I Gde Sastra Winata1* , I Nyoman Gede Budiana1
, I Nyoman Gede Budiana1 , I Made Jawi2
, I Made Jawi2  and Ketut Suwiyoga1
and Ketut Suwiyoga1
1Department of Obstetrics and Gynaecology of Sanglah General Hospital/Faculty of Medicine, Udayana University, Denpasar, Indonesia.
2Department of Pharmacology of Faculty of Medicine of Udayana University, Denpasar, Indonesia.
Corresponding Author E-mail: sastra@unud.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2425
Abstract
This study aimed to describe Paclitaxel-Carboplatin chemotherapy as neoadjuvant chemotherapy in stage IB3, IIA2 and IIB cervical cancer. The review was conducted by collecting journals from previous studies discussing neoadjuvant chemotherapy in cervical cancer stages IB3, IIA2, and IIB and in this case specifically discussing Paclitaxel-Carboplatin chemotherapy. Neoadjuvant chemotherapy refers to systemic therapy intended to reduce the size of the tumour before the definitive operation. Several studies have shown that neoadjuvant chemotherapy has greater advantages than surgery alone for early stage cancers (IB3, IIA2, and IIB). Paclitaxel and Carboplatin are known chemotherapeutic agents that can be used as neoadjuvant chemotherapy. Neoadjuvant Chemotherapy regimen Paclitaxel Carboplatin is one of the options in performing therapy for early stage cervical cancer which can be very helpful in healing and cancer-free patient condition. Neoadjuvant chemotherapy followed by radical surgery has significant benefits that have been described in several previous studies. Neoadjuvant Chemotherapy regimen Paclitaxel Carboplatin may be used as therapy regimen for early stage cervical cancer with all advantage compared to only surgery. Thus, this type of regimen can be used to decrease mortality and morbidity in patient with stadium IB3, IIA2 and IIB cervical cancer.
Keywords
Carboplatin; Neoadjuvant Chemotherapy; Paclitaxel; Stadium IB3, IIA2 and IIB cervical cancer
Download this article as:| Copy the following to cite this article: Winata I. G. S, Budiana I. N. G, Jawi I. M, Suwiyoga K. Neoadjuvant Chemotherapy in Stadium IB3, IIA2 and IIB Cervical Cancer a Narrative Review. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Winata I. G. S, Budiana I. N. G, Jawi I. M, Suwiyoga K. Neoadjuvant Chemotherapy in Stadium IB3, IIA2 and IIB Cervical Cancer a Narrative Review. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3ucHucP |
Introduction
Cervical cancer is a malignant tumour in the cervix caused by the infection of human papilloma virus (HPV). Cervical cancer is the most commonly found gynecological cancer and is the main cause of pain and death in women, especially in developing countries. Until now, the handling of cervical cancer is still very developing and in some early stages there are still many controversies, one of which is an IB3, IIA2 and IIB stage.
Cervical cancer is ranked fourth most often found in women after breast cancer, colorectal and lung. The incidence of cervical cancer has increased since the last two decades. The incidence of cervical cancer globally is 13.1 cases per 100,000 women, which vary in each country. The incidence of cervical cancer in the world based on the International Agency for Research On Cancer (IARC) in 2015 is 17 cases per 100,000 women1. Bulky cervical cancer or cervical cancer with a mass size of ≥ 4 cm, i.e. IB3, IIA2 and IIB stadium are specialized problems related to the controversy of handling and the patient’s prognosis. The prognosis of IB3 Stadium Cervical Cancer, IIA2 and IIB is worse than the initial stage to IB2 regarding the incidence of local recurrence and higher lymph node metastases.
Until now the IB3 stage cervical cancer management strategy, IIA2 and IIB is still controversy, where chemoradiation, operations radical hysterectomy or neoadjuvant chemotherapy which is then continued with radical hysterectomy can be done2. The National Comprehensive Cancer Network (NCCN) recommends therapeutic modalities in cervical cancer this type is direct radical hysterectomy, radical hysterectomy after neoadjuvant chemotherapy or concurrent chemoradiotherapy3,4. In Sanglah Hospital, cervical cancer therapy follows the recommendations issued by Federation Internationale de Gynecologie et d’obstetrique (FIGO). But specifically for Cervical Cancer Stadium IIB Gynecology Gynecology Oncology Division RSUP Sanglah Establish the handling of Cervical Cancer Stadium IIB includes the provision of Platinum-Based Neoadjuvant chemotherapy followed by radical hysterectomy in cases that allow operations or radiotherapy if they do not allow operation. The neoadjuvant chemotherapy regimens are often used include Cisplatin, Paclitaxel, Topotecan, Vinorelbine, Gemcitabine and iFosfamide5. In research by Mori et al. (2010), the provision of Paclitaxel regimens and carboplatin per week continued with radical operations in Locally Advanced Cervical Cancer (LACC) patients is a promising therapy with a better prognosis6. Neoadjuvant chemotherapy is able to reduce the risk of lymph node metastasis, parametrial infiltration and tumour size so that it can increase the survival rate, the quality of life of the patient and reduce the need for post-operating radiation therapy7.
