Uma Narayanamurthy  , Barathane D
, Barathane D , Sidharth Karthik and Sabari Anandh J V*
, Sidharth Karthik and Sabari Anandh J V*
Department of Pharmacology, MGMCRI, Sri Balaji Vidyapeeth (deemed to be University), Puducherry, India.
Corresponding Author E-mail: crony.8681@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2508
Abstract
Background: Seaweeds (Marine macro algae) are multicellular marine organism and are vital constituents of the of marine ecosystem, which are abundant in the coastal areas of the world. They are tremendous source of many bioactive metabolites and have been shown to exhibit a wide range of therapeutic properties, including anti-cancer, anti-oxidant, anti-inflammatory and anti-diabetic activities. Several Asian cultures have a strong tradition of using different varieties of seaweed extensively in cooking as well as in herbal medicines preparations. As such, seaweeds have been used to treat a wide variety of health conditions such as cancer, digestive problems, and renal disorders. These plants contain important phytochemical constituents and have various potential biological activities. References regarding the use of algae in Ayurveda and Siddha system of medicine has been reported since time immemorial, but their phytochemical properties have not been reported. Aim: To identify the phytochemical constituents present in the Acoathophora deilei (Red alage) using Preliminary phytochemical and GC-MS analysis. Methods: The shade-dried of red algae were extracted with methanol and the crude methanolic extract was subjected to GC-MS analysis to identify the various bioactive components Results: Phytochemical investigations suggests that the Acoathophora deilei showed the presence of phytochemicals like alkaloids, phytosterols, flavonoids and diterpenes, which may contribute to its biological activities. GC-MS analysis showed the presence of 28 different compounds. The main chemical constituents found in high percentage are Hexadecanoic acid methyl ester, 2-ethyl butyric acid octadecyl ester, hexadecanoic acid, 9-octadecanoic acid methyl ester and 1,2 – Benzene dicarboxylic acid. Conclusion: Thus, the present analytical study of Acoathophora deilei on phytochemical and GC-MS analysis provides an important novel information to support further ongoing studies to evaluate structure of bioactive compound and its pharmacological activities.
Keywords
Acoathophora deilei; GC-MS analysis; GC-MS fingerprinting; Marine seaweeds; Phytochemical analysis
Download this article as:| Copy the following to cite this article: Narayanamurthy U, Barathane D, Karthik S, Anandh J. V. S. Preliminary Phytochemical and GC-MS Analysis of Marine Seaweed- Acoathophora deilei (Red alga). Biomed Pharmacol J 2022;15(3). |
| Copy the following to cite this URL: Narayanamurthy U, Barathane D, Karthik S, Anandh J. V. S. Preliminary Phytochemical and GC-MS Analysis of Marine Seaweed- Acoathophora deilei (Red alga). Biomed Pharmacol J 2022;15(3). Available from: https://bit.ly/3atOLy3 |
Introduction
In traditional medicine, drugs are categorized into 3 groups, namely herbal products, mineral and animal products. The source of herbal products includes not only the higher plants but also the lower plants like seaweeds (Marine macro algae) 1. Algae are alternatives, especially marine algae, are least explored for their medicinal uses 2. The marine algae are new potential source and it is rich in various bioactive compounds. Seaweeds are treasured resources that belong to the plant kingdom – thallophyta, which had primitive group of non-flowering plants (Cryptogams) without true root, stem and leaves. Seaweeds occur in the intertidal, superficial and upto 180m depth of the sea and also in the backwaters. They develop on rocky shores, corals, solid rock layer, small stones and on other plants. Based on the presence of pigments, stored food materials, morphological and anatomical characters, they are categorized into four major groups such as Chlorophyceae (Green seaweeds), Phaeophyceae (Brown seaweeds), Rhodophyceae (Red seaweeds) and Cyanophyceae (Blue green algae). Marine algae are used for the preparation of various food items such as jelly, jam, chocolate, pickle, soup, salad, vegetable and porridge. Seaweeds are also utilized as animal fodder and as fertilizer for various crops 3. To explore various nutritional benefits of seaweed consumption, there is a need for more evidence relating to the properties of seaweeds on human health. So, the current study was performed to analyze various phytoconstituents present in the methanolic extract of Acoathophora deilei (Red algae). GC-MS fingerprinting was also done to recognize various phytochemicals present in red algae.
