Manuscript accepted on :12-09-2022
Published online on: 20-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Maysaa Kadhim Al-Malkey
Second Review by: Dr. Mohsen Tabasi
Final Approval by: Dr. Patorn Piromchai
Maha J Hashim* and Jeffrey R Fry
and Jeffrey R Fry
School of Life Sciences, University of Nottingham, University Park, Nottingham NG7 2RD, UK
Corresponding Author E-mail: mahajalal_73@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2478
Abstract
NAD(P)H:quinone oxidoreductase NQO1 is a phase ll enzyme that catalyzes the linked intracellular conversion of NADPH2 to NADP and duroquinone (DQ) to hydroduroquinone (DQH2) in cells. There are different methods to determine NQO1 activity. The classic NQO1 enzyme assay is the usual method for measuring NQO1 activity in cell lysates. We chose to determine the intact-cell activity to investigate the effect of the four compounds Quercetin (Q), Epigallocatechin-3-gallate, (EGCG), indole-3-carbinol (I3C), and Sulforaphane (SFN) in stimulating NQO1 activity. In brief, DQ-mediated reduction of the cell-membrane-imperative secondary electron acceptor, ferricyanide, was used to quantify intact-cell NQO1 activity. This approach involves adding quinone duroquinone to the cells and then measuring the appearance rate of the two-electron reduction product, durohydroquinone, by its ferricyanide reduction. In conclusion, I3C and SFN did not interfere with the enzymatic reaction. In contrast, Q and EGCG can interfere with the enzymatic reaction of NQO1 because Q and EGCG possess quinone structures, unlike I3C and SFN, which do not have the same shape.
Keywords
Duroquinone (DQ); EGCG; HepG2 cells; I3C; NQO1; SFN
Download this article as:| Copy the following to cite this article: Hashim M. J, Fry J. R, Evaluation of a Direct Cellular Assay for NQO1 in the Presence of Phytochemicals.Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Hashim M. J, Fry J. R, Evaluation of a Direct Cellular Assay for NQO1 in the Presence of Phytochemicals.Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3DBrq9S |
Introduction
Experimental studies in cell culture and animal models pointed to the crucial effects of natural agents in our diet to protect, reduce, and prevent diseases considered dangerous to human life, such as cancers 1, 8,9,10,11. Recently, evidence of the protective effects of phytochemicals, such as SFN, increases due to awareness of induction of phase ll detoxifying enzymes, NQO1 in particular 2. On the other side, reports showed that the diet enriched with catechins might affect the ability of the organism to detoxify free radicals and environmental xenobiotics through a significant decrease in NQO1 activity due to an unclear reason. Therefore there is a persistent need to further studies to establish a cell-based assay for NQO1 as part of screening programs for modulation of NQO1 activity 3. A recent cell-based assay for NQO1 is described by Bongard 4, which is based on the modulation of duroquinone. NQO1 is a phase ll enzyme that catalyzes the linked intracellular conversion of NADPH2 to NADP and duroquinone (DQ) to hydroduroquinone (DQH2) in the cell. The DQH2 product leaves the cell and is converted to DQ, during which ferricyanide [Fe (CN6)-3] is reduced to ferrocyanide [Fe (CN6)-4]. This enzymatic reaction can be prevented by dicumoral, the inhibitor of NQO1 (Fig.1).