The search for original research articles discussing neoadjuvant chemotherapy in cervical cancer stages IB3, IIA2, and IIB and in this case specifically discussing Paclitaxel-Carboplatin chemotherapy were conducted on January19th of 2021. The search was conducted on Cochrane Center Register of Controlled Trial (CENTRAL) and PubMED databases and used MeSH and text words to generate a four-check subset including cervical cancer, neoadjuvant, paclitaxel, and carboplatin. The retrieval formula for this research was generated using “AND” to connect all four subsets. We also expand the search using secondary search by scanning the references of the relevant articles and from the clinical trials or systematic reviews that have previously published. There is no limitation of the year published. We exclude non relevant articles that does not match specifically with our interest. Pharmacokinetics and pharmacodynamics of Paclitaxel and Carboplatin in affecting angiogenesis, mitosis, and tumour instability were studied, compared and explored. After that, it was described about the specific effect of this chemotherapy on cervical cancer stages IB3, IIA2, and IIB.
Neoadjuvant chemotherapy in IB3, IIA2 and IIB Stadium Cervical Cancer
Neoadjuvant chemotherapy refers to systemic therapy intended to reduce the size of the tumour before the definitive operation8. The provision of neoadjuvant chemotherapy is estimated to provide a better life expectancy than adjuvant chemotherapy, this is due to neoadjuvant chemotherapy can reduce genetic heterogeneity. Neoadjuvant chemotherapy followed by operations reportedly increasing the progression free survival (PFS) and diseases free survival (DFS) of 88.1% and 60.5%9. In addition, neoadjuvant chemotherapy can also be used to optimize the operative approach, and monitor response and adjust the use of chemotherapy regimens. This can be used at the beginning of the regiment, or if it fails to achieve the optimal response, and to choose chemotherapy or other additional therapies10.
Historically, Cisplatin, Paclitaxel, and iFosfamide are considered as the most active platinum agent drug with a response rate of 20% in cervical cancer9. The use of Cisplatin 100 mg/m2 shows a higher level of response compared to 50 mg/m2, but in research by Bonomi et al.in 198511 reported the existence of nephrotoxicity and myelosuppression on the use of high-dose cisplatin. Besides Cisplatin, iFosfamide derived from alkylation agents also shows the level.
The response is 20% at a dose of 1.2 g/m2 for 5 days. Doxorubicine is an anthracycline antibiotic drug which shows a response rate of 20% in cervical cancer, but the heaviest side effects of the use of doxorubicin are cardiotoxicity that can be fatal. Paclitaxel from the Taxane group showed a response rate of 27%9.
Combination therapy shows more effective results in attacking heterogeneous cell populations. The use of multiple regiments with different working mechanisms can minimize the occurrence of drug resistance, reduce the dosage and toxicity of each chemotherapy drug12. Giving Paclitaxel 60 mg/m2 and Cisplatin 60 mg/m2 was carried out in 43 cervical cancer patients by Park et al. (2002) produced downstaging in 31 patients13. Hornychová et al. (2008) gives Neoadjuvant chemotherapy combination of Cisplatin regimen 75 mg/m2 with iFosfamide 2 g/m2 in patients with cervical squamous cell carcinoma, and 75 mg/m2 cisplatin with 35 mg/m2 doxorubicin in patients with adenocarcinoma14. Reported by 69.5% of patients who received a combination neoadjuvant chemotherapy experienced a reduction of tumours of more than 50% and 11.3% of patients reported no discovery of residual malignancies on pathological examinations. Meanwhile Angioli et al. (2013) provide a combination of Cisplatin 60 mg /m2 and ethoposide 100 mg/m2 which then continues with surgery9.
Lately, the combination of Paclitaxel Carboplatin’s combination every 3 weeks shows an effect similar to the combination of Cisplatin Paclitaxel every 3 weeks in recurrent cervical cancer10. In addition, the side effects of nausea, neurotoxicity, muscular-toxicity, and nephrotoxicity from carboplatin are relatively lower than Cisplatin. Research by Singh et al. (2013) using the Neoadjuvant Chemotherapy regimen Paclitaxel Carboplatin obtained a 67.8% of patients giving a good response to neoadjuvant chemotherapy, where the complete response was 7.1% and partial of 60.7%15. Salihi et al. (2017) conducted a prospective study and reported that the combination of Paclitaxel Carboplatin provides a clinical response rate to reach 89% lower the size of the tumour so that it is very good in patients who are then operated16. Research by Mori et al. (2010) also reported a good chemotherapy response of Paclitaxel Carboplatin combination, where 84% was obtained complete and partial response6. In Sanglah General Hospital, based on the Clinical Practice Guide Number YM.01.02 / ppk.xiv.6.1 / 35842/2018, Paxus Carboplatin is a choice of neoadjuvant chemotherapy in cervical cancer. Neoadjuvant chemotherapy will be given as many as 3 series before evaluation of operation and followed by radical acts of hysterectomy and removal of lymph nodes in the pelvis area17.