Materials and Methods
Plant material and extraction
deilei seaweed was collected from Rameswaram costal area, Tamil Nadu, India and it was authenticated by Dr. S. Bragadeeswaran, Marine Biologist, Centre for advanced studies in marine biology, Annamalai University, Parangipettai, Tamil Nadu. The sample was thoroughly washed with seawater to remove epiphytes followed by tap water, to remove the salts and other extraneous materials. The seaweed was washed with water, shade dried and powdered coarsely. Maceration with 95% methanol at room temperature for 72 hrs. and crude extract was obtained and the procedure was repeated till exhaustion of the material. Thereafter, the methanolic extract was distilled and dried under reduced pressure to get methanolic extract.
Phytochemical Analysis
Methanolic extract of Acoathophora deilei (Red algae) powder was subjected to various chemical tests (qualitative) to develop profiles of the extract for its chemical composition 4.
Phytochemical Screening
Qualitative phytochemical analysis of methanolic extract of Acoathophora deilei was performed based on the method of sofowara (1993), Trease & Evans (1975) and Harborne (1973) 5,6,7.
Detection of alkaloids
Methanolic extract of A.deilei was dissolved in adequate quantity of diluted HCl and filtered.
Mayer’s Test
Methanolic extract was treated with Mayer’s reagent (Potassium Mercuric Iodide). The presence of alkaloids was confirmed by the formation of a yellow-coloured precipitate.
Wagner’s Test
Methanolic extract was treated with Wagner’s reagent (Iodine in Potassium Iodide). The presence of alkaloids was confirmed by the formation of a brown/reddish precipitate.
Dragendroff’s Test
Methanolic extract was treated with Dragendroff’s reagent (solution of Potassium Bismuth Iodide). The presence of alkaloids was confirmed by the formation of a red precipitate.
Hager’s Test
Methanolic extract was treated with Hager’s reagent (saturated picric acid solution). Formation of the yellow-coloured precipitate indicates the presence of alkaloids.
Detection of carbohydrates
Methanolic extract was dissolved in 5 ml distilled water and filtered. The filtrate was used to test for the presence of carbohydrates.
Molisch’s Test
Methanolic extract was treated with 2 drops of alcoholic α-naphthol solution in a test tube. The presence of carbohydrates was confirmed by the formation of a violet ring at the junction.
Benedict’s test
Methanolic extract was treated with Benedict’s reagent and heated gently. Formation of orange red precipitate is an indication of the presence of reducing sugars.
Fehling’s Test
Methanolic extract was hydrolysed with dilute HCl, neutralized with alkali and heated with Fehling’s A & B solutions. The presence of reducing sugars was confirmed by the formation of red precipitate.
Detection of glycosides
Methanolic extract was hydrolysed with dilute HCl, and then subjected for glycosides test.
Modified Borntrager’s Test
Methanolic extract was treated with ferric chloride solution and immersed in boiling water for about 5 minutes. Obtained mixture was then cooled and extraction was done with equal volume of benzene. The benzene layer was separated and treated with ammonia solution. The presence of anthranol glycosides was confirmed by the formation of rose-pink colour.
Legal’s Test
Methanolic extract was treated with sodium nitropruside in pyridine and sodium hydroxide. The presence of cardiac glycosides was confirmed by the appearance of pink to blood red colour.
Detection of saponins
Froth Test
Methanolic extract was diluted with 20ml of distilled water and this was mixed for 15 minutes. The presence of saponins was confirmed by the formation 1 cm layer of froth.
Foam Test
Using 2 ml of distilled water was mixed with 0.5 mg of methanolic extract and shaken. Formation and persistence of foam for ten minutes will indicate the presence of saponins.
Detection of phytosterols
Salkowski’s Test
Methanolic extract was treated with chloroform and filtered. The filtrated extract was treated with few drops of conc. H2SO4, shaken and allowed to stand. Appearance of golden yellow colour is the indication of the presence of phytosterols.
