic compounds, flavonoids, ethyl gallate, anthocyanidins and anthocyanins17. Hence, it becomes our interest to disclose the in-vitro potentiality of the methanolic leaves extract of C. odontophyllum Miq as a potential skin cancer chemoprevention agent through its antioxidant profile. In previous study18, the methanolic leaves extract had shown no cytotoxicity at lower range of concentrations against UVB irradiated B164A5 melanoma cells. Hence, in this study, IC10, 0.17 mg/mL have been used to treat the cells.
In this study, UVB irradiated B164A5 melanoma cells were used as a testing vehicle. B164A5 melanoma cell is one of the most common and versatile models in skin cancer studies19,20 & 21. The cell line is derived from the skin melanoma of a C57BL/6 strain mouse. It has fibroblast-like characteristic and melanin-producing capacity22. Additionally, there is a significant cell viability reduction post 24 hours of UVB radiation. Hence, this cell model can be employed for photo-protective effects study23.
Materials and Methods
Plant materials and preparation of extracts
Leaves of C.odontophyllum Miq were authenticated by Herbarium Universiti Kebangsaan Malaysia in Bangi, Malaysia with voucher specimen no. UKMB 40052. The leaves were thoroughly rinsed and cut into small pieces. The leaves pieces were dried in an oven at 45 ⁰C until a constant weight was obtained. Finally, the leaves were ground by using a blender. The ground leaves powder was subjected for solvent extraction. The extraction was carried out by using methanol through the succession method24. In the ratio of 1 part of leaves powder (89.69 g) were soaked in 5 part of methanol (450 mL). By using the magnetic stirrer, the mixture was mixed for 24 hours. After that, the mixture was filtered by using filter paper to collect the residue and filtrate. The residue was dried and mixed with another 450 mL methanol for the second round of extraction. Both filtrates were mixed and filtered by filter paper and subjected under reduced pressure through a rotary evaporator. The methanolic extracted crude was air dried and stored at 4⁰C.
Leaves extract stock solution (100 mg/mL) preparation
About 100 mg of extract powder was weighed and dissolved in 1 mL of 100 % dimethyl sulfoxide (DMSO). The mixture was then mixed vigorously by using an electronic vortex for 30 minutes. By using the 0.22 µm filter membrane, the mixture was filtered for sterility. The filtered extract solution was kept at -20⁰C until further use.
Cell culture
The B164A5 murine melanoma cell line was purchased from the European Collection of Authenticated Cell Culture (ECACC). The cells were cultivated in Dulbecco’s Modified Eagle Medium (DMEM). This culture media was enriched with 10 % fetal bovine serum, 1 % PenStrep, glucose and L-glutamine. The cells were incubated in a humidified atmosphere at 37⁰C in 5 % CO2. Sub-culture was done when the cell confluency had reached 80 %.
Cell lysate preparation
The cell lysate was prepared according to Inayat-Hussain et al25 and Jung et al26. 1 x 105 cells/mL cells were seeded on 6 wells plate and incubated for 24 hours at 37 ⁰C in 5 % CO2. On the next day, the cell culture media was discarded and replaced with 1 mL phosphate-buffered saline(PBS). The cells were subjected to UVB exposure at 30 mj/cm2 for 36.4 seconds. After the UVB exposure, the PBS was discarded and the cells were treated with 0.17 mg/mL leaves extract, 50 µg/mL ascorbic acid as a positive control and untreated cells (only cell culture media) were regarded as negative control. The cell plates were further incubated for 24 hours at 37 ⁰C in 5 % CO2. On the following day, the media was discarded and washed with PBS. The cells were collected through the trypsinization process. The cells were then transferred to centrifuge tubes and centrifuged at 3000 rpm for 3 minutes. The supernatant was removed and the cell pellet was washed with PBS by centrifugation too. After that, the cell pellet was lysed with 100 µL chilled lysis buffer ( 0.1 % Triton X-100 in 10 mM phosphate buffer, pH 7.2). The lysed cells were subjected to 3 cycles of sonication and 20 seconds for each cycle. Centrifugation with microcentrifuge at 13000 x g for 10 minutes at 4 ⁰C was followed. The supernatant (cell lysate) was collected and stored at -80 ⁰C for up to 2 days. The cell lysate was used for antioxidant and oxidative stress assays.