Effects of Neoadjuvant Chemotherapy Followed by Radical Surgery versus Primary Surgical Treatment Alone on Patients with Cervical Carcinoma
Neoadjuvant chemotherapy is used to reduce tumour volume before surgery or radiation therapy. In patients with advanced cervical cancer, 2-3 cycles of neoadjuvant chemotherapy can improve the chemotherapy rate of surgical resection. Previous studies have shown that neoadjuvant chemotherapy suppresses tumor micrometastasis and can increase resection by reducing tumour volume. Recent Clinical trials of phase II ASCO have also shown that cervical cancer has a high response rate to neoadjuvant chemotherapy and that chemotherapy toxicity is acceptable. In addition, neoadjuvant chemotherapy has been shown to reduce lymph node metastasis and parametric infiltration in patients with cervical cancer. Neoadjuvant chemotherapy regimens, cycles, and drug doses varied from study to study, making it difficult to draw specific conclusions in previous meta-analyses10.
Some studies suggested that neoadjuvant chemotherapy was associated with a decrease in bleeding and improved likelihood of laparoscopic surgery compared with primary surgical treatment alone13. Although data regarding effect of tumour size following neoadjuvant therapy on pathologic complete response in cervical cancer was still limited, it is found that, indeed, tumour size after neoadjuvant chemotherapy was independently correlated with pathologic complete response in breast cancer after controlling for receptor status14. Further researches are warranted.
Paclitaxel-Carboplatin Chemotheraphy
Paclitaxel
Paclitaxel is a metabolite of the isoprenoid group, more precisely it is pseudoalkaloids of expenses consisting of a Taxane ring and a group of n-benzoylphenylisoserine, with molecular formula C47H51NO14. Paclitaxel shows the unique pharmacological properties in inhibiting mitosis, in contrast to Vinka alkaloids and derivates of colchicine which inhibits the formation of microtubules, paclitaxel increases the polymerization of microtubules during cell division which will lead to multipolar division causing cell death. Paclitaxel is a chemotherapy agent commonly used as cervical, breast, ovarian and lung cancer therapy18,19,20,21.
Pharmacokinetics Paclitaxel
Paclitaxel is a white crystalline powder that has a high molecular weight (853.9 g/mol), is highly lipophilic with a very low solubility in air (0.7 g/L), and has a melting point close to 216oC19. Paclitaxel has very lipophilic and hydrophobic properties so it needs to be dissolved in Cremophor EL® solvent which consists of a mixture of polyoxyethylated oil and ethanol in a 1:1 ratio. However, this solvent can cause hypersensitivity reactions if given intravenously for a long time. Therefore, prophylactic antihistamines (H1 and H2 receptor antagonists histamine) and glucocorticoids are given to reduce the possibility of hypersensitivity reactions following paclitaxel infusion. To overcome this problem, another formulation of paclitaxel, namely nab-paclitaxel consisting of nanoparticles bound to albumin and paclitaxel was developed to prevent post-infusion hypersensitivity reactions so that it does not require the administration of antihistamine prophylaxis19.
Paclitaxel is generally given intravenously over 3 hours, but it can also be given intraperitoneally. Paclitaxel dose 135 – 175 mg/m2 every 3 weeks. paclitaxel The dose for primary disease is 80 mg/m2 for 3 consecutive weeks on a 21 day schedule or commonly called a “solid dose”, while for recurrent disease it is given in a 28 day schedule. The pharmacokinetics of these drugs have a high standard deviation. The terminal half-life ranged from 1.3-8.6 hours (mean 5 hours) and the steady-state volume of distribution was ~87.1 mL/min/m18.
Paclitaxel is metabolized mostly by cytochrome P450 2C8 to the neoadjuvant chemotherapy metabolite 6-hydroxypaclitaxel and to a lesser extent by cytochrome 3A4 to 3′-phenyl-hydroxypaclitaxel. Paclitaxel is a substrate for the ATP-binding cassette (ABC) efflux transporter so that on oral administration, the ABC efflux transporter from paclitaxel will return to the intestinal lumen causing very low oral bioavailability. Therefore, intravenous administration is required in the administration of paclitaxel. Several studies have revealed that paclitaxel undergoes hepatic uptake due to the interaction of the organic anion polypeptide 1B3 influx transporter. Apart from being a substrate for ABC efflux transporters, paclitaxel is also a substrate for organic anion transporter 2 (SLC22A7) in the kidney (The presence of this transporter activity in the liver and kidneys contributes to the distribution and elimination of paclitaxel and influences the variability in the pharmacokinetics of paclitaxel19.
Paclitaxel activates the pregnane X receptor which induces upregulation of key enzymes for drug metabolism such as cytochrome 3A4 and the ABC1 transporter. However, administration of paclitaxel every 1, 2, or 3 weeks was not associated with changes in drug metabolism over time, meaning minimal autoinduction that occurred at standard doses19. Plasma clearance of paclitaxel is biphasic. The first decrease in volume occurs rapidly as a consequence of drug distribution from the central compartment and drug elimination from the peripheral compartment18.
Pharmacodynamics Paclitaxel
In adequate concentrations, paclitaxel works to induce multipolar cell division by binding to microtubule subunits, namely -tubulin. Paclitaxel and other microtubule-stabilizing agents bind to microtubules causing dynamic suppression and microtubular stabilization. A study states that the impact of microtubule-stabilizing agents inhibits the process of cell division and affects cell signaling pathways such as apoptosis18. The process of cell mitosis then stops which will cause cell death during the mitosis process or the cell will continue to the next process called mitotic slippage. In mitotic slippage, a cell can enter the G1 phase of the cell cycle without going through anaphase or cytokinesis to produce a single cell that is tetraploid. These cells have the possibility to stop proliferating, die after mitotic slippage, or enter the next cycle. The factors that determine the outcome of this mitotic slippage process are still not clearly understood, but it is known that drug concentration and time of cell exposure influence cell death18,21.
