Sathya G R1 , Priscilla Johnson2*
, Priscilla Johnson2* , Subhasis Das1
, Subhasis Das1 , Rajagopalan B3
, Rajagopalan B3 , Rekha D1
, Rekha D1 , Lavanya Sekhar2
, Lavanya Sekhar2 and M. Manikandan4
and M. Manikandan4
1Department of Physiology, Pondicherry Institute of Medical Sciences, Ganapathichetikulam, Puducherry -605014. India
2Department of Physiology, Sri Ramachandra Medical College and Research Institute, SRIHER, , Porur, Chennai- 600116, India
3Department of Chest and TB, Sri Ramachandra Medical College and Research Institute, SRIHER, , Porur, Chennai- 600116, India
4Department of Community Medicine, Pondicherry Institute of Medical Sciences, Ganapathichetikulam, Puducherry -605014. India
Corresponding Author E-mail: priscillajohnson@sriramachandra.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2461
Abstract
Background and objectives: Asthma is a chronic inflammatory airway disease which requires biomarkers that reflect refractoriness to conventional therapy with inhalational steroids. Periostin is an extracellular matrix protein that is secreted in the airway epithelium, in response to stimulation by Interleukin -13 (IL-13). IL-13 is a cytokine that mediates airway inflammation following the Type 2 immune response. Both these biomarkers can be used to signify type 2 inflammatory response, which reflects steroid hypo-responsiveness in asthmatics. The objectives of the study were to: 1. Obtain the reference value of serum Periostin and IL-13 levels in healthy south Indian adult population 2. To compare the reference range of Periostin and IL-13 with that of the asthmatics on inhalational corticosteroids. Methodology: The study was carried out among 50 asthmatics and 50 healthy volunteers aged between 25 to 65 years. After procuring the informed consent, the Pulmonary Function test assessment was done to recruit the study subjects. The blood sample was collected for assessment of Serum Periostin and IL-13. Results: The median (IQR) baseline level of Serum Periostin among control group and in asthmatics was 13.2 (8.8-28.1) ng/ml and 16.7 (10.9-20.7) respectively. Also, the median (IQR) baseline level of Serum IL-13 among healthy individuals and among asthmatics was 42.9 (37.8-52.4) pg/ml and 73.5 (60.0-91.1) pg/ml respectively, which was statistically significant. Conclusion: The obtained baseline values of Serum IL-13 and Periostin could be of clinical utility in asthmatics. The validity of the data obtained from this study can be tested out on larger study populations.
Keywords
Asthma; interleukin-13; inhalational steroid; Periostin
Download this article as:| Copy the following to cite this article: Sathya G. R, Johnson P, Das S, Rajagopalan B, Rekha D, Sekhar L, Manikandan M. Activities of Periostin and Inteleukin-13 among Asthma Patients in Comparison with Healthy people in Southern India. Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Sathya G. R, Johnson P, Das S, Rajagopalan B, Rekha D, Sekhar L, Manikandan M. Activities of Periostin and Inteleukin-13 among Asthma Patients in Comparison with Healthy people in Southern India. Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3PS7uD5 |
Introduction
Bronchial asthma is a type 2 inflammatory disease of the airways characterized by shortness of breath, wheeze, and airflow obstruction. Globally, asthma ranks as the 14th leading chronic disease affecting 334 billion people worldwide. Though the number of deaths has dropped, the number of people affected based on quality of life and the economic burden has increased, viz asthmatics in Europe, account for huge amount spent on treatment (509 Euro per patient). In India, studies have shown asthma as the 25th leading cause of Disability Adjusted life years (DALYs) and studies by Barnet et al, Murray et al and Global asthma report 2011 have reported that bronchial asthma contributes to about 1% of the total DALYs. 1-4 People diagnosed with asthma are conventionally treated with inhalational corticosteroids (ICSs) or beta 2 agonists. Based on an estimation in the year 2015, the amount spent on asthma treatment in India is approximately about 139.45 billion INR [≈ 2.3 billion USD]. Studies have suggested that if all the asthmatics receive medications as per the evidence-based guidelines, the treatment cost is likely to come down. 5 The modality of asthma treatment is aimed at: the suppression of inflammation associated with asthma, the reduction of bronchial hyper-reactivity with the use of leukotriene antagonists/Long-acting beta-2 sympathomimetic agents in combination with ICSs.