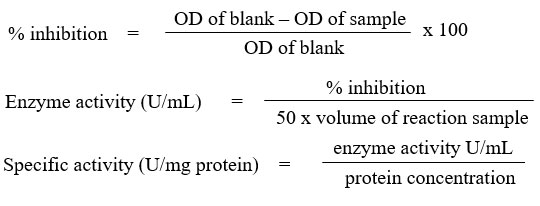
Superoxide dismutase (SOD) assay
The assay was carried out by referring to Beyer & Fridovich27 with slight modifications. 1 mL of aliquots containing (27 mL 50mM, pH 7.8 phosphate buffer, 1.5 mL 300 mg/10mL L-methionine, 1 mL 14.1 mg/10mL nitro blue tetrazolium chloride (NBT) and 0.75 mL 1 % Triton X-100) was added into the test tubes and followed with 20 µL sample. As for blank, 20 µL phosphate buffer was added instead of sample. Then, 10 µL 4.4 mg/100mL riboflavin was added to all the tubes. After mixing well, about 250 µL mixtures were transferred into the 96 wells plate. The plate was then placed in a box illuminated with 20 W lamp for 7 minutes. Absorbance reading was taken at 560 nm. The SOD activity was calculated as follows and expressed in U/mg protein.
Catalase assay
The test was done as described by Aebi28. To the test tubes, about 100 µL sample was added then followed with 800 µL 50 mM phosphate buffer, pH 7.0 and 100 µL 0.02 % Triton X-100. The mixture was allowed at room temperature for 10 minutes. Then, 2 mL 30 mM hydrogen peroxide was added into the tubes and mixed well. Without any delays, absorbance reading was taken at 240 nm for each 1 minute for 4 minutes. The decrease in absorbance was recorded and the activity of catalase was determined as below formula and expressed as U/mg protein.
Malondialdehyde (MDA) assay
Ledwozyw et al29 method was applied in carrying MDA assay. A calibration curve (0 – 25 nmol/mL) was constructed before sample analysis. 1,1,3,3-tetraethoxypropane (TEP) was used as a standard solution for calibration curve. As for sample analysis, 0.5 mL sample and 2.5 mL 19.93 % trichloroacetic acid (TCA) were added into a test tube. The mixture was mixed well and incubated for 15 minutes at room temperature. About 1.5 mL 0.05 M thiobarbituric acid (TBA) was added into the mixture and placed in the 100 ⁰C boiling waterbath for 1 hour. After that, the test tubes were be cooled at room temperature. About 4 mL n-butanol was added and mixed vigorously for 3 minutes. The tubes then were centrifuged at 3000 rpm for 10 minutes. The supernatant layer was collected for absorbance reading at 532 nm. The MDA level was calculated against the calibration curve and expressed as nmol/mg protein.
Protein carbonyl (PC) assay
The protein carbonyl level was according to Levine et al30 with some modifications. About 200 µL sample was pipetted into two test tubes separately. About 800 µL 2,4-dinitrophenylhydrazine (DNPH) was added into sample tubes whereas 800 µL 2.5 M hydrochloric acid (HCl) into the tube which regarded as blank. All the tubes were subjected for one hour dark room temperature incubation. For each 15 minutes interval during incubation period, each tube was briefly vortex. 1 mL 20 % TCA was added and mixed. The tubes were incubated for 5 minutes on ice. By using microcentrifuge, centrifugation was done at 10 000 x g for 10 minutes at 4⁰C. The supernatant was removed and the pellet suspended with 1 mL 10 % TCA. The tube was placed on ice and left for 5 minutes. The tubes were then centrifuged with microcentrifuge as described previously. The supernatant was removed and the pellet was mixed with 1 mL of ethanol / ethyl acetate (1:1) mixture. The mixture was re-suspended with spatula and vortex thoroughly. The tubes were then centrifuged again. The previous steps ( begin with ethanol / ethyl acetate mixture) were repeated for 2 times. After the final microcentrifugation, the pellet was re-suspended with 500 µL guanidine hydrochloride and mixed. Again, the tubes were then centrifuged. A volume of 200 µL supernatant was transferred into the 96 wells plate and absorbance readings were taken at 370 nm. The protein carbonyl level was calculated as below formula and expressed as nmol/mg protein.