Another study states that this bond will lead to the formation of abnormal spindle fibers during the mitotic process and due to the presence of these spindle threads, additional spindle poles are formed during the mitotic process. Cells that enter anaphase, there will be separation of chromosomes in various directions (multipolar cleavage), which will have an impact on random chromosome segregation, then result in partial cytokinesis failure. The result of this partial cytokinesis failure is the presence of aneuploid cells resulting from division. These cells will then die. prevents the breakdown of microtubules so that aberrant clusters of microtubules and microtubule structures are found in the mitotic phase. This prevents mitosis and is followed by apoptosis20,21.
The cause of apoptosis by paclitaxel is not clearly known, but it is suspected that apoptosis occurs due to the activation of the transcription factor p53 (Figure 1), in addition to recent research that paclitaxel increases the production of Reactive Oxygen Species (ROS) and overexpression of genes and proteins associated with damage. on the endoplasmic reticulum (ER). Damage to the ER can lead to the release of Ca2+ and cause mitochondrial damage due to excess Ca2+. This will lead to an increase in ROS production. In a study conducted on dogs, it was shown that there was a decrease in the expression of the anti-apoptotic protein B-cell Leukaemia 2 (Bcl-2) in tumour cells, as well as over-expression of the pro-apoptotic protein Bcl-2-associated X (BAX). These changes lead to mitochondrial apoptosis through disruption of the Mitochondrial Membrane Potential (MMP) and the release of cytochrome C from the mitochondria into the cytoplasm, as well as the cleavage of the protein caspase-3. However, the possibility of mitochondrial apoptosis due to increased ROS is not completely clear22.
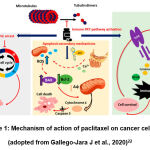 |
Figure 1: Mechanism of action of paclitaxel on cancer cells (adopted from Gallego-Jara J et al., 2020)22 |
In the immune system, paclitaxel causes macrophage stimulation resulting in the secretion of TNF- or IL-2 cytokines that trigger the activation of NK cells, dendritic cells, and cytotoxic T lymphocytes that cause tumour cell eradication. In addition, paclitaxel acts directly by binding to Toll-like receptors on the surface of dendritic cells, resulting in antigen-presenting cell (APC) maturation23. Paclitaxel has also been shown to decrease VEGF and Ang-1 expression in cervical cancer cells, and increase TSP-1 secretion in the tumour microenvironment24.
Carboplatin
Carboplatin is an agent of the platinum coordination complex group. Platinum coordination complexes covalently bind to nucleophilic sites on DNA and have many of the same pharmacological attributes as alkylating agents, but platinum coordination complexes do not form carbonium ion intermediates like alkylating agents as adjuvant therapy or salvage therapy for epithelial ovarian cancer12, 25.
Pharmacokinetics of Carboplatin
Carboplatin is a platinum-based chemotherapy regimen that is known to enter cells through a simple passive diffusion mechanism. There are several studies that reveal uptake and efflux mechanisms in transporter processes and regulation that contribute to drug accumulation in resistant cells26.
Transporters for the metabolism of drugs from metal groups such as copper transporters (CTR1, ATP7A and ATP7B). The CTR1 transporter is known to mediate Carboplatin influx and the ATP7B and ATP7A transporters contribute to the removal of copper from cells. The process of uptake and efflux of Carboplatin is thought to be related to copper metabolism so that the absorption of copper and Carboplatin can influence each other27.
The dose of intravenous carboplatin was calculated to achieve an “area under the curve” (AUC) target of 6, based on the glomerular filtration rate (GFR). The Calvret equation is a commonly used formula for calculating doses, i.e., total carboplatin dose [mg] = AUC x [GFR + 25], but in clinical practice, the estimated creatinine clearance (CrCl) is often used instead of GFR and can be calculated by the Cockroft equation. -Gault (CrCl = [140 – age] x body weight [kg]/0.72 x serum creatinine level [mg/100 mL]). Carboplatin infusion is given for 30-60 minutes and the dose is repeated every 3 to 4 weeks12.
Pharmacodynamics of Carboplatin
Carboplatin works by penetrating cell membranes, carboplatin will undergo hydrolysis so that it becomes positively charged, then binds covalently at the location of N7 purine bases forming monoadducts or intra and inter diadduct chains. This process gives rise to interactions between DNA or DNA-proteins. This binding between DNA and carboplatin can cause lesions on DNA so that it causes crosslinking between DNA (interstrand cross-linking) which is the most cytotoxic effect because it interferes with cell replication (G2/M growth stops) causing apoptosis or necrosis of cancer cells22,27.