Various studies have concluded that biomarkers could aid in the identification of asthmatics based on phenotype, severity and refractoriness to the conventional management. However, there is great challenge in identification of the asthmatics who are hypo-responsive to steroids. Evidence suggests that biomolecules like Periostin (POSTN) and Interleukin -13 (IL-13) can be used as markers to Type 2 (Th2) inflammatory process that led to eosinophilic recruitment , inflammation and thereby leading to airway remodeling. Studies have also shown that measurement of periostin, IgE, IL-13 and the blood eosinophil count could help in identification of asthmatics who remain as poor responders to steroids and also could possibly express enhanced response to treatment with monoclonal antibodies. 6-15 But, limited data are available with respect to a diagnostic test with better sensitivity in diagnosing steroid hypo-responsive asthma. Hence, the present study was carried out Periostin and IL-13 as a novel biomarker which can be used to develop a new treatment strategy by categorizing the asthmatics.
Role of Periostin and Interleukin in the pathogenesis of Asthma
Periostin is found in healthy individuals as well. It is derived from periodontal ligament and is expressed in epithelial cells, osteoblasts, fibroblasts etc. At the time of disease onset in an individual, an allergen finds its way into the body and mediates type 2 inflammatory response by activating the mast cells, lymphocytes, neutrophils. These immune cells once activated start producing chemical substances like interleukin- 4, 5 and 13 which further causes eosinophil recruitment. The eosinophils in turn produces Periostin which has varied effects. The POSTN thus produced act via autocrine mechanism causing further eosinophil recruitment. It exhibits extracellular inter-linking with fibronectin, tenascin and collagen and act via Transforming growth Factor to bring about sub epithelial fibrosis and airway remodeling leading to refractoriness to routine therapy with inhalational corticosteroids. 16-21 IL-13 is an immune cell cytokine that enhances IgE production and brings about airway inflammation, hyper reactivity and increased mucus production. 22,23 The genetic expression of IL-13 on chromosome 5q31 is strongly linked in individuals with asthma.
Methodology
This prospective cohort study was conducted among individuals aged 25 to 65 years. The sample size was calculated considering alpha – 5%; power – 80%; Since it was a follow up study, the attrition rate was kept at 20%. Based on this, 50 healthy individuals and 50 asthmatics were enrolled in this study. The study subjects were categorized into two groups, viz asthmatics and healthy controls by subjecting them to pulmonary function test and by following the GINA guidelines. People who were devoid of respiratory symptoms suggestive of asthma and had a normal Pulmonary function test were categorized as healthy individuals. Persons who had symptoms of cough for more than two weeks, a history of wheeze or shortness of breath was considered to have asthma and were subjected to a pre and post broncho-dilator pulmonary function testing using 400 micro- litre of Salbutamol using a spacer. Those persons who showed a 12% and 200 ml reversibility of FEV1% were confirmed as asthmatics and were included in the study group. Following this, 5 ml of blood sample was collected from these individuals in the assessment of serum Periostin and serum Interleukin – 13. People with morbid obesity, diabetes mellitus, pregnant ladies, cardiovascular morbidities and immunodeficiency diseases were excluded from the study.
Assay for Biomarkers
The serum levels of Periostin were measured using the Human Periostin ELISA Kit, Thermo Scientific, Pierce™ and ab100553-IL-13 (Interleukin-13).
Serum Biomarkers
For the testing of serological markers, the blood samples were collected, centrifuged, sera separated and stored in a deep freezer at -20 degree Celsius. When a sufficient number of samples got collected, the samples were individually tested for serum Periosin and Interleukin-13 respectively.
Serum Periostin
Procedure
All reagents, samples and standards were prepared as instructed in the manual. 100μL of standard was added to the samples placed in the wells. It was then covered with plate & incubated at room temperature for 2.5 hours. The plate was washed four times. Then 100μL Biotinylated antibody was added to the wells. Again, it was covered with plates and incubated at room temperature for 1 hour. The plates were washed four times. 100μL of StreptavidinHRP Reagent was added to each well and was covered with plates and incubated at room temperature for 45 minutes. The plates were washed four times. Then 100μL TMB Substrate was added to each well. The plates were developed at room temperature in the dark for 30 minutes. 50μL of Stop Solution was added to each well. The absorbance was calculated using calorimetry.