Several methods are introduced to determine NQO1 activity. The classic assay is the purposive method for measuring NQO1 activity in cell lysates. In contrast, the intact-cell activity is the inference that represents the functional results for other compounds, such as antioxidant activity or toxicity of NQO1-catalyzed activation of the compound under study 5. Therefore, we chose to determine the intact-cell action to investigate the effect of the four phytochemicals Q, EGCG, I3C, and SFN, that possess antioxidants activity 6,7,12 in stimulating NQO1 activity. In brief, DQ-mediated reduction of the cell-membrane-imperative secondary electron acceptor, ferricyanide, was used to quantify intact-cell NQO1 activity. This approach involves adding quinone duroquinone to the cells and then measuring the appearance rate of the two-electron reduction product, durohydroquinone, by its ferricyanide reduction 4.In a related study of phytochemicals with NOQ1, the induction of phase ll enzyme, in particular, NQO1 prevents the generation of ROS and toxic semiquinone radicals by reducing compounds with a quinone structure due to two-electron transfer 3. We attempted to assess NQO1 activity in intact cells in the absence and presence of phytochemicals by determining ferric ion reduction. The principle of this assay is the ability of NQO1 to catalyze the conversion of duroquinone to hydroduroquinone where this enzymatic reaction is linked with reducing ferricyanide to ferrocyanide 4; therefore, lower the reduction reaction rate of iron is considered an important sign of NQO1 activity. In the absence of duroquinone, evaluation of Q and EGCG in inducing NQO1 in intact HepG2 cells may seem impossible because each interferes with the reduction reaction resulting in an effect on NQO1 assessment while I3C and SFN are not. The overlapping caused by Q and EGCG in the enzymatic reaction may be that each possesses a quinone structure where I3C and SFN are missing 6 (Fig.3). Therefore, the prolonged incubation for Q and EGCG with cells may allow them to penetrate inside the cells and be reduced by NQO1 as quinone compounds, subsequently leading to reduced ferric to ferrous.
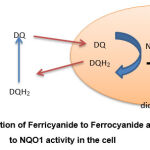 |
Figure 1: reduction of Ferricyanide to Ferrocyanide according to NQO1 activity in the cell |
Materials and methods
Chemicals
All chemicals are from Sigma Chemical Company, Pool, and Dorset, U.K.
Treatment of cells
HepG2 (ATCC USA HB-8065) in 24-well plates for 24 hours and then incubated with phytochemicals (100 μg/ml of Q, 100 μg/ml of EGCG, 25 μg/ml of I3C, and 1 μg/ml of SFN) for 20 hours in complete culture medium before incubation with 1 ml HBSS/Hepes solution per well by itself or containing 600 μM ferricyanide or 600 μM ferricyanide + 50 μM DQ 4. HBSS/Hepes solution had Hanks’ Balanced Salt Solution with 10 mM HEPES buffer pH 7.4 and 5.5 mM glucose. Incubation at 37ºC was for up to 60 minutes. Culture wells were washed three times extensively with PBS after incubation with phytochemicals to prevent the interference between these compounds and ferricyanide. In a related experiment, HepG2 cells were incubated as described above with no prior treatment with phytochemicals. The absorbance of ferricyanide in the medium was measured spectrophotometrically at 412 nm using a spectrophotometer: Multiskan Ascent manufactured by Thermo Electron Corporation.
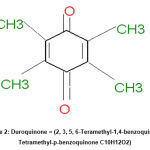 |
Figure 2: Duroquinone = (2, 3, 5, 6-Teramethyl-1,4-benzoquinone, Tetramethyl-p-benzoquinone C10H12O2). |
Results
Results demonstrated that ferricyanide did not reduce during incubation with HepG2 cells alone (Fig 3, green square). Still, it was efficiently reduced in the presence of duroquinone (Fig3, red circle), where the absorbance was falling from about 0.14 to about 0.02 after incubation for one hour ((Fig.3). In cells pretreated with Q for 20 hours, ferricyanide reduces to ferrocyanide in the absence of duroquinone (Fig. 3a), the extent of which was more significant in the presence of duroquinone (Fig. 3a). Results were identical with cells pre-incubated with EGCG. However, the rate of ferricyanide reduction in the absence of duroquinone was somewhat less than observed in cells pretreated with Q (Fig 3b).On the other hand, in the absence of duroquinonewe couldn’t observe the direct reduction of ferricyanide in cells pretreated with either I3C or SFN (Fig. 3c and d), respectively. In comparison, the removal of ferricyanide in the presence of duroquinone in cells pretreated with I3C or SFN was no more significant than that in cells not treated with these compounds. These results suggested that the rate of duroquinone-mediated reduction of ferricyanide in HepG2 cells was not enhanced after treatment with any of the four phytochemicals known to induce NQO1 activity. The results also indicated that the two direct antioxidants of the four phytochemicals tested (i.e., Q and EGCG) interfered in the assay. They mediated ferricyanide reduction in the absence of duroquinone.
