Manuscript accepted on :08-06-2022
Published online on: 28-06-2022
Plagiarism Check: Yes
Reviewed by: Dr. Nataliya Kitsera
Second Review by: Dr. Nicolas Padilla
Final Approval by: Dr. H Fai Poon
Maysaa A. M. Alhar
Horticulture Department, Faculty of Agriculture, University of Kufa, Iraq.
Corresponding Author E-mail: maysaa.alhar@uokufa.edu.iq
DOI : https://dx.doi.org/10.13005/bpj/2447
Abstract
This study was conducted to create a new reagent from Anil-Azo compounds and study their biological impacts on two types of bacteria. For the first step, four reagents had been created by reaction of p-phenyl diamine in acidic form with 2-formyl-4-methyl phenol in neutral medium to create reagent {1}, which used to produce reagent {2} by reacting it with amino benzothiazole over four hours in the presence of glacial CH3COOH. The reagent {1} was also used to form reagent {3} by reacting it with amino imidazole over two hours. Finally, reagent {3} had been generated by reacting reagent {1} with naphthyl amine (0.2 mol) over four hours in the presence of glacial CH3COOH. The UV-visible spectrum was showed that a new ligand was created between 190-600 nm in reagent {2}, {3} and {4} while reagent {1} was appeared in 519-600 nm area. FTIR spectrum showed that many new coordinate bonds had been formed in different locations. Also, the chromatographic separation study showed that reagent {4} was separated faster than other reagents. Study of compounds stability showed that all reagents were stable in methanol, ethanol, DMSO and DMF. Study of chemical-physical peripteries showed that percentage of reagents’ yield ranged between 80-70%. The assessment of the formulated reagents against various kinds of bacteria was carried out using a medium (agar) via numerous processes. Microbial inhibition was tested at three concentrations: 30, 50 and 70 micrograms, with a blank solvent (DMSO), for bacteria Staphylococcus aureus, E. coli and Streptococcus pneumonia with an incubation period of 24 hours at 37℃. The results of biological impacts showed that reagent {2} showed more inhibition Staphylococcus aureus and Streptococcus pneumonia.
Keywords
Analytical; Antimicrobial; Bacteria; Reagent
Download this article as:| Copy the following to cite this article: Alhar M. A. M. A Study of the Spectral, Chromatographic and Solubility Characteristics of New Analytical Reagents from Anil-Azo and Their Biological Effects on Bacteria. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Alhar M. A. M. A Study of the Spectral, Chromatographic and Solubility Characteristics of New Analytical Reagents from Anil-Azo and Their Biological Effects on Bacteria. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/39TEIlP |
Introduction
Analytical reagents are used in different methods of chemical separation because these techniques make use of the polar groups in the prepared reagents. For example, chromatography(1) depends mainly on the nature of the active and polarised group included in each prepared reagent. This directly affects the process of chromatographic separation of reagents like Anil-Azo compounds, as these reagents involve functional groups.(2) Anil-Azo compounds are coloured and active reagents. Chromatographic separation is called colour separation, and it is a method used to separate substances and chemical elements mixed with each other.(3). It depends on the idea that chemical components of differing proportions can be easily separated, as long as the required means are available. Examples of substances(4) that can be separated include solids from liquids and solids from gases.(5) The first studies related to this method of separating chemicals dates to 1901 AD, when the chemist Tsweet attempted to separate the pigments of plants. He named them chromatographs in relation(6) to the word ‘chroma’, which means colour,(8) and ‘graph’, which means writing. Following the emergence of many chemical developments(9) in the field of methods of separating chemical elements,(22-25) the chromatographic method(15) has become one of the most successful.(17) Gas chromatography is used in airports to detect bombs and is also used in forensic medicine in many different ways. It is used to analyse fibres on a person’s body as well as to analyse blood found at a crime scene.(18) In gas chromatography, helium is used to move a gaseous mixture through a column of sorbent materials.(19) Therefore, chromatography is an important method for separation and analysis.
Materials and Methods
Numerous investigations other than measurements have been attempted using several techniques carried out at the University of Tehran (Iran), in particular, the separation technique using chromatography instrumentation (modern method), and other analytical studies such as solubility, physical properties, infrared measurement and UV-visibility (UV-Vis), carried out in Iraq.