Serum IL-13
Procedure
The standard or samples were added to each well. It was then incubated at room temperature. Prepared biotin antibody and then the prepared Streptavidin solution were added to each well and incubated at room temperature. Then TMB One-Step Development Solution was added to each well and incubated at room temperature. The reaction is brought to end by adding stop solution to each well. Reading were obtained using calorimetry at 450nm immediately.
Statistical Analysis
The data were entered in excel and was analyzed using SPSS -21.0. Descriptive Statistics viz
Mean, Standard Deviation, Median, IQR were calculated. Mann-Whitney U test was used to compare the mean score of Serum Periostin and Serum IL-13 between healthy individuals and asthmatics.
Results
The median (IQR) value of serum Interleukin – 13 was 42.9 (37.8-52.4) pg/ml in healthy individuals and 73.5 (60.0-91.1) pg/ml among asthmatics. There was significant difference between both the groups. (p < 0.0001). (Figure 1) Also, the median (IQR) value of serum Periostin was 13.2 (8.8 – 28.1) ng/ml in healthy individuals and 16.7 (10.9–20.7) ng/ml among asthmatics. There was no significant difference between both the groups. (p=0.269). (Figure 2).
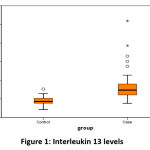 |
Figure 1: Interleukin 13 levels. |
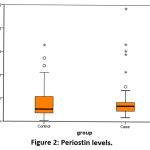 |
Figure 2: Periostin levels. |
Discussion
The gold standard treatment that is being followed to treat asthmatics is the use of inhalational glucocorticoids. However, it doesn’t always infer complete relief in roughly around 5 to 10% of the asthmatics. This type of refractory asthma can be identified using various tools like FENO, blood or sputum eosinophil count, biomarkers like IgE, Periostin and IL-4, 5,13. Despite the availability of these tests, the biopharmaceutical medication targeting the asthmatics using a newer modality of management had not yielded promising results due to the underutilization of the biomarker assays. The refractoriness to asthma management varies based on the pathogenesis. Eosinophil mediated hyper responsiveness of airway would require targeted therapy using biomolecule. This suggests that there is a need of assessment of more sensitive biomarkers that is highly feasible to identify the level of control of asthma activity. 23-31 This would pave the way for better asthma control based on genotyping and severity.
Among them, studies recommend the assessment of serum Periostin mediated by IL-13 as a multicellular protein that terminates in the formation of sub epithelial fibrosis. 32 Research that utilizes the pathogenetic role of POSTN and IL-13 in the categorization of asthmatics remains as an area of active research during the last decade. 21,33-34
The present study projected the distribution of serum Periostin among asthmatics: 16.7 (10.9–20.7) ng/ml and healthy individuals: 13.2 (8.8 – 28.1) ng/ml. This was similar to other studies. 11,12,21 Though the raw data seems to show that there is a difference in the periostin levels between both the groups, probably due to an insufficient number of study population, we were unable to yield statistically significant result. The study was carried out by adjusting for the possible errors that could happen. However, the process of sample collection, storage, processing and immunoassay were done using a standardize protocol.
The study found the levels of IL-13 among the asthmatics: 73.5 (60.0-91.1) pg/ml and healthy individuals was 42.9 (37.8-52.4) pg/ml. There was significant difference between the serum IL-13 levels between both the groups. Various studies that have been attempted to find the link between the IL-13 and asthma have yielded conflicting results. Few studies have shown IL-13 as a molecule that is responsible for the raised IgE levels and hyper-reactivity whereas few studies have shown IL-13 to have either a protective role or no association in the pathogenesis of asthma. 35-39 Studies have shown that persons with elevated interleukin levels showed a good response to treatment with monoclonal antibodies. Identification of such phenotypes who would respond better to targeted therapy, would mediate improvement in airway inflammation, thereby, decrease the number of exacerbations and provide a better quality of life.
Conclusion
The baseline values of serum IL-13 and Periostin were obtained from healthy individuals and was compared with asthmatics. Serum periostin and IL-13 can be considered as biomarkers to identify asthmatics who are steroid resistant. The study can be conducted on a large scale and values, thus obtained could be clinically utilized in targeting refractory asthma and thus change the management strategy.
