Manuscript accepted on :25-07-2022
Published online on: 10-08-2022
Plagiarism Check: Yes
Reviewed by: Dr. Shabana Khatoon
Second Review by: Dr. Guy-Armel
Final Approval by: Dr. H Fai Poon
E. Yu. Radoustskaya, Ya. I. Onischuk and I. I. Novikova
Federal Budgetary Scientific Institution "Novosibirsk Research Institute of Hygiene" of Rospotrebnadzor, Novosibirsk, Russian Federation
Corresponding Author E-mail: aizman.roman@yandex.ru
DOI : https://dx.doi.org/10.13005/bpj/2504
Abstract
The article is devoted to the current medical and social problem - vibration disease, which takes an important place in the clinic of occupational pathology. The syndromological classification of the vibration disease of the last revision is given here. Particular attention is paid to the pharmacotherapy of neuropathic manifestations of the disease. The article presents the data of the performed clinical studies of Cokarnit and alpha-lipoic acid preparations efficiency in patients with professional vegetative-sensory polyneuropathy of limbs. Clinical efficacy of Cocarnit with a possibility of using Cocarnit as monotherapy as well as complex therapy (Cocarnit and lipoic acid) in neuropathic pain syndrome associated with peripheral polyneuropathy of professional genesis was determined.
Keywords
Alpha-Lipoic Acid Preparations; Cocarnitis; Electromyography; M-Response Amplitude; Occupational polyneuropathy; Peripheral Nerve Impulse Velocity; Vibration Disease
Download this article as:| Copy the following to cite this article: Radoustskaya E. Y, Onischuk Y. I, Novikova I. I. Pharmacotherapy of Distal Autonomic Sensory Polyneuropathies in Vibration Disease. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Radoustskaya E. Y, Onischuk Y. I, Novikova I. I. Pharmacotherapy of Distal Autonomic Sensory Polyneuropathies in Vibration Disease. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3SDSaeY |
According to Rosstat, the number of working population in Russian Federation (RF) was over 70 million[1] in 2019 1, 2.. The number of workers exposed to harmful and/or hazardous substances and production factors with a risk of developing occupational and occupation conditional diseases is just over 10 million people 1.. In 2021, the frequency of occupational lesions from the effects of physical factors remains quite high, where vibration disease occupies the second rank place – 46.82%, and with functional overstrain of individual organs and systems, where mono-and polyneuropathies occupy the third place – 19.85% 3.
According to the type of exposure to vibration, there is vibration disease caused by local vibration, by general vibration and by combined vibration 4. Localized vibration is transmitted via the hands of the person working, while general vibration is transmitted via the supporting surfaces to the body of a person standing or sitting. The leading clinical symptom of vibration disease is peripheral nerve damage. When exposed to local vibration, these are polyneuropathies of the upper extremities, and when exposed to general vibration, there are the polyneuropathies of the upper and lower extremities 5, 6.
Polyneuropathy in vibration disease is a serious medical problem as it is often accompanied with severe neuropathic pain, which leads to a significant reduction in the quality of patients’ life 7.
Pharmacotherapy of any polyneuropathy, including polyneuropathy due to vibration exposure, involves two vectors of action: reduction of pain syndrome (symptomatic therapy) and restoration of the affected nerves (pathogenetic therapy) 8, 10. In this regard, the treatment of polyneuropathy should be comprehensive and combined, including drugs aimed at different sides of the pathological process. One of these complex drugs is Cocarnit, which is a combination of trifosadenine, cocarboxylase, cyanocobalamin and nicotinamide. Each of the above components of Cocarnit performs a specific function in the metabolic processes of nervous tissue. Many clinical studies performed by domestic and foreign professionals have demonstrated the efficacy and safety of Cocarnit in the treatment of patients with diabetic polyneuropathy 11- 15. The preparations of lipoic acid have also shown the pronounced neuroprotective effect 16, 17. They act as antioxidant, have neurotrophic effect, reduce endoneural hypoxia and ischemia, increase the concentration of antioxidant glutathione, thereby reducing manifestations of polyneuropathy in the form of paresthesia, burning sensation, pain and numbness of the extremities; normalizing energy metabolism and nerve impulse conduction 18, 22.