Libermann Burchard’s test
Methanolic extract was treated with chloroform and filtered. The filtrate was treated with few drops of acetic anhydride, boiled and cooled. conc. H2SO4 was added. The presence of phytosterols was confirmed by the formation of brown ring at the junction.
Detection of phenols
Ferric Chloride Test
Methanolic extract was treated with 3-4 drops of ferric chloride solution. Appearance of bluish black colour is the indication for the presence of phenols.
Detection of tannins
Gelatin Test
Methanolic extract was added to 1% gelatin solution containing sodium chloride. The presence of tannins was confirmed by the formation of white precipitate.
Detection of flavonoids
Alkaline Reagent Test
Methanolic extract was treated with few drops of NaOH solution. Formation of intense yellow colour, which becomes colourless on addition of diluted acid, indicates the presence of flavonoids.
Lead acetate Test
Methanolic Extract was treated with few drops of lead acetate solution. Appearance of precipitates of yellow colour indicates the presence of flavonoids.
Detection of proteins and amino acids
Xanthoproteic Test
Methanolic extract was treated with few drops of concentrated Nitric acid. The presence of proteins was confirmed with the appearance of yellow colour.
Ninhydrin Test
0.25% w/v ninhydrin reagent was added to the extract and boiled for few minutes. Appearance of blue colour showed the presence of amino acid.
Detection of diterpenes
Copper acetate Test
Extract was dissolved in water and treated with 3-4 drops of copper acetate solution. Appearance of emerald green colour showed the presence of diterpenes.
Gas Chromatography- Mass Spectroscopy
Column Chromatography and Analysis Derivatization procedure
GC-MS analysis of the active fractions of A.deilei seaweed was performed using GC SHIMADZU QP2010system and gas chromatograph interfaced to a mass spectrometer (GC-MS) Acoathophora deilei (Red algae) was extracted and concentrated by using rotary evaporator. The 1.5 ml upper layer of extract was taken in funnel and added 100µl N, O-Bis (trimethylsilyl) trifluoroacetamide, trimethyl chlorosilane (BSTFA+TMCS) and 20µl pyridine and heated at 60°c for 30 minutes. To this aceto nitrile was added and filtered into a conical flask. 50µl BSTFA+TMCS was added to the filtrate and heated at 60°c in a water bath for 30 minutes. Filtered using 0.45µ membrane filter to a vial 8.
Identification of components
Interpretation of mass spectrum GC-MS was done using the database of National Institute Standard and Technique (NIST08s), WILEY8 and FAME having more patterns. The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST08s, WILEY8 and FAME library. The name, molecular weight, molecular formula and structure of the component of the test material was as certained.
Results And Discussion
The phytochemical screening showed that methanolic extract of Acoathophora deilei was found to contain alkaloids, carbohydrates, phytosterols, flavonoids and diterpenes (Table -1). They were known to exhibit various medicinal and pharmacological activities 5. Previous studies reported the presence of different phytochemical compounds of seaweeds collected from the coastal regions of the world 9,10. Flavonoids have been proved with antitumor and anti-oxidant properties 11. Alkaloids was found to have antimicrobial 12,13,14,cytotoxic15 and antispasmodic properties 16,17.
Table 1: Qualitative phytochemical analysis of Acoathophora deilei.
|
Phytochemicals |
Extracts | |
| Observations | Results | |
| Alkaloids:
Wagner’s test Mayer’s Test Dragendroff’s Test Hager’s Test |
Reddish Brown Solution precipitate
Formation of yellow coloured precipitate Formation of red coloured precipitate Formation of yellow coloured precipitate |
Present Present Present Present |
| Flavonoids:
Lead acetate test Alkaline Reagent |
Formation of yellow colour Formation of intense yellow colour, which becomes colourless on addition of dilute acid |
Present Present |
| Phytosterols:
Salkowski’s Test : Libermam Burchard’s Test |
Appearance of golden yellow colour precipitate Formation of brown ring at the junction indicates |
Present Present |
| Diterpenes:
Copper acetate Test |
Formation of emerald green colour |
Present |
| Carbohydrates:
Molisch’s test Benedict’s Test Fehling’s Test |
Formation of violet ring at the junction indicates No Orange red precipitate No formation of red coloured precipitate |
Present Absent Absent |
| Glycosides:
Modified Borntrager’s Test Legal’s Test |
No formation of rose-pink colour precipitate No formation of pink to blood red colour precipitate |
Absent Absent |
| Saponins:
Froth Test FoamTest |
No formation of thin layer precipitate Don’t produced persists for 10 mintues |
Absent Absent |
| Phenols:
Ferric chloride test |
No formation of bluish black colour precipitate |
Absent |
| Tannins:
Gelatin Test |
No formation of white colour precipitate |
Present |
| Proteins and Amino acids:
Xanthoproteic test Ninhydrin Test |
No formation yellow colour precipitate No formation of blue colour precipitate |
Absent Absent |
GC-MS Interpretation
The components present in the crude extract of Acoathophora deilei (Red algae) were identified by GC-MS analysis. The Chromatograph is shown in figure-1. The various components with their retention time (RT), molecular formula, molecular weight (MW) and percentage composition in the crude extract of the drug was presented in Table -2.