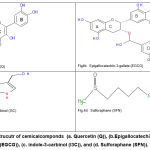 |
Figure 4: Strucutr of cemicalcomponds (a. Quercetin (Q)), (b.Epigallocatechin-3-gallate (EGCG)), (c. indole-3-carbinol (I3C)), and (d. Sulforaphane (SFN)). |
Discussion
NAD(P)H:quinone oxidoreductase (NQO1) is a two-electron quinone reductase found in many tissues and cells. This phase II enzyme catalyses the reduction of a diverse range of physiological, pharmacological, and toxicological quinones and other redox-active substances. One of the many roles in which NQO1 has been implicated is as a target for bioreductive activation of certain anticancer drugs and antioxidants, as well as a means of xenobiotic detoxification, with induction or genetic overexpression representing potential means of exploiting these functions [4]. Q and EGCG have direct antioxidant properties that can be used in cellular research. The hydroxyl groups present in Q and EGCG may be responsible for their free radical scavenging activity. Q has 5 groups on its structure, whereas EGCG has 9 groups. These groups represent potential attack sites for free radicals, resulting in the radicalization of all hydroxyl groups.
In the present study, we aimed to use the four compounds Quercetin (Q), Epigallocatechin-3-gallate, (EGCG), indole-3-carbinol (I3C), and Sulforaphane (SFN) as antioxidants against oxidative stress for further investigations into the stimulation of the NQO1 enzyme mechanism as measured by the DQ-mediated reduction of ferricyanide as described by Bongard et al. 4. We incubated HepG2 cells with ferricyanide in the absence of phytochemicals to ensure the reduction of Fe3+ is according to the NQO1 activity, not because of external interference with enzymatic reaction. I3C and SFN did not affect Fe+3 reduction, so any reduction to Fe+3 may attribute to the activity of NQO1( 3c,3d). Therefore, the enzymatic reaction of NQO1 catalyzes the conversion of duroquinone to hydroduroquinone, and the linked conversion of ferricyanide is reduced to ferrocyanide. On the other hand, the ferric ion is reduced to ferrous in HeG2 cells treated with Q and EGCG, and the reduction reaction may attribute to the effects of these antioxidants, not NQO1(Fig 3a, 3b). The suggestion for the mechanism for Q and EGCG action is due to their direct antioxidant activity 6. The ferric ion can be reduced to ferrous by Q and EGCG (not the duroquinone) through accepting an electron from them, and subsequently, they interfere with an enzymatic reaction.
Evidence of the protective effects of phytochemicals, such as SFN, has recently increased due to increased awareness of the induction of phase ll detoxifying enzymes, particularly NQO1 2. On the other hand, studies have shown that a diet high in catechins may impair the organism’s ability to detoxify free radicals and environmental xenobiotics by causing a significant decrease in NQO1 activity for unknown reasons. As a result, there is a continuing need for research to develop a cell-based assay for NQO1 as part of screening programs for modulation of NQO1 activity 3.
Additionally, Q and EGCG possess quinone structures (Fig.2, 4a, and 4b), missing in SFN and I3C (Fig. 4c,4d). The similarity in structure between Q, EGCG from the side with duroquinone from the other side allows for the direct antioxidants to be reduced by NQO1 instead of duroquinone via two-electron transfer consequently. Moreover, the long incubation time of HepG2 cells with Q and EGCG (20 hours) 7 allowed them to penetrate the cell and remain inside and act as a substrate of NQO1 and involved reduction reaction even though PBS extensively washed the treated cells. On the other hand, it seems possible that the cell-based assay can further study the modulation of NQO1 activity by compounds such as I3C and SFN, which act by indirect antioxidant mechanisms.
Conclusion
Q and EGCG can interfere with the enzymatic reaction of NQO1 that assessed by DQ-mediated reduction of ferricyanide in intact cells. This interference suggests a significant limitation in using this assay to screen for induction of NQO1 activity by plant-derived chemicals and extracts.