Paclitaxel Carboplatin Chemotherapy Relationship to Angiogenesis, Mitosis and Tumour Genetic Instability
Paclitaxel causes autophagy by increasing levels of protein 5 and Beclin-1. Protein 5 and Beclin-1 are proteins required for the formation of autophagosomes. This leads to increased expression of p53 and LC3B thereby regulating the initiation of autophagy. Paclitaxel also significantly reduces the density of micro vessels in tumours, and reduces the synthesis of Vascular Endothelial Growth Factor (VEGF) in vivo. The antiangiogenic effect of paclitaxel is thought to be caused by the accumulation of paclitaxel in endothelial cells and carboplatin can increase p53 expression through DNA damage, resulting in acetylation of lysine associated with proapoptotic genes and preventing cells from entering the G2-M phase. Carboplatin induces ERK activation, which is a factor that increases the p53 response to DNA damage caused by carboplatin thereby preventing the development of cancer cells28.
VEGF-C expression in bulky cervical cancer is triggered by cell hypoxia, resulting in an increase in Hypoxia Inducible Factors-1α (HIF-1α) and upregulation of VEGF gene expression levels, as well as an increase in transcriptional activity in the VEGF signal transduction pathway. VEGF-C is a VEGF derivative that plays a role in vasculogenesis and lymphangiogenesis, while HIF-1α is a transcription factor that plays a role in the regulation of neovascularization. HIF-1α levels are always increased by cancer cells to maintain oxidative metabolism and promote the growth and metastasis of cancer cells under hypoxic conditions29. Neoadjuvant chemotherapy is given with the aim of causing more neoplastic cell death thereby reducing tumour size in the cervix, improving irregular vascularization and oxygen imbalance resulting in high oxygenation and an indirect decrease in HIF-1α expression followed by a decrease in VEGF-C gene transcription30. In a study by Kartikasari et al. (2018), 25 patients were given Paclitaxel-Carboplatin, and the expression of VEGF-C decreased significantly from an average of 6.16 to 4.2031.
Ki-67 protein is significantly associated with cell proliferation and acts in the mitotic phase, namely G1, S, G2, and M, and maintains DNA structure32. High levels of Ki-67 indicate that tumour cells have a fast cell cycle. Prevention of depolymerization by paclitaxel led to the cessation of late G2/M phase cell cycle progression followed by apoptosis. Under normal conditions, the phosphorylation of Ki-67 by CDK-1 occurs in the M phase so that the cell can pass through the mitotic state. Decreased tumour cell proliferation as indicated by a decrease in Ki-67 after paclitaxel administration, then along with tumour cell apoptosis and tumour cell necrosis due to VEGF inhibition increased the response to neoadjuvant chemotherapy for the better33. The mechanism of regulation of Ki-67 levels is thought to be controlled by p53. In HeLa cell studies, it was found that p53 interacts with Sp-1 thereby suppressing the transcription of Ki-67.
Effectiveness of Paclitaxel-Carboplatin Neoadjuvant Chemotherapy in Stage IB3, IIA2 and IIB Cervical Cancer
Neoadjuvant chemotherapy followed by radical surgery has significant benefit in early-stage cervical cancer. Research conducted in Thailand by Prueksaritanond et al. (2012)34, compared neoadjuvant chemotherapy followed by radical hysterectomy versus radical hysterectomy alone in patients with stage IB3, IIA2, and IIB cervical cancer. A total of 80 patients were included in the study, with the result that the need for postoperative adjuvant chemoradiation was reduced in patients receiving neoadjuvant chemotherapy compared to radical hysterectomy alone (27.5% versus 57.5%, p = 0.0007). The systematic review by Rydzewska et al. (2012)35 involving six clinical trials reported an increase in DFS and PFS in patients receiving preoperative neoadjuvant chemotherapy. In addition, there was also a significant reduction in the incidence of pelvic and parametrial lymph node infiltration in patients receiving preoperative neoadjuvant chemotherapy compared to those undergoing radical hysterectomy alone.
A randomized clinical study conducted by Gupta et al. (2018), on 633 patients with cervical cancer stages IB3, IIA2, and IIB to determine the effectiveness of neoadjuvant chemotherapy and surgery compared to chemoradiation36. The study was conducted for 12 years, with an average duration of following patients for 58.5 months. A total of 316 patients were in the neoadjuvant chemotherapy group with surgery and 317 patients in the chemoradiation group. The five-year survival rate in the neoadjuvant chemotherapy and surgery group was 69.3% compared with 76.7% in the chemoradiation group (hazard ratio, 1.38; 95% CI, 1.02 to 1.87; P = 0.038) . The corresponding five-year survival rates were 75.4% and 74.7%, respectively (hazard ratio, 1.025; 95% CI, 0.752 to 1.398; P = 0.87). When compared between the neoadjuvant chemotherapy group with surgery and the chemoradiation group, the level of toxicity to surrounding organs such as the rectum, bladder and vagina in neoadjuvant chemotherapy followed by surgery was lower than chemoradiation.
In a study conducted by Liu et al. (2018) comparing the efficacy between neoadjuvant chemotherapy followed by radical hysterectomy surgery with primary surgical therapy for cervical cancer stages IB3, IIA2, and IIB. In 303 patients, it was found that patients who received neoadjuvant chemotherapy before surgery had less bleeding, shorter operation duration and better tumour efficacy with neoadjuvant chemotherapy37.