 |
Figure 1: Gc-Ms Analysis of Methanolic Extract of Acoathophora Deilei. |
Table 2: Retention time (rt), Molecular formula, Molecular weight (mw) and Percentage composition in the crude extract of the drug.
| SI.No | Retention time | Compound Name | Molecular formula | Molecular weight | Percentage area peak |
| 1. | 7.112 | Acetamide,NN-diethyl- | C6H13NO | 115 | 0.77 |
| 2 | 7.379 | Acetamide, N- Ethyl- | C4H9NO | 87 | 0.40 |
| 3 | 13.560 | Caryophyllene | C15H24 | 204 | 0.40 |
| 4 | 14.542 | Phenol,3,5-bis(1,1-dimethyethyl) | C14H22O | 206 | 0.36 |
| 5 | 15.531 | Diethyl phthalate | C12H14O4 | 222 | 1.79 |
| 6 | 17.052 | Methyl tetradecanoate | C15H30O2 | 242 | 1.16 |
| 7 | 17.817 | 1-Heptadecene | C17H34 | 238 | 1.27 |
| 8 | 18.137 | Pentadecanoic acid, Methyl ester | C16H32O2 | 256 | 0.24 |
| 9 | 18.276 | Neophytadiene | C20H38 | 278 | 0.42 |
| 10 | 18.331 | 2-Pentadecanone,6,10,14-trimethyl- | C18H36O | 268 | 0.84 |
| 11 | 18.664 | 8-Octadecanone | C18H36O | 268 | 0.64 |
| 12 | 19.043 | Octadecane,1-Chloro- | C18H37Cl | 288 | 0.39 |
| 13 | 19.176 | Hexadecanoic acid, Methyl ester | C17H34O2 | 270 | 28.58 |
| 14 | 19.513 | Dibutyl Phthalate | C18H37CL | 288 | 26.93 |
| 15 | 19.863 | 1-Heneicosanol | C21H44O | 312 | 1.65 |
| 16 | 20.379 | Hexadecanoic acid,2-hydroxy-,Methyl ester | C17H34O3 | 286 | 1.36 |
| 17 | 20.643 | 10-Nonadecanone | C19H38O | 282 | 0.27 |
| 18 | 20.872 | 9-Octadecanoic acid, methyl ester | C19H36O2 | 296 | 7.20 |
| 19 | 21.105 | Methyl stearate | C19H38O2 | 298 | 1.59 |
| 20 | 21.217 | Cis-Vaccenic acid | C18H34O2 | 282 | 0.92 |
| 21 | 21.432 | Octadecanoic acid | C18H36O2 | 284 | 1.30 |
| 22 | 21.730 | n-tetracosanol-1 | C24H50O | 354 | 1.32 |
| 23 | 21.855 | 2-Ethylbutyric acid,octadecyl ester | C24H48O2 | 368 | 12.74 |
| 24 | 22.296 | 5, 8, 11, 14- Eicosatetraenoic acid. Ethyl ester | C22H36O2 | 332 | 0.33 |
| 25 | 23.137 | Silane,methylvinyl(4-methylpent-2-yloxy)ethoxy | C11H42O2Si | 216 | 0.57 |
| 26 | 23.444 | n-tetracosanol | C24H50O | 354 | 1.11 |
| 27 | 24.558 | 1,2 Benzendicarboxylic acid | C24H38O4 | 390 | 4.92 |
| 28 | 25.027 | Octacosanol | C24H38O4 | 390 | 0.55 |
28 components were identified in the drug. The major compounds were hexadecanoic acid methyl ester (28.58%), followed by Dibutyl phthalate (26.93%), 2-Ethyl butyric acid, octadecyl ester ( 12.74%) ,9-Octadecanoic acid, methyl ester (7.20%), and 1,2- Benzendicarboxylic acid (4.92%) and their pharmacological importance activity in Table 3 .All other components were less than 4% and hence found to be less significance as their bioavailability is negligible. Figure (2 to 29) shows the mass spectrum and structures of the major phytol compounds. The biological activities listed are based on Dr.Dukes phytochemical and ethnobotanical databases by Dr. Jim Duke of the agricultural Research service, USDA.