Limitations of the study
The study was carried out among a less number of people and the result procured cannot be extrapolated to the entire population. Also, serum periostin is elevated only in a small subset of asthmatics. But in the present study, phenotype subgroup with equal number of subjects in each group was not considered. The recruitment of study subjects based on reversibility testing in PFT was challenging. Hence it is recommended to carryout study on a larger scale and considering equal number of study subjects in each phenotype subgroup.
Acknowledgement
We, the authors immensely thank the funding agency for providing us with the necessary funding to conduct the study and special thanks to the people who took part in the study as study subjects and cooperated for the smooth conduct of the study.
Conflict of Interest
There is no Conflict of interest.
Funding Sources
The study was funded by SERB (Science and Engineering Research Board, New Delhi), (EMR/2017/0004010).
References
- Global Asthma Network. Global asthma report 2014. Global burden of disease due to asthma. http://www.globalasthmareport.org/ burden/burden.php (accessed July 29, 2015).
- Accordini S, Corsico AG, Braggion M, et al. The cost of persistentasthma in Europe: an international population-based study Int Arch Allergy Immunol 2013; 160: 93–101.
CrossRef - Barnett SB, Nurmagambetov TA. Costs of asthma in the United States: 2002-2007. J Allergy Clin Immunol.2011;127:145–52.
- Murray CJ, Lopez AD. Measuring the global burden of disease. N Engl J 2013;369:448–57.
CrossRef - Murthy KJR, Sastry JG. Economic burden of asthma. Back-ground papers; Burden of disease in India. [Last accessed on 2014 Mar 5]. Available from:http://www.who.int/macrohealth/action/NCMH_Burden%20of%20disease_%2829%20Sep%202005%29.pdf
- Dina R. Mohammeda , Amira Y. Abdelnabyb , Enas A. El Zamranb , Ibrahim S. Ibrahim. Role of serum periostin as a biomarker in diagnosis of bronchial asthma. The Egyptian Journal of Chest Diseases and Tuberculosis; 2018, 67:4–8.
CrossRef - Inoue T, Akashi K, Watanabe M. Periostin as a biomarker for the diagnosis of pediatric asthma. Pediatr Allergy Immunol 2016; 27: 521–526
CrossRef - Shimaa Khaled Ismail , Mohamed Sanad Nagiub, Heba Gamal Anany, and Ayman Abdel Rahman Allam. Serum Periostin Level Interpretation in Asthmatic Children. European Journal of Molecular & Clinical Medicine.2021; 8(3):4444-51.
- Taylor DR. Using biomarkers in the assessment of airways disease. J Allergy Clin Immunol 2011; 128: 927– 934
CrossRef - Szefler SJ, Wenzel S, Brown R, et al. Asthma outcomes: biomarkers. J Allergy Clin Immunol 2012; 129: Suppl. 3, S9– S23
CrossRef - Petsky HL, Cates CJ, Lasserson TJ, et al. A systematic review and meta-analysis: tailoring asthma treatment on eosinophilic markers (exhaled nitric oxide or sputum eosinophils). Thorax 2012; 67: 199–208.
CrossRef - Malerba M, Ragnoli B, Radaeli A, et al. Usefulness of exhaled nitric oxide and sputum eosinophils in the long-term control of eosinophilic asthma. Chest 2008; 134: 733–738.
CrossRef - Hanania NA, Wenzel S, Rosén K, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187: 804–811
CrossRef - Corren J, Lemanske RF, Hanania NA, et al. Lebrikizumab treatment in adults with asthma. N Engl J Med 2011; 365: 1088–1098.
CrossRef - Hanania NA, Noonan M, Corren J, et al. Lebrikizumab in moderate-to-severe asthma: pooled data from two randomised placebo-controlled studies. Thorax 2015; 70: 748–756.
CrossRef - Li W, Gao P, Zhi Y, Xu W, Wu Y, Yin J, Zhang J. Periostin: its role in asthma and its potential as a diagnostic or therapeutic target. Respiratory Research. 2015;16(1):57.
CrossRef - Jia G, Erickson RW, Choy DF, et al. Periostin is a systemic biomarker of eosinophilic airway inflammation in asthmatic patients. J Allergy Clin Immunol 2012; 130: 647– 654.
CrossRef - Nair P, Kraft M. Serum periostin as a marker of TH2-dependent eosinophilic airway inflammation. J Allergy Clin Immunol 2012; 130: 655–656.