In the systematic review and meta-analysis conducted by Ye et al. (2020), to assess the efficacy and safety between neoadjuvant chemotherapy followed by radical hysterectomy with chemoradiation for cervical cancer stages IB3, IIA2, and IIB. This study found that patients who were given neoadjuvant chemotherapy before surgery had better OS and PFS and lower organ toxicity compared to chemoradiation38.
Predictors of Neoadjuvant Chemotherapy Success in Stage IB3, IIA2 and IIB Cervical Cancer
Age
Age has a significant influence on the incidence of cervical cancer. Therefore, age is an important factor to consider in predicting the success of neoadjuvant chemotherapy therapy accompanied by radical surgery in cases of cervical cancer. The study conducted by Jin Zhou et al. (2016), showed that the overall response rate in younger patients, aged <35 years, was higher than in older patients. It was concluded that young patients with cervical cancer at stages IB3 and IIA2 had a better clinical response than older patients39.
Parity
Not many studies have discussed the relationship between parity and response to neoadjuvant chemotherapy. Analysis of research conducted by Khatimah and Muhammad (2019) showed that there was no significant relationship between parity and response to neoadjuvant chemotherapy in early-stage cervical cancer40.
Tumour size
The study by Sardi et al. (1997) showed that in stage IB2 tumours measuring more than 4 cm, the response to neoadjuvant chemotherapy was 83.6%. Meanwhile, tumour size 6 cm gave a better therapeutic response than tumour size > 6 cm with a ratio of 50%: 74.3%. However, the analysis conducted by Khatimah and Muhammad (2019)40stated that there was no significant relationship between tumour size and neoadjuvant chemotherapy response41.
Degree of differentiation
Differentiation is the process of maturation of immature cells into mature cells that have specific functions. In cancer, this process describes how much the tumour tissue looks like the normal tissue of origin. Well-differentiated cancer cells look like normal cells and tend to grow and metastasize more slowly than poorly differentiated or undifferentiated cancer cells42. In cervical cancer, the degree of differentiation can be a predictor of prognosis. Not many studies have discussed the relationship between the degree of differentiation and the success of neoadjuvant chemotherapy, but the research conducted by Matsuo et al. (2018) stated that there was no significant relationship between the degree of differentiation and the response to neoadjuvant chemotherapy43.
Histopathological type
Squamous cell carcinoma is the main histologic type that accounts for three-quarters of all cervical cancers. Adenocarcinoma and adenosquamous cell carcinoma represent 10–15% and other histology represents the remaining 10–15%. In general, histologic type did not significantly affect chemotherapy response. However, the squamous cell type has a better response to neoadjuvant chemotherapy, which is 84% versus 70%44. There are differences of opinion regarding the effect of histological type on the prognosis of cervical cancer. Some studies have found that adenocarcinoma has a poor prognosis, whereas other studies have found no evidence of histopathological type as a prognostic factor45. Another study concluded that cervical cancer with histopathological types of small cell carcinoma and adenocarcinoma correlated with poorer survival46.
Conclusion
Neoadjuvant Chemotherapy regimen Paclitaxel Carboplatin is one of the options in performing therapy for early stage cervical cancer which can be very helpful in healing and cancer-free patient condition. Neoadjuvant chemotherapy followed by radical surgery has significant benefits that have been described in several previous studies. Neoadjuvant chemotherapy can help reduce the need for postoperative adjuvant chemoradiation in patients compared to radical hysterectomy alone, improve five-year survival and reduce toxicity to surrounding organs such as the rectum, bladder and vagina.
Acknowledgement
None
Conflict of Interest
There is no conflict of interest
Funding Sources
There is no funding source
References
- Arbyn M, Weiderpass E, Bruni L, et al. Estimates of incidence and mortality of cervical cancer in 2018: a worldwide analysis. Lancet Glob Health.; 8(2):e191-e203 (2018).
CrossRef - Matsuzaki S, Klar M, Mikami M, et al. Management of Stage IIB Cervical Cancer: an Overview of the Current Evidence. Curr Oncol Rep.; 22(3):28 (2020).
CrossRef - Shen J, Yin Q, Chen L, Zhang Z, Li Y. Co-delivery of paclitaxel and survivin shRNA by pluronic P85-PEI/TPGS complex nanoparticles to overcome drug resistance in lung cancer. Biomaterials.;33(33):8613-24 (2012).
CrossRef - Wang Y, Yu YH, Shen K, Xiao L, Luan F, Mi XJ, Zhang XM, Fu LH, Chen A, Huang X. Cervical cancer screening and analysis of potential risk factors in 43,567 women in Zhongshan, China. Asian Pac J Cancer Prev.;15(2):671-6 (2014).
CrossRef - Scatchard K, Forrest JL, Flubacher M, Cornes P, Williams C. Chemotherapy for metastatic and recurrent cervical cancer. Cochrane Database Syst Rev,; 10(10):CD006469 (2012).
CrossRef - Mori Y, Nishimura T, Kitano T, Yoshimura K, Matsumoto S, Kanai M, Hazama M, Ishiguro H, Nagayama S, Yanagihara K, Teramukai S, Chiba T, Sakai Y, Fukushima M. Oxaliplatin-Free Interval as a Risk Factor for Hypersensitivity Reaction among Colorectal Cancer Patients Treated with FOLFOX. Oncology.; 79, 136–143 (2010).