Statistical analysis
The SPSS v25 software was used in analysing the results. Each data was presented as the mean ± standard error of mean (SEM) of triplicates from 3 different experiments (n=3). Independent t-test was used for mean comparisons. p value <0.05 was considered as statistically significant.
Result and Discussion
As human skin is the external largest barrier, undoubtedly it can be continuously subjected to many extrinsic noxious insults which include chemicals, viruses and UV radiation. In addition, these are all contributing factors, particularly UVB radiation, to skin cancer31 & 32. Exposure of UVB can cause tremendous production of free radicals which will lead to oxidative damages. To counteract the oxidative stress, an effective antioxidant defense system is required33.
In our body, we have a collections of antioxidant defense system which is categorised into first, second, third and fourth line defense antioxidants. The first line is the only enzymatic antioxidants defense system that comprises of SOD, catalase and glutathione peroxidase. These enzymes are very effective to neutralize any molecules that have the potential to become free radicals or the free radicals that have the capability to produce other free radicals34.
Hence, it is relevant to find a chemoprevention agent that can provide an effective antioxidant activity. Previous numerous studies have shown that agents from natural resources such as medicinal plants, herbs and their phytochemicals have provided promising chemopreventive effects against many cancers35. In this study also it was found the antioxidants potentiality of the Canarium odontophyllum Miq. leaves extract against UVB induced B164A5 melanoma cells.
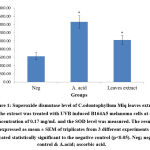
SOD is known as the first detoxification enzyme as well as the most powerful endogenous cellular antioxidant34. It was found that the extract had significantly induced the SOD level (1023.02 ± 106.74 U/mg protein) and the activity was significantly (p<0.05) higher than the negative control (625.35 U/mg protein) (Figure 1). The SOD acts by catalysing the dismutation of superoxide radicals into hydrogen peroxide and molecular oxygen which reduces the superoxide radical’s action33. Observed findings in this study were in agreement with Shakirin et al16 where the kernel oil extracted from Canarium odontophyllum Miq. fruit was also significantly enhanced the SOD level in healthy rabbits in-vivo model and provided a protective effect.
In addition, it was also found that the extract was able to catalyse the decomposition of hydrogen peroxide into water and molecular oxygen. It can be proven through catalase assay in which the level was increased up to 0.12 ± 0.003 U/mg protein and it was three folds higher than the negative control, 0.04 ± 0.008 U/mg protein (Figure 2). By decomposing the hydrogen peroxide, it completed the detoxification process which initiated by SOD34. The significant finding was similar to Gazuwa et al36 where the Canarium schweinfurthii oil had successfully induced the blood catalase level in paraquat-induced lipid peroxidation rats model.
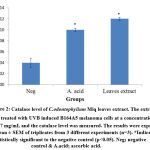 |
Figure 2: Catalase level of C.odontophyllum Miq. leaves extract. The extract was treated with UVB induced B164A5 melanoma cells at a concentration of 0.17 mg/mL and the catalase level was measured. |
Above findings were also supported with our oxidative damage markers’ evaluation. Reactive oxygen species (ROS) generated during UVB exposure would damage the cell membrane and organelles which lead to lipid peroxidation and protein oxidation by-products such as MDA and PC respectively37,38 & 39. From this study investigation, both MDA and PC were significantly (p<0.05) reduced with the value of 1.181 ± 0.037 and 1.69 ± 0.296 nmol/mg protein respectively (Figure 3 & 4). The results were in accordance with Budin et al40 where the aqueous extract of leaves of Canarium odontophyllum Miq. had provided a protective effect by reducing the MDA and PC level in streptozotocin-induced diabetic rats.