The aim of the study was to investigate the efficacy of Cocarnit monotherapy and the synergistic effect of combined Cocarnit and alpha-lipoic acid in treatment of patients with vibration disease presenting the symptoms of polyneuropathy combined with assessment of motor, sensory and autonomic fibres using electromyography.
Material and methods
The materials of the present study are derived from a clinical and neurophysiological examination carried out for over 4 years (2018-2021) with three groups of patients with upper and lower extremeties polyneuropathy, differing in the content of the treatment courses. The first group of patients (n=19) received treatment with Cocarnit; the second group (n=22) with lipoic acid preparations; the third group (n=9) with lipoic acid preparations and Cocarnit together. The study took into account that Cocarnit and lipoic acid affect different mechanisms of the etiopathogenesis of distal polyneuropathy, therefore, the therapeutic effect was achieved by different ways. All patients were observed in an occupational disease clinic for vibration disease from general and local vibration exposure.
In the first group of 19 patients, 5 patients had the first degree of disease accompanied by syndrome of sensory (vegetative-sensory) polyneuropathy of lower and upper extremities; the remaining patients had the second degree of disease, 13 of them had polyneuropathy of lower and upper extremities combined with radiculopathy of lumbosacral level and one patient had polyneuropathy of lower and upper extremities combined with sensory and vegetative-trophic disturbances. The age of the patients in the first group was from 49 to 75 years; the duration of the disease was 4 to 31 years.
In the second group of 22 patients, 4 patients had the first-degree disease accompanied by syndrome of sensory (vegetative-sensory) polyneuropathy of lower and upper extremities; the remaining patients had the second-degree, manifested by syndrome of polyneuropathy of lower and upper extremities combined with radiculopathy of lumbosacral level. The age of patients ranged from 54 to 70 years; the duration of the disease ranged from 4 to 25 years.
In the third group, 2 patients had the first degree of the disease – syndrome of sensory (vegetative-sensory) polyneuropathy of lower and upper extremities; the rest of the patients had the second degree of the disease, among them 5 patients had syndrome of polyneuropathy of lower and upper extremities combined with radiculopathy of lumbosacral level, and 2 patients had syndrome of polyneuropathy of lower and upper extremities combined with sensory and vegetative-trophic disorders. Age ranged from 51 to 75 years; duration of disease was from 4 to 27 years.
The criteria for inclusion in the study were the presence of vibration disease with autonomic-sensory polyneuropathy and the patients’ informed consent. Exclusion criteria were contraindications for intaking Cocarnitp (registration certificate no. LP-002839 dated 23.01.2015) and lipoic acid preparations, taking gabapentin, and preparations containing vitamin B. The patients did not take non-steroidal anti-inflammatory drugs or analgetics during the study.
All patients underwent a standard neurological examination. The main tool used to assess pain was the validated PAIN DETECT questionnaire (PDQ) 23. The main performance criterion was the dynamics of the neuropathic symptoms on the DN4 scale and on the NSS (Neurological Symptoms Score). Patients underwent stimulation electromyography with assessment of motor and sensory fibres of the peripheral nerves in order to examine the peripheral nerves 24- 26.
The examination was performed under standard conditions on Neuron-MP 4 electroneuromyoanalyzer, Neurosoft, Russia. The condition of motor fibers of median, ulnar, tibial and peroneal nerves was evaluated. Stimulation electromyography data allowed us to analyze the amplitude of motor response (M-response) and the speed of impulse conduction. The following were taken as normal: M-response amplitude of ulnar nerve – not less than 6 mV, median and tibial nerves – not less than 4 mV, peroneal nerve – not less than 3 mV; peripheral nerve motor fiber impulse conduction velocity of upper extremities at least 50 m/s, lower extremities at least 40 m/s; distal latency for median and ulnar nerves at 3.5 ms, for peroneal and tibial nerves at 4 ms 27-29.
Peripheral sensory nerve conduction was investigated for the median, ulnar, calf and superficial peroneal nerves. Impulse conduction velocity, distal latency, and sensory response (S-response) amplitude were determined for sensory fibres. The S-response amplitude of at least 10 µV for the median and ulnar nerves and at least 5 µV for the calf and superficial peroneal nerve was considered normal; the impulse conduction speed along the sensory fibers of peripheral nerves of the upper extremities was not lower than 48 m/s, lower extremities not lower than 38 m/s [30, 32].