CrossRef - Candelaria M, Cetina L, Garcia-Arias A, et al. Radiation-sparing managements for cervical cancer: a developing countries perspective. World J Surg Oncol.; 4:77. Published 2006 Nov 13 (2006). doi:10.1186/1477-7819-4-77
CrossRef - Masood S. Neoadjuvant chemotherapy in breast cancers. Womens Health (Lond Engl).; 12, 480–491 (2016).
CrossRef - Angioli R, Plotti F, Montera R, Aloisi A, Luvero D, Capriglione S, Terranova C, De Cicco Nardone C, Muzii L, Benedetti-Panici P. Neoadjuvant chemotherapy plus radical surgery followed by chemotherapy in locally advanced cervical cancer. Gynecol Oncol.;127(2):290-6 (2012).
CrossRef - Hayes DF, Schott AF. Neoadjuvant Chemotherapy: What Are the Benefits for the Patient and for the Investigator? JNCI Monographs.; 36–39 (2015).
CrossRef - Bonomi P, Blessing JA, Stehman FB, Di Saia PJ, Walton L, Major FJ. Randomized trial of three cisplatin dose schedules in squamous-cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol.; 3(8):1079-85 (1985).
CrossRef - Hoffman BL, et al. William gynecology 3rd edition. New York: McGraw-Hill Education.; p657-678 (2015).
- Zhao H, He Y, Yang S, Zhao Q, Wu Y. Neoadjuvant chemotherapy with radical surgery vs radical surgery alone for cervical cancer: a systematic review and meta-analysis. Onco Targets Ther.;12:1881-1891 (2019).
CrossRef - Hornychová H, Melichar B, Tomšová M, Mergancová J, Urminská H, Ryška A. Tumour-Infiltrating Lymphocytes Predict Response to Neoadjuvant Chemotherapy in Patients with Breast Carcinoma. Cancer Investigation.; 26, 1024–1031 (2008).
CrossRef - Singh RB, Chander S, Mohanti BK, Pathy S, Kumar S, Bhatla N, Thulkar S, Vishnubhatla S, Kumar L. Neoadjuvant chemotherapy with weekly paclitaxel and carboplatin followed by chemoradiation in locally advanced cervical carcinoma: A pilot study. Gynecologic Oncology.; 129, 124–128 (2013).
CrossRef - Salihi R, Leunen K, Moerman P, Amant F, Neven P, Vergote I. Neoadjuvant Weekly Paclitaxel-Carboplatin Is Effective in Stage I–II Cervical Cancer. Int J Gynecol Cancer.; 27, 1256–1260 (2017).
CrossRef - Zusterzeel PLM, Aarts JWM, Pol FJM, Ottevanger PB, van Ham MAPC. Neoadjuvant Chemotherapy Followed by Vaginal Radical Trachelectomy as Fertility-Preserving Treatment for Patients with FIGO 2018 Stage 1B2 Cervical Cancer. Oncologist.;25(7):e1051-e1059 (2020). doi:10.1634/theoncologist.2020-0063
CrossRef - Alves RC, Fernandes RP, Eloy JO, Salgado HRN, Chorilli M. Characteristics, Properties and Analytical Methods of Paclitaxel: A Review. Crit Rev Anal Chem.; 48(2):110-118 (2018).
CrossRef - Stage TB, Bergmann TK, Kroetz DL. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin Pharmacokinet.; 57(1):7-19 (2018).
CrossRef - Weaver BA. How Taxol/paclitaxel kills cancer cells. Mol Biol Cell.; 25(18):2677-2681 (2014).
CrossRef - Zasadil LM, Andersen KA, Yeum D, Rocque GB, Wilke LG, Tevaarwerk AJ, Raines RT, Burkard ME, Weaver BA. Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci Transl Med.; 6(229):229ra43 (2014).
CrossRef - Gallego-Jara J, Lozano-Terol G, Sola-Martínez RA, Cánovas-Díaz M, de Diego Puente TA. Compressive Review about Taxol®: History and Future Challenges. Molecules.; 25, 5986 (2020).
CrossRef - Nguyen HD et al. Partial Surface Modification of Low Generation Polyamidoamine Dendrimers: Gaining Insight into their Potential for Improved Carboplatin Delivery. Biomolecules.; 214(9) (2019).
CrossRef - Bocci G, Di Paolo A, & Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis.; 16(3), 481–492.
CrossRef - LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. Platinum Coordination Complexes.; PMID: 31644095 (2012).
- Hall MD, Okabe M, Shen DW, Liang XJ, Gottesman MM. The role of cellular accumulation in determining sensitivity to platinum-based chemotherapy. Annu Rev Pharmacol Toxicol.; 48:495-535 (2008).
CrossRef - Sousa GF, Wlodarczyk SR, Monteiro G. Carboplatin: molecular mechanisms of action associated with chemoresistance. Brazilian Journal of Pharmaceutical Sciences.; 50(4), pp. 693-701 (2014).