Ascorbic acid was well known as a potent antioxidant which could scavenge the free radicals by donating electrons and reduced the oxidative stress41. Hence, ascorbic acid was regarded as a positive control in our study. Through our findings, the ascorbic acid had significantly (p<0.05) increased the antioxidant activities and also significantly (p<0.05) reduced the oxidative stress. In comparison, our extract had exhibited a comparable antioxidant activity to ascorbic acid.
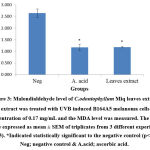 |
Figure 3: Malondialdehyde level of C.odontophyllum Miq. leaves extract. The extract was treated with UVB induced B164A5 melanoma cells at a concentration of 0.17 mg/mL and the MDA level was measured. |
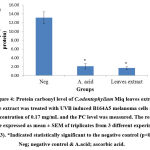 |
Figure 4: Protein carbonyl level of C.odontophyllum Miq. leaves extract. The extract was treated with UVB induced B164A5 melanoma cells at a concentration of 0.17 mg/mL and the PC level was measured. |
Based on the previous studies42 & 43, the phytochemical screening in methanolic extract of leaves from C. odontophyllum Miq. had shown that it contained saponin, terpenoid, tannin, phenolic compounds and flavonoid, without the presence of alkaloid. Based on this study, it can postulated that those phytochemicals were responsible for the chemopreventive activity through their antioxidant mechanism. The extract could be considered to have a specific photo-protective mechanism as the extract had the capability to increase the antioxidant enzymes, SOD and catalase.
Conclusion
In conclusion, the methanolic leaves extract from C. odontophyllum Miq. had shown its promising protective effects against UVB induced B164A5 melanoma cells. Based on these findings, the extract possessed an effective antioxidant mechanism to be developed as a potential skin cancer chemoprevention agent.
Acknowledgment
This research was funded by grant of DIP-2018-034. We also would like to acknowledge the Sarawak Biodiversity Centre, Malaysia for the permit for export and the permit for research and development with permit number SBC-2020-EP-58-MWH and SBC-2019-RDP-20-MWH respectively.
Conflict of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Funding Sources
There is no funding source.
References
- Abdulhamid I. A. M, Sahiner A and Rahebi J. New auxiliary function with properties in nonsmooth global optimization for melanoma skin cancer segmentation. BioMed Research International. 2020.
- Penta D, Somashekar B. S and Meeran S. M. Epigenetics of skin cancer: Interventions by selected bioactive phytochemicals. Photodermatology, Photoimmunology & Photomedicine. 2018; 34(1): 42-49.
- World Cancer Research Fund International, “Skin Cancer Statistics,” https://www.wcrf.org/dietandcancer/cancer-trends/skin-cancer-statistics.
- Globocan 2020, International Agency for Research on Cancer, https://gco.iarc.fr/today/data/factsheets/populations/458-malaysia-fact-sheets.pdf.
- Gandini S, Autier P and Boniol M. Reviews on sun exposure and artificial light and melanoma. Progress in biophysics and molecular biology. 2011; 107(3): 362-366.
- Berwick M, Erdei E, Hay J. Melanoma epidemiology and public health. Dermatologic Clinics. 2009; 27: 205-214.
- Narayanan D. L, Saladi R. N and Fox J. L. Ultraviolet radiation and skin cancer.International J. of Dermatology. 2010; 49: 978-986.
- Mohania D, Chandel S, Kumar P, Verma V, Digvijay K, Tripathi D, ChoudhuryK, Mitten S. K and Shah D. Ultraviolet radiations: skin defense-damage mechanism.In Ultraviolet Light in Human Health, Diseases and Environment. 2017; 71-87.
- D’Orazio J, Jarrett S, Amaro-Ortiz A and Scott T. UV radiation and the skin.International Journal of Molecular Sciences. 2013, 14, 12222-12248.
- De Jager T. L, Cockrell A. E and Du Plessis S. S. Ultraviolet light induced generation of reactive oxygen species. In Ultraviolet Light in Human Health, Diseases and Environment. 2017, 15-23.