Clinical and neurophysiological examinations were carried out twice: before the drugs intaking in 2018 and after 4 years, in 2021.
Cocarnit was administered in the recommended doses according to the “Instructions for Use of Medicinal Products for Medical Use” deep intramuscularly into the gluteal muscle with 2 ml once daily for 9 days, followed by 9 injections with a frequency of 2 times/week. These courses were administered twice a year annually for 4 years (from 2018 to 2021). Alpha-lipoic acid was administered by infusion at a dose of 600 mg/day for 10 days, followed by oral intake at the same dose for 1 month. These courses were administered twice a year also for 4 years.
Each patient had a Pain Detect pain score card, a DN4 and NSS score for the severity of neuropathic symptoms, and a neurophysiological examination protocol. Patients were fully informed about the nature and mechanism of action of the medication, its efficacy and possible side effects, and filled out an informed consent form for the study.
Statistical processing of the results was carried out using Excel Worksheet software from Microsoft Office 2013. Statistical processing was performed using descriptive statistics, calculating the mean (M), standard deviation (SD) and the criterion of reliability (t). Differences at the 95% significance level at p < 0.05 were considered significant.
Results and discussion
Analysis of the clinical picture revealed that most patients had sensory and autonomic disturbances in the distal upper and lower extremities, spreading proximally. Virtually all of the patients studied suffered from all types of sensation: vibration, temperature, pain and tactile sensation.
Symptoms such as pain, paresthesia or dysaesthesia were diagnosed before treatment in all patients studied, with varying degrees of severity from mild to moderate. The neuropathic component of pain, as assessed by the Pain Detect questionnaire, was detected in 80% of subjects, and with DN4 in 100%.
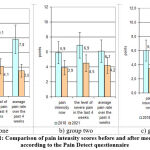 |
Figure 1: Comparison of pain intensity scores before and after medication according to the Pain Detect questionnaire. |
Statistically significant differences in scores during the observation period were observed in groups one and three (p < 0.05).
The results show that the use of Cokarnit, both alone and in combination with lipoic acid, is effective in reducing the severity of the pain syndrome (Fig. 1).
The results were confirmed by electromyography. Standard electrode patterns of J.A.De Lisa (1987) were used [33]. In all the muscles studied, there was no significant intermittent asymmetry, which made it possible to analyzing their average total value. The amplitude of the M-response reflects the integrity of the axons of the motor nerve innervating the given muscle. The amplitudes of the M response during distal nerve stimulation were assessed, since there was a little difference between the amplitudes of the M response during proximal and distal nerve stimulation.
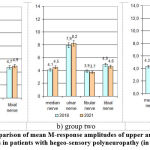 |
Figure 2: Comparison of mean M-response amplitudes of upper and lower limb nerves in patients with hegeo-sensory polyneuropathy (in µV). |
When analyzing the data, it was clear that there was a decrease in the signs of axonopathy in all groups and an overall increase in the amplitude of the M-responses (p < 0.05), but in groups 1 and 3 the efficiency was more pronounced (p < 0.05) – Fig.2.
Impulse conduction velocity along the median, ulnar, peroneal and tibial nerves before and after therapy was analyzed to assess conduction along the sensory fibers of peripheral nerves. It should be noted that according to the literature, the minimum allowable value of sensory impulse velocity in a healthy person for the peripheral nerves of the hands is 48 m/s, lower extremities – 38 m/s [31, 35].
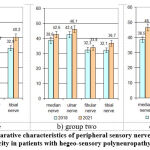 |
Figure 3: Comparative characteristics of peripheral sensory nerve fibre conduction velocity in patients with hegeo-sensory polyneuropathy (m/s). |
The observation revealed a statistically significant increase in impulse conduction velocity along all studied nerves during treatment (p<0.05), with a significant advantage in group 1 and particularly in group 3 (Fig.3).