CrossRef - Sun H, Xin J, Lu Z, Wang N, Liu N, Guo Q. Potential Molecular Mechanisms for Improved Prognosis and Outcome with Neoadjuvan Chemotherapy Prior to Laparoscopical Radical Hysterectomy for Patients with Cervical Cancer. Cell Physiol Biochem.; 32(5):1528–40 (2013).
CrossRef - Zhou J. et al. Young Cervical Cancer Patients May Be More Responsive than Older Patients to Neoadjuvan Chemotherapy Followed by Radical Surgery. PLoS ONE.; 11(2). (2016).
CrossRef - Department of Obstetrics dan Gynecology, Faculty of Medicine, Universitas Sebelas Maret, Wiraswesty I, Dr. Moewardi Hospital, Surakarta, Respati SH. The Effect of Neoadjuvant Chemotherapy on HIF-1α Expression in Cervical Uterine Cancer. INDONES J MED.; 3, 119–124 (2018).
CrossRef - Kartika, UK. Ekspresi Vascular Endothelial Growth Factor-C di Jaringan pada Pasien Karsinoma Serviks Sel Skuamosa yang Diberi Kemoterapi Neoadjuvan. Universitas Sebelas Maret.; p1-46 (2018).
- Pan D, Wei K, Ling Y, Su S, Zhu M, Chen G. The Prognostic Role of Ki-67/MIB-1 in Cervical Cancer: A Systematic Review with Meta-Analysis. Med Sci Monit Int Med J Exp Clin Res.; 21:882–9 (2015).
CrossRef - Kumari M, Ray L, Purohit M, Patnaik S, Pant A, Shukla Y, Kumar P, Gupta K. Curcumin loading potentiates the chemotherapeutic efficacy of selenium nanoparticles in HCT116 cells and Ehrlich’s ascites carcinoma bearing mice. Eur J Pharm Biopharm.; 117, 346–362 (2017).
CrossRef - Prueksaritanond N, Chaisarn P, Yanaranop M. The efficacy of neoadjuvan paclitaxel-carboplatin chemotherapy followed by radical hysterectomy compared to radical hysterectomy alone in bulky stage IB2-IIA cervical cancer. J Med Assoc Thail Chotmaihet Thangphaet.; 95 Suppl 3:S55-61 (2012).
- Rydzewska L, Tierney J, Vale CL, Symonds PR. Neoadjuvan chemotherapy plus surgery versus surgery for cervical cancer. Cochrane Database Syst Rev.; 12(12):CD007406 (2012).
CrossRef - Gupta S, Maheshwari A, Parab P, Mahantshetty U, Hawaldar R, Sastri Chopra S, et al. Neoadjuvan Chemotherapy Followed by Radical Surgery Versus Concomitant Chemotherapy and Radiotherapy in Patients With Stage IB2, IIA, or IIB Squamous Cervical Cancer: A Randomized Controlled Trial. J Clin Oncol Off J Am Soc Clin Oncol.; 36(16):1548–55 (2018).
CrossRef - Liu F, Jin T, Liu L, Xiang Z, Yan R, Yang H. The role of concurrent chemotherapy for stage II nasopharyngeal carcinoma in the intensity-modulated radiotherapy era: A systematic review and meta-analysis. PLoS ONE.; 13, e0194733 (2018).
CrossRef - Ye Q. et al. Neoadjuvan Chemotherapy Followed by Radical Surgery versus Radiotherapy (with or without Chemotherapy) in Patients with Stage IB2, IIA, or IIB Cervical Cancer: A Systematic Review and Meta-Analysis. Disease Markers.; pp. 1–7 (2020).
CrossRef - Khatimah GH, Muhammad S. Hubungan Tipe Histopatologi dengan Respon Kemoterapi Neoadjuvant pada Kanker Serviks Stadium IB2 dan IIA2. AOGJ.; 3, 63–81 (2019).
CrossRef - Sardi JE, Giaroli A, Sananes C, Ferreira M, Soderini A, Bermudez A, Snaidas L, Vighi S, Rueda NG, di Paola G. Long-Term Follow-up of the First Randomized Trial Using Neoadjuvant Chemotherapy in Stage Ib Squamous Carcinoma of the Cervix: The Final Results. Gynecologic Oncology.; 67, 61–69 (1997).
CrossRef - National Cancer Institute. Definition of differentiation. (2011); Available at: https://www.cancer.gov/publications/dictionaries/cancer terms/def/differentiation [Accessed: 27 January 2021]
- Matsuo K. et al. Association of tumour differentiation grade and survival of women with squamous cell carcinoma of the uterine cervix. Journal of Gynecologic Oncology.; 29(6) (2018).
CrossRef - World Health Organization. Cervical cancer treatment by FIGO stage [Internet]. Comprehensive Cervical Cancer Control: A Guide to Essential Practice. 2nd edition. World Health Organization; (2020) [cited 2021 Jan 11]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK269617/
- Vizcaino AP et al. International trends in the incidence of cervical cancer: I. Adenocarcinoma and adenosquamous cell carcinomas. Int J Cancer Res.; 75(4), pp. 536–545 (1998).
CrossRef - Vinh-Hung V, et al. Prognostic value of histopathology and trends in cervical cancer: a SEER population study. BMC Cancer.; 7, p. 164 (2013).
CrossRef