Table 3: Important major compounds with their pharmacological activity of Acoathophora Deilei.
| S.NO | Chemical compounds | Molecular formula | Pharmacological activity |
| 1. | Hexadecanoic acid, methyl ester | C16H32O2 | Anti-bacterial and antifungal activity [18]. |
| 2. | 9-octadecanoic acid, methyl ester | C19H36O2 | Anti-inflammatory, anti-androgenic, Anti- cancer activity, dermatitigenic, 5-alpha reductase inhibitor, anemiagenic and insectifuge activity [15]. |
| 3. | 1,2 – Benzene dicarboxylic acid | C24H38O4 | Neurodegenerative disorders, Anti-cancer activity. |
 |
Figure 2: Acetamide N,N diethyl |
 |
Figure 3: Acetamide, N –Ethyl |
 |
Figure 4: Caryophyllene. |
 |
Figure 5: Phenol, 3,5-bis (1,1-dimethylethyl). |
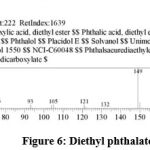 |
Figure 6: Diethyl phthalate. |
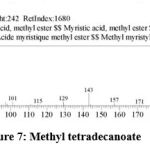 |
Figure 7: Methyl tetradecanoate. |
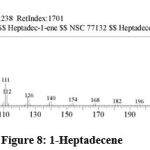 |
Figure 8: 1-Heptadecene. |
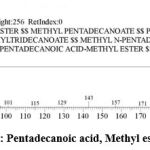 |
Figure 9: Pentadecanoic acid, Methyl ester. |
 |
Figure 10: Neophytadiene. |
 |
Figure 11: 2-Pentadecanone,6,10,14-trimethyl |
 |
Figure 12: 8-Octadecanone. |
 |
Figure 13: Octadecane,1-Chloro. |
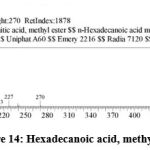 |
Figure 14: Hexadecanoic acid, methyl ester. |
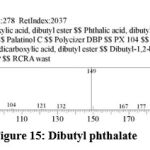 |
Figure 15: Dibutyl phthalate. |
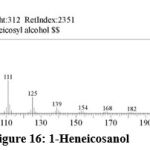 |
Figure 16: 1-Heneicosanol. |
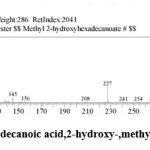 |
Figure 17: Hexadecanoic acid,2-hydroxy-,methyl ester. |
 |
Figure 18: 10-Nonadecanone. |
 |
Figure 19: 9-Octadecenoic acid, methyl ester,(E) |
 |
Figure 20: Methyl stearate. |
 |
Figure 21: Cis-Vaccenic acid. |
 |
Figure 22: Octadecanoic acid. |
 |
Figure 23: N-tetracosanol-1. |
 |
Figure 24: 2-Ethylbutyric acid, Octadecyl ester. |
 |
Figure 25: 5,8,11,14-Eisosatetraenoic acid,Ethyl ester. |
 |
Figure 26: Silane, methyl vinyl(4-methylpent-2-yloxy) ethoxy. |
 |
Figure 27: N-tetracosanol. |
 |
Figure 28: 1,2Benzenedi Carboxy acid. |
 |
Figure 29: Octacosanol. |
Conclusion
Phytochemical analysis of Acoathophora deilei. shown the presence of various metabolites such as flavonoids, alkaloids, phytosterols and Diterpenes are present. GC-MS analysis showed the presence of 28 different compounds of varied nature in methanolic extract of Acoathophora deilei. The present study is the first report to be published till date related to the GC-MS analysis of Acoathophora deilei, and it is a significant source for novel bio active compounds. Future studies (in vitro and in vivo) are required to prove the potential medicinal properties of the Acoathophora deilei (red algae).