Acknowledgment
The authors thank The University of Nottingham and the School of Life Sciences (UK) for their scientific support. The author Dr. Maha J Hashim would like to thank her supervisor in Ph.D. (including this research), Dr. Jeffrey R Fry at the School of Life Sciences, The University of Nottingham (UK), for all his guidance and assistance. Also, big thanks from Dr. Maha J Hashim to her father Mr. Jalal Hashim Mohammed Tabana, in Iraq, who has funded her Ph.D. study (including this research) with all living costs in the United Kingdom for five years.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Shokouhi G, Tubbs RS, Shoja MM, et al. (2008) The effects of aerobic exercise training on the age-related lipid peroxidation, Schwann cell apoptosis and ultrastructural changes in the sciatic nerve of rats. Life Sciences 82(15-16):840-846 doi:DOI 10.1016/j.lfs.2008.01.018.
CrossRef - Tarozzi A, Morroni F, Merlicco A, et al. (2009) Sulforaphane as an inducer of glutathione prevents oxidative stress-induced cell death in a dopaminergic-like neuroblastoma cell line. J Neurochem 111(5):1161-71 doi:10.1111/j.1471-4159.2009.06394.x.
CrossRef - Wiegand H, Boesch-Saadatmandi C, Regos I, Treutter D, Wolffram S, Rimbach G (2009) Effects of Quercetin and Catechin on Hepatic Glutathione-S Transferase (GST), NAD(P)H Quinone Oxidoreductase 1 (NQO1), and Antioxidant Enzyme Activity Levels in Rats. Nutrition and Cancer-an International Journal 61(5):717-722 doi:Doi 10.1080/01635580902825621.
CrossRef - Bongard RD, Krenz GS, Gastonguay AJ, Williams CL, Lindemer BJ, Merker MP (2011) Characterization of the threshold for NAD(P)H:quinone oxidoreductase activity in intact sulforaphane-treated pulmonary arterial endothelial cells. Free Radic Biol Med 50(8):953-62 doi:10.1016/j.freeradbiomed.2011.01.009.
CrossRef - Lee YY, Westphal AH, de Haan LH, Aarts JM, Rietjens IM, van Berkel WJ (2005) Human NAD(P)H:quinone oxidoreductase inhibition by flavonoids in living cells. Free Radic Biol Med 39(2):257-65 doi:10.1016/j.freeradbiomed.2005.03.013.
CrossRef - Hashim MJ, Fry JR. Evaluation of Direct and Indirect Antioxidant Properties of Selected Four Natural Chemical Compounds: Quercetin, Epigallocatechin-3-Gallate, Indole-3-Carbinol and Sulforaphane by DPPH Radical Scavenging Assay. J Biomed Res Environ Sci. 2020 Dec 15; 1(8): 389-392. doi: 10.37871/jbres1170 Article ID: JBRES1170.
CrossRef - Hashim MJ, Fry JR. A Cell-Based Assay for Antioxidant Behaviour of Phytochemicals: Influence of Exposure Time and Presence of Serum. J Biomed Res Environ Sci. 2021 July 27; 2(7): 610-617. doi: 10.37871/jbres1286 Article ID: JBRES1286, Available at: https://www.jelsciences.com/articles/jbres1286.pdf.
CrossRef - Gopalakrishnan A, Tony Kong AN (2008) Anticarcinogenesis by dietary phytochemicals: cytoprotection by Nrf2 in normal cells and cytotoxicity by modulation of transcription factors NF-kappa B and AP-1 in abnormal cancer cells. Food Chem Toxicol 46(4):1257-70 doi:10.1016/j.fct.2007.09.082
CrossRef - Stoll BA (1998) Breast cancer and the Western diet: Role of fatty acids and antioxidant vitamins. European Journal of Cancer 34(12):1852-1856 doi:Doi 10.1016/S0959-8049(98)00204-4.
CrossRef - Surh YJ (2008) NF-kappa B and Nrf2 as potential chemopreventive targets of some anti-inflammatory and antioxidative phytonutrients with anti-inflammatory and antioxidative activities. Asia Pac J Clin Nutr 17 Suppl 1:269-72.
- Agudo A, Cabrera L, Amiano P, et al. (2007) Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: findings from the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Spain). American Journal of Clinical Nutrition 85(6):1634-1642.
CrossRef - Maha J Hashim, Jeffrey R Fry. Influence of Extracellular Protein on the Cytoprotectiv Effects of Two Model Phytochemicals. Mol Biol. 2019;8(1). doi: 10.4172/2168 9547.1000227.