CrossRef - Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA 2007; 104: 15858–15863.
CrossRef - Takayama G, Arima K, Kanaji T, et al. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 2006; 118: 98–104.
CrossRef - Woodruff PG, Modrek B, Choy DF, et al. T-helper type 2-driven inflammation defines major subphenotypes of asthma. Am J Respir Crit Care Med 2009; 180: 388–395
CrossRef - Wynn TA (2003) IL-13 effector functions. Annu Rev Immunol 21:425–456.
CrossRef - Elias JA, Lee CG, Zheng T, Shim Y, Zhu Z (2003) Interleukin-13 and leukotrienes: An intersection of pathogenetic schema. Am J Respir Cell Mol Biol 28:401–404.
CrossRef - James A, Janson C, Malinovschi A, Holweg C, Alving K, Ono J,et al. Serum periostin relates to type-2 inflammation and lung function in asthma: data from the large population-based cohort Swedish GA (2)LEN. Allergy. 2017; 72:1753–60.
CrossRef - Noguchi T, Nakagome K, Kobayashi T, Uchida Y, Soma T, Nakamoto H, et al. Periostin upregulates the effector functions of eosinophils. J Allergy Clin Immunol. 2016;138:1449–52.
CrossRef - Fingleton J, Braithwaite I, Travers J, et al. Serum periostin in obstructive airways disease. EurRespirJ2016; 47:1383–91.doi:10.1183/13993003.01384-2015.
CrossRef - Boushey HA, Sorkness CA, King TS, et al; National Heart, Lung, and Blood Institute’s Asthma Clinical Research Network. Daily versus as-needed corticosteroids for mild persistent asthma. N Engl J Med. 2005;352(15):1519–28.
CrossRef - Szefler SJ, Martin RJ, King TS, et al; Asthma Clinical Research Network of the National Heart Lung, and Blood Institute. Significant variability in response to inhaled corticosteroids for persistent asthma. J Allergy Clin Immunol. 2002;109(3):410–8.
CrossRef - Powell H, Gibson PG. Initial starting dose of inhaled corticosteroids in adults with asthma: A systematic review. Thorax 2004;59(12):1041–5.
CrossRef - Kroegel C. Global Initiative for Asthma (GINA) guidelines: 15 years of application. Expert Rev Clin Immunol. 2009;5(3):239–49.
CrossRef - Gibson PG. Monitoring the patient with asthma: An evidence-based approach. J Allergy Clin Immunol. 2000;106(1 Pt 1):17–26.
CrossRef - Yuyama N, Davies DE, Akaiwa M, et al. Analysis of novel disease-related genes in bronchial asthma. Cytokine. 2002;19(6):287–96.
CrossRef - Grunig G, et al. (1998) Requirement for IL-13 independently of IL-4 in experimental asthma. Science 282:2261–2263.
CrossRef - Zimmermann N, et al. (2003) Dissection of experimental asthma with DNA microarray analysis identifies arginase in asthma pathogenesis. J Clin Invest 111:1863–1874
Crossref - Dixit P, Awasthi S, Agarwal S. Association of Interleukin genes polymorphism with asthmasusceptibility in Indian Children: A case- control study. Annals of Human Biology; 2014:1-8.
- Howard DT, Koppelman GH, Xu J, Zheng SL, Postma SD, Meyers DA et al. Gene-gene interaction in asthma: IL 4ra and IL 13 in a Dutch population with asthma. Am J Hu m Genet 2002; 70:230–236.
CrossRef - Kim BH, Lee CY, Lee SW, Jung J, Jin SH, Kim JH et al. Gene–gene interaction between IL-13and IL-13Ra1 is associated with total IgE in Korean children with atopic asthma. J Hum Genet 2006; 51:1055–1062.
CrossRef - Battle CN, Choudhry S, Tsai JH, Eng C, Kumar G, Beckman K et al. Ethnicity-specific Gene–Gene Interaction between IL-13 and IL-4R among African Americans with Asthma.. Am J Respir Crit Care Med 2007; 175: 881–887.
CrossRef - Nagarkatti R, Kumar R, Sharma KS, Ghosh B. Association of IL 4 gene polymorphisms with asthma in north Indians. Int Arch Allergy Immunol 2004;134:206–212.
CrossRef