- Gupta S and Mukhtar H. Chemoprevention of skin cancer: current status and future prospects. Cancer and Metastasis Reviews. 2002; 21: 363-380.
- Piccolella S and Pacifico S. Plant-derived polyphenols: a chemopreventive and chemoprotectant worth-exploring resource in toxicology. In Advances in molecular toxicology. 2015; 9: 161-214.
- El-Harakeh M, Al-Ghadban S and Safi R. Medicinal plants towards modeling skin cancer. Current Drug Targets. 2020.
- Shakirin F. H, Prasad K. N, Ismail A, Yuon L. C and Azlan A. Antioxidant capacity of underutilized Malaysian Canarium odontophyllum (dabai) Miq. fruit. J. of Food Composition and Analysis. 2010; 23: 777-781.
- Prasad K. N, Chew L. Y, Khoo H. E, Kong K. W, Azlan A. and Ismail A. Antioxidant capacities of peel, pulp, and seed fractions of Canarium odontophyllumMiq. fruit. Journal of Biomedicine and Biotechnology. 2010.
- Shakirin F. H, Azlan A, Ismail A, Amom Z, and Yuon L. C. Protective effect of pulp oil extracted from Canarium odontophyllum Miq. fruit on blood lipids, lipid peroxidation, and antioxidant status in healthy rabbits. Oxidative Medicine and Cellular Longevity. 2012.
- Chew L. Y, Khoo H. E, Amin I, Azrina A and Lau C. Y. Analysis of phenolic compounds of dabai (Canarium odontophyllum Miq.) fruits by high-performance liquid chromatography. Food Analytical Methods. 2012; 5: 126-137.
- Ghazali, A. R.; Soon, K. S.; Basri, D. F.; Ali, N. A. Cytotoxicity and glutathione level of methanolic leaves extract from Canarium odontophyllum Miq. (Dabai) against UVB induced B164A5 melanoma cell lines. Proceeding book : Bridging Traditional Knowledge & Natural Products Innovations Towards Wellness and Shared Prosperity. 2021, 66-70.
- Oprean C, Ivan A, Bojin F, Cristea M, Soica C, Draghia L, Caunii A, Paunescu V and Tatu C. Selective in vitro anti-melanoma activity of ursolic and oleanolic acids. Toxicology Mechanisms and Methods. 2018; 28(2): 148-156.
- Sturza A, Pavel I, Ancușa S, Danciu C, Dehelean C, Duicu O and Muntean D.Quercetin exerts an inhibitory effect on cellular bioenergetics of the B164A5 murine melanoma cell line. Molecular and Cellular Biochemistry. 2018; 447: 103-109.
- Danciu C, Vlaia L, Fetea F, Hancianu M, Coricovac D. E, Ciurlea S. A, SoicaC. M, Marincu I, Vlaia V, Dehelean C. A and Trandafirescu C. Evaluation of phenolic profile, antioxidant and anticancer potential of two main representants of Zingiberaceae family against B164A5 murine melanoma cells. Biological Research. 2015; 48: 1-9.
- Danciu C, Falamas A, Dehelean C, Soica C, Radeke H, Barbu-Tudoran L, Bojin F, Pinzaru S. C and Munteanu M. F. A characterization of four B16 murine melanoma cell sublines molecular fingerprint and proliferation behavior. Cancer Cell International. 2013; 13: 75.
- Pavel I. Z, Iftode O. A, Pinzaru I, Coricovac D, Moaca A, Farcas C, Simu S. C, Soica C, Dehelean C and Motoc A. Skin Specific Cells and UVB Damage An experimental assessment. REVISTA DE CHIMIE. 2017; 68: 1229-1233.
- Basri D. F and Fan S. H. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian journal of Pharmacology. 2005; 37(1): 26.
- Inayat-Hussain S. H, Chan K M, Rajab N. F, Din L. B, Chow S. C, Kizilors A, Farzaneh F and Williams G. T. Goniothalamin-induced oxidative stress, DNA damage and apoptosis via caspase-2 independent and Bcl-2 independent pathways in Jurkat T-cells. Toxicology letters. 2010; 193: 108-114.