Acknowledgement
We acknowledge the authorities of Central Inter-Disciplinary Research Facility, SBV University and the Central Council for Research in Siddha, Chennai for the facilities and support provided at the time of our research.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Murugeshamuthaliyar Gunapadam thathu –Geevan 2nd, Directorate of Indian Medicine & homeopathy Thurai Publication, third edition 2008.
- Rizvi SI,Mishra N.Traditional Indian medicines used for the management of diabetes mellitus. J Diabetes Res,vol 13;pp1-11, 2013. http://dx.doi.org/10.1155/2013/712092.
CrossRef - Kaliaperumal. N., Seaweeds diversity, resources, utilization conservation and cultivation. In: Abstracts of the National seminar on Marine resources: Sustainable utilization and Conservation, organized by department of Plant Biology and Biotechnology. St. Mary’s College Thooothukudi. P.1;2009.
- Prashant T, Kumar B,Mandeep K, Gurpreet K, Harleen K: Phytochemical Screening and Extraction : A Review ; Internationale Pharmaceutica Sciencia ;; Vol 1,Issue 1;PP 98-106,Jan- March 2011.
- Sofowara A . Medicinal plants and Traditional medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria. p. 289. 1993
- Trease GE and Evans WC; Pharmacognosy 11th edition; Brailiar Tiridel can.Macmilian publishers 1989.
- Harborne J B: Phytochemical methods, chapman and hall, London 1973.
- Aneesh. T.P,Elizabeth T, Della G T, Anandan R : GC-MS analysis of Phytochemical compounds present in the rhizomes of Nervilia Aragoana GAUD ; Asian Journal of Pharmaceutical and Clinical Research, Vol 6,Supp 3,;pp-68-74,2013.
- Sachithananthan K., Sivapalan A. Antibacterial properties of some marine algae of Sri Lanka. Bulletin of Fisheries Research Station, Sri Lanka.; 26:5-9;1975
- Vidhyavathi, N and Sridhar, K.R. Seasonal and Geographical variations in the antimicrobial activity of seaweeds from the Mangalore coast in India. Mar;34: 279-284; 1991.
CrossRef - Cody V; Middleton E; Harborne JB; Baretze A. Plant flavonoids. In: Biology and medicine II: Biochemical, cellular and medicinal properties. Alan R. Liss Inc, New York, 330,1988.
- Omulokoli E, Khan B, Chhabra SC. Antiplasmodial activity of four Kenyan medicinal plants. Ethnopharmacol. 56: 133-137; 1997.
CrossRef - Cowan, M.M. Plant products as antimicrobial agents. Clinical Microbiology Reviews; 12: 564-582; 1999.
CrossRef - Srivastava, N., K. Saurav, V. Mohanasrinivasan, K. Kannabiran and M. Singh. Antibacterial Potential of Macroalgae Collected from the Mandapam Coast, India British J. Pharmacol. and Toxicol., 1(2): 72-76; 2010.
- Nobori T; Miurak K; Wu DJ; Takabayashik CA; Carson DA. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers Nature: 46, 753-756;1994
CrossRef - Stray F. The natural guide to medicinal herbs and plants. Tiger Books International, London:12-16; 1998.
- DE Okwu and ME Okwu. Phytochemicals and vitamin content of indigenous spices of South Eastern Nigeria. J. Sustain. Environ., 6(2): 140-147; 2004.
- Krishnamoorthy.K, Subramaniam .P; Phytochemical profiling of leaf, stem an tuber parts of solena amplexicaulis (lami) Gandhi using GC-MS: International scholarly research notices, vol pp1-13; 2014.
CrossRef