Assessment of the distal latency of the M-response involved recording the pulse conduction from the stimulation point to the neuromuscular synapse. In the literature, the mean distal latency for the median nerve is 3.7 ms, upper limit – 4.2 ms; for the ulnar nerve 2.5 ms, upper limit – 3.3 ms; for the peroneal nerve – 3.7 ms, upper limit – 4.8 ms; for the tibia nerve -3.9 ms, upper limit – 5.1 ms (Stohr, Bluthard, 1992). The study showed a reduced level of distal M-response latency from the median and tibial nerves, while values from the ulnar and peroneal nerves were within normal limits. Therapy had a positive effect on the distal peripheral nerve latency, although the median and tibial nerves did not reach normal values, although the group 3 was the closest to normal (Figure 4).
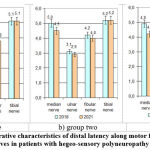 |
Figure 4: Comparative characteristics of distal latency along motor fibres of peripheral nerves in patients with hegeo-sensory polyneuropathy (ms). |
The study demonstrated a significant efficacy of monotherapy with Cocarnit and combination therapy with Cocarnit and alpha-lipoic acid in the treatment of polyneuropathy symptoms in patients with vibration disease.
Conclusion
There were no side-effects and all patients tolerated treatment well, so all the patients recruited successfully completed the study.
Cocarnit has shown a significant effect in neuropathic pain associated with peripheral polyneuropathy. It contributes to a statistically significant reduction not only in the overall Pain Detect score, but also in pain intensity. The use of Cocarnit allows reducing the dose of a concomitant analgetic drug or cancelling it.
The combined use of Cocarnit and alpha-lipoic acid has shown a clear improvement in motor and sensory peripheral nerve fibres in the upper and lower extremities, with a predominant effect on severe pathologically modified nerve fibres, as evidenced by the clinic.
Cocarnit, especially in combination with alpha-lipoic acid preparations, can be recommended as a metabolic agent improving trophic function of peripheral nerve axons for long-term treatment of polyneuropathy of upper and lower extremities in patients with vibration disease.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Report of the Chief freelance specialist of the occupational pathologist of the Ministry of Health of Russia for 2019. Analysis of information on the state of the accompanying medical direction, study of domestic and foreign experience in the field of healthcare organization, medical technologies and methods of prevention, diagnosis and treatment of diseases, medical rehabilitation. URL: https://minzdrav.gov.ru/vneshtatnye-spetsialisty/glavnyy-vneshtatnyy-spetsialist-38/plan-38. (In Russian)
- Labor and employment in Russia. 2021: Stat.sat./Rosstat 78. M., 2021. – 177 р. URL: https://rosstat.gov.ru/storage/mediabank /Trud_2021.pdf]. (In Russian)
- On the state of sanitary and epidemiological welfare of the population in the Russian Federation in 2021: State Report. Moscow: Federal Service for Supervision of Consumer Rights Protection and Human Well-being, 2022. – 340 p. URL: https://rospotrebnadzor.ru/upload/iblock/594/sqywwl4tg5arqff6xvl5dss0l7vvuank/Gosudarstvennyy-doklad.-O-sostoyanii-sanitarno_epidemiologicheskogo-blagopoluchiya-naseleniya-v-Rossiyskoy-Federatsii-v-2021-godu. pdf. (In Russian)
- Baraeva, R. A. Endothelial dysfunction in vibration disease [Electronic resource] / R. A. Baraeva, S. A. Babanov // Modern aspects of health saving: collection of materials of the jubilee. Scientific and practical conf. with the international participation dedicated to the 55th anniversary of the medical professor of the Faculty of the BSMU, Minsk, May 23-24, 2019 / edited by A.V. Sikorsky, A.V. Gindyuk, T. S. Borisova. – Minsk, 2019. –528-532 pp. (In Russian)
- Occupational pathology. National leadership / Ed. acad. RAMS N.F. Izmerova. – M.: GEOTAR-Media, 2011. – 784 p. (In Russian)
- Babanov, S. A., Azovskova, T. A., Vakurova, N. V., & Baraeva, R. A. On the modern aspects of the classification of vibration disease // Health and safety in the workplace: collection of scientific papers. / Min. labor and Social protection Republic Belarus. State enterprise “Republican Labor Protection Center of the Ministry of Labor of Social Protection Republic Belarus”. “Scientific research. Institute of Occupational Medicine named after Academician N.F. Izmerov”; /Chief editor I.V. Bukhtiyarov, T.M. Rybin. – Minsk: LLC Polikraft, 2019. – Vol. 1. Issue 3. – P. 28.] (In Russian)
- Grebenkov S. V., Kochetova O. A., Milutka E. V., Malkova N. Yu. Professional polyneuropathy: a modern view of the problem in Russia and abroad. Literature review //Hygiene and sanitation. – 2019. – Vol. 98. – No. 6. – 631-635 pp. DOI: http://dx . doi . org/10.18821/0016-9900-2019-98-6-631-635 (In Russian).