- Jung K, Seidel B, Rudolph B, Lein M, Cronauer M. V, Henke W, Hampel G, Schnorr D and Loening S. A. Antioxidant enzymes in malignant prostate cell lines and in primary cultured prostatic cells. Free Radical Biology and Medicine. 1997; 23:127-133.
- Beyer Jr. W. F and Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Analytical biochemistry. 1987; 161: 559-566.
- Aebi H. Catalase in vitro. In Methods in Enzymology. 1984; 105: 121-126.
- Ledwozyw A, Michalak J, Stepień A, Kadziołka A. The relationship between plasma triglycerides, cholesterol, total lipids and lipid peroxidation products during human atherosclerosis. International J. of Clinical Chemistry. 1986; 155: 275.
- Levine R. L, Garland D, Oliver C. N, Amici A, Climent I, Lenz A. G, Ahn B.W, Shaltiel S and Stadtman E. R. Determination of carbonyl content in oxidatively modified proteins. Methods in Enzymology. 1990; 186: 464-478.
- Islam S. U, Ahmed M. B, Ahsan H, Islam M, Shehzad A, Sonn J. K and LeeY. S. An Update on the Role of Dietary Phytochemicals in Human Skin Cancer: New Insights into Molecular Mechanisms. Antioxidants. 2020; 9: 916.
- Nagapan T. S, Ghazali A. R, Basri D. F and Lim W. N. Photoprotective effect of stilbenes and its derivatives against ultraviolet radiation-induced skin disorders. Biomedical and Pharmacology J. 2018; 11: 1199-1208.
- Yin S, Wang Y, Liu N, Yang M, Hu Y, Li X, Fu Y, Luo M, Sun J and Yang X. Potential skin protective effects after UVB irradiation afforded by an antioxidant peptide from Odorrana andersonii. Biomedicine & Pharmacotherapy. 2019; 120: 109535.
- Ighodaro O. M and Akinloye O. A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. of Medicine. 2018; 54: 287-293.
- Roy A, Jauhari N and Bharadvaja N. Medicinal plants as a potential source of chemopreventive agents. In Anticancer Plants: Natural Products and Biotechnological Implements. 2018; 109-139.
- Gazuwa S. Y, Jaryum K. H and Datit T. Antioxidant properties of Canarium schweinfurthii,(including its quality indices), and Elaeis guineensis oils on rats exposed to paraquat-induced lipid peroxidation. J. of Pharmacy and Biological Sciences. 2015; 2: 24-28.
- Wang Z, He Z, Emara A. M, Gan X and Li H. Effects of malondialdehyde as a byproduct of lipid oxidation on protein oxidation in rabbit meat. Food Chemistry. 2019; 288: 405-412.
- Zhu X, Li N, Wang Y, Ding L, Chen H, Yu Y and Shi X. Protective effects of quercetin on UVB irradiation‑induced cytotoxicity through ROS clearance in keratinocyte cells. Oncology Reports. 2017; 37: 209-218.
- Aryal B, Rao V. A. Specific protein carbonylation in human breast cancer tissue compared to adjacent healthy epithelial tissue. PloS One. 2018; 13.
- Budin S. B, Kumar S, Warif M. A, Saari S. M and Basri D. F. Protective effect of aqueous extracts from Canarium odontophyllum Miq. leaf on liver in streptozotocin-induced diabetic rats. Life Sci. Med. Biomed. 2018, 2: 1-5.
- Padayatty S. J, Katz A, Wang Y. Eck P, Kwon O, Lee J. H, Chen S, Corpe C,Dutta A,Dutta S. Kand Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J. of the American College of Nutrition. 2003; 22: 18-35.
- Basri D. F, Shabry A. S. M and Meng C. K. Leaves extract from Canarium odontophyllum Miq.(dabai) exhibits cytotoxic activity against human colorectal cancer cell HCT 116. Natural Products Chemistry & Research. 2015.
- Basri D. F and Nor N. H. M. Phytoconstituent screening and antibacterial activity of the leaf extracts from Canarium odontophyllum Miq. American Journal of Plant Sciences. 2014; 5: 2878.