CrossRef - Clinical guidelines on neurology of the European Federation of Neurological Communities. Edited by Gilhus N.E., Barnes M.P., Brainin M. (scientific editor of the Russian edition Nikitin S.S.). – M.: LLC “ID “ABC-press”, 2012. (In Russian)
- Babanov S.A. Vakurova N.V., Azovskova T.A. Vibration disease. Optimization of diagnostic and therapeutic measures. Samara: Etching,-2012.-160 p. (In Russian)
- Ziegler, D., Papanas, N., Schnell, O., Nguyen, B. D. T., Nguyen, K. T., Kulkantrakorn, K., & Deerochanawong, C. Current concepts in the management of diabetic polyneuropathy //Journal of Diabetes Investigation. – 2021. – Т. 12. – №. 4. – С. 464-475. DOI:https://doi.org/10.1111/jdi.13401
CrossRef - Radoutskaya E.Yu., Kruglikova N.V. Kokarnit in the treatment of professional polyneuropathies // Nervous diseases. No. 2.- 37-42 pp. (In Russian)
- Parpauţ C., Vudu L.. Evaluarea eficacităţii preparatului Cocarnit în tratamentul polineuropatiei distale la pacienţii cu diabet zaharat tip 2. In: Arta Medica. 2014, nr. 2(53), pp. 93-95. https://repository.usmf.md/handle/20.500.12710/14050
- Kotov, S. V., Isakova, E. V., Leidvoll, V. Y., Belova, Y. A., Volchenkova, T. V., Borodin, A. V., & Shvedov, V. A. The efficacy of cocarnit in diabetic neuropathy //Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova. – 2018. – Т. 118. – №. 1. – С. 37-42. DOI: 17116/jnevro20181181137-42 (In Russian)
CrossRef - Gatckikh, I. V., Brickman, I. N., Gazenkampf, K. A., Petrova, M. M., Veselova, O. F., Shalda, T. P., . & Potupchik, T. V. Dynamics of neurologic disturbances on the background of combined therapy in patients with type 2 diabetes //Zhurnal Nevrologii i Psikhiatrii Imeni SS Korsakova. – 2018. – Т. 118. – №. 6. – С. 31-36. DOI: 17116/jnevro20181186131(In Russian)
CrossRef - Batrac, G. A., Metelkina, N. F., Brodovskaya, A. N., & Andrianova, E. A. Features of diabetic polyneuropathy in patients with diabetes mellitus according to the results of electroneuromyography //Consilium Medicum. – 2020. – Т. 22. – №. 2. – С. 45-49. DOI: https://doi.org/10.26442/20751753.2020.2.200087 (In Russian)
CrossRef - Tóth F., Cseh E. K., Vécsei L. Natural molecules and neuroprotection: Kynurenic acid, pantethine and α-lipoic acid //International Journal of Molecular Sciences. – 2021. – Т. 22. – №. – С. 403. DOI: https://doi.org/10.3390/ijms22010403
CrossRef - Rochette L., Ghibu S. Mechanics insights of alpha-lipoic acid against cardiovascular diseases during COVID-19 infection //International Journal of Molecular Sciences. – 2021. – Т. 22. – №. – С. 7979. DOI: https://doi.org/10.3390/ijms22157979
CrossRef - Xia, D., Zhai, X., Wang, H., Chen, Z., Fu, C., Zhu, M. Alpha lipoic acid inhibits oxidative stress‐induced apoptosis by modulating of Nrf2 signalling pathway after traumatic brain injury //Journal of Cellular and Molecular Medicine. – 2019. – Т. 23. – №. 6. – С. 4088-4096. DOI: https://doi.org/10.1111/jcmm.14296.
CrossRef - Giustina, A. D., de Souza Goldim, M. P., Danielski, L. G., Garbossa, L., Junior, A. N. O., Cidreira, T., … & Petronilho, F. Lipoic acid and fish oil combination potentiates neuroinflammation and oxidative stress regulation and prevents cognitive decline of rats after sepsis //Molecular Neurobiology. – 2020. – Т. 57. – №. 11. – С. 4451-4466.
CrossRef - Ding, Y., Zhang, Y., Zhang, W., Shang, J., Xie, Z., & Chen, C. Effects of lipoic acid on ischemia-reperfusion injury //Oxidative Medicine and Cellular Longevity. – 2021. – Т. 2021. DOI: https://doi.org/10.1155/2021/5093216
CrossRef - Salehi, B., Berkay Yılmaz, Y., Antika, G., Boyunegmez Tumer, T., Fawzi Mahomoodally, M., Lobine, D., … & Sharifi-Rad, J. Insights on the use of α-lipoic acid for therapeutic purposes //Biomolecules. – 2019. – Т. 9. – №. – С. 356., https://doi.org/ 10.3390/ biom9080356
CrossRef - Agathos, E., Tentolouris, A., Eleftheriadou, I., Katsaouni, P., Nemtzas, I., Petrou, A., … & Tentolouris, N. Effect of α-lipoic acid on symptoms and quality of life in patients with painful diabetic neuropathy //Journal of International Medical Research. – 2018. – Т. 46. – №. 5. – С. 1779-1790. DOI: https://doi.org/10.1177/0300060518756540
CrossRef - Freynhagen R. et al. Pain DETECT: a new screening questionnaire to identify neuropathic components in patients with back pain //Current medical research and opinion. – 2006. – Т. 22. – №. 10. – С. 1911-1920.
CrossRef - Panggabean, A. S., Dananjoyo, K., Malueka, R. G. O-NE005. Electroneuromyography as a predictor of peripheral neuropathy symptoms after two years follow-up in farmers exposed to pesticide: A cohort study. Clinical Neurophysiology. 2021; 132(8):e70. https://doi.org/10.1016/j.clinph.2021.02.135
CrossRef - Poroshina M. M., Vlasova E. M., Perevalov A. Y. Optimization of diagnostic system of hand diseases in employees working under combined effects of vibration and physical overloads. Russian Journal of Occupational Health and Industrial Ecology; 2019; 11: 950-955. https://doi.org/10.31089/1026-9428-2019-59-11-950-955 (In Russian)
CrossRef - Valls-Sole J., Leote J., Pereira P. Antidromic vs orthodromic sensory median nerve conduction studies //Clinical neurophysiology practice. – 2016. – Т. 1. – С. 18-25. https://doi.org/10.1016/j.cnp.2016.02.004
CrossRef - Michell A. Understanding EMG. – OUP Oxford, 2013.
CrossRef - Buchner H., Margold M. Methodik der Neurografie und Myografie zur Identifikation pathologischer Befunde //InFo Neurologie+ Psychiatrie. – 2020. – Т. 22. – №. 3. – С. 36-49.
CrossRef - Stålberg, E., van Dijk, H., Falck, B., Kimura, J., Neuwirth, C., Pitt, M., … & Zwarts, M. Standards for quantification of EMG and neurography //Clinical Neurophysiology. – 2019. – Т. 130. – №. 9. – С. 1688-1729.
CrossRef - Geht B. M. Theoretical and clinical electromyography. – Science. branch, 1990. (In Russian)
- Geht B.M. Electromyography in the diagnosis of neuromuscular diseases. / B.M. Geht, L.F. Kasatkina, M.I. Samoilov, A.G. Sanadze. – Taganrog: TRTU Publishing House, – 1997. – 370 p. (In Russian)
- Stöhr M., Pfister R. Klinische Elektromyographie und Neurographie-Lehrbuch und Atlas. – Kohlhammer Verlag, 2014.
- De Lisa J.A. Techniques of nerve conduction studies. Manual / J.A. De Lisa. – New York: Raven Press Ltd., 1987. – 278 p.
- Mallik A., Weir A. I. Nerve conduction studies: essentials and pitfalls in prctice. Journal of Neurology, Neurosurgery & Psychiatry. 2005;76(2): ii23-ii31. http://dx.doi.org/10.1136/jnnp.2005.069138
CrossRef - Kimura J. Electrodiagnostics in diseases of nerves and muscles: principles and practice. 2013.