Formation of Analytical Reagents:
Formation of Reagent {1}
To form reagent {1}, p-phenyl diamine (0.1 mol) was dissolved in 2 ml of HCl. A solution of sodium nitrite in ace path was then added. This was added to a basic solution of 0.02 mol of (2-formyl-4-methyl phenol) in natural medium. After that, it was filtrated, washed and dried to formulate new reagent {1} for use in this experiment. 10-11
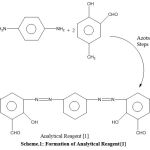 |
Scheme 1: Formation of Analytical Reagent{1}. |
Formation of Reagent {2}
To form reagent {2}, 0.1 mol of reagent {1} was reacted with amino benzothiazole (0.2 mol) over four hours in the presence of glacial CH3COOH. The next steps were filtration, aeration and finally recrystallisation to form new reagent {2} for use in this experiment. 10, 11
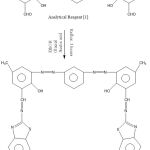 |
Scheme 2: Formation of Analytical Reagent {2}. |
Formation of Reagent {3}
To form reagent {3}, 0.1 mol of reagent {1} was reacted with amino imidazole (0.2 mol) over two hours in the presence of glacial CH3COOH. The next steps were filtration, aeration and finally recrystallisation to form new reagent {3} for use in this experiment.10-11
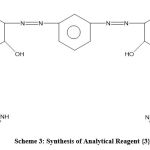 |
Scheme 3: Synthesis of Analytical Reagent {3}. |
Formation of Reagent {4}
To form reagent {4}, 0.1 mol of reagent {1} was reacted with naphthyl amine (0.2 mol) over four hours in the presence of glacial CH3COOH. The next steps were filtration, aeration and finally recrystallisation to form new reagent {3} for use in this experiment.10-11
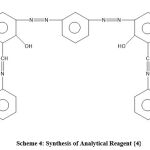 |
Scheme 4: Synthesis of Analytical Reagent {4}. |
Results and Discussion
Scientific progress is limitless and constantly progressing. One of the most important methods of separation in analytical chemistry is currently the chromatographic method, in which colours and chemicals are separated from each other.
Spectral Recognition of Chemical Reagents
UV-Visible Recognition
The structures of the new reagents were demonstrated with UV-Vis spectrophotometry with ideal concentration. All spectral information is presented in figures 1-4:
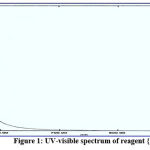 |
Figure 1: UV-visible spectrum of reagent {1}. |
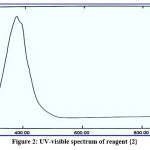 |
Figure 2: UV-visible spectrum of reagent {2}. |
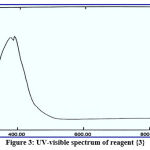 |
Figure 3: UV-visible spectrum of reagent {3}. |
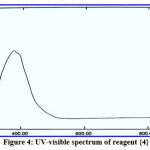 |
Figure 4: UV-visible spectrum of reagent {4}. |
FTIR Analysis
This chemical analysis was performed in the range 400–4000 cm-1 to improve the analytical reagents. Through this type of measurement, many bands appeared, such as bands at (1487, 1510), (1484, 1517), (1472, 1500) and (1442, 1518) cm-1 for azo groups (-N=N-) in reagents {1}, {2}, {3} and {4} respectively, bands at (3417, 3424, 3404 and 3415) cm-1 for hydroxyl group of phenol (-OH) in reagents {1}, {2}, {3} and {4} respectively, a band at (1710) cm-1 for carbonyl group of aldehyde (-CO-H) in reagent {1}, which disappeared, and further bands appeared at (1610, 1622, 1608) cm-1 for imine group of schiff base (-CH=N-) in reagents{2}, {3} and {4}. This provides a complete explanation of spectra, as supported by the literature,17 and shown in figures (5-8).
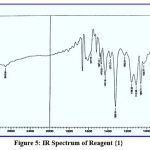 |
Figure 5: IR Spectrum of Reagent {1}. |
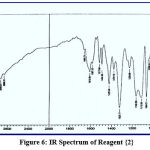 |
Figure 6: IR Spectrum of Reagent {2}. |
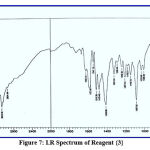 |
Figure 7: I.R Spectrum of Reagent {3}. |
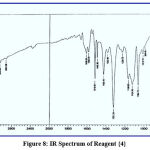 |
Figure 8: IR Spectrum of Reagent {4}. |
Chromatographic Separation for Analytical Reagents
Chromatography is a commonly used analytical technique to separate a chemical mixture into its individual components, so that these components can be accurately analysed. There are many types of chromatography, e.g. liquid chromatography, gas chromatography, ion exchange chromatography and affinity chromatography, but all of these use the same basic principles. Chromatography is a separation technique with which every organic chemist and biochemist is familiar. In agreement with other studies,7 using diluted concentrations of reagents {1}, {2}, {3} and {4} and an injection of nitrogen gas at a flow speed of 25 ml/min, the injection temperature was 25℃ higher than the temperature of the separation column. Using a flame ionisation detector, the temperature was 50℃ higher than that of the column. The column temperature gradually increased from 90℃ to 160℃. The data in figures 9, 10, 11 and 12 show that reagent {4} separated faster than the other compounds due to its structure being less polarised and therefore having less interaction with the separation column. This was followed by reagents {2}, {3} and lastly {1}, which has the most polarised structure.
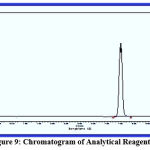 |
Figure 9: Chromatogram of Analytical Reagent {1}. |
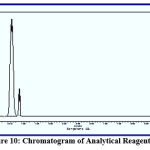 |
Figure 10: Chromatogram of Analytical Reagent {2}. |
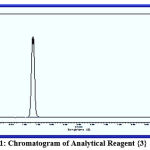 |
Figure 11: Chromatogram of Analytical Reagent {3}. |
 |
Figure 12: Chromatogram of Analytical Reagent {4}. |
Study of Solubility in Various Solvents
The formulated reagents were noted to have good solubility in some solvents according to their polarity. This is due to the functional groups in the reagents and the structure of the active groups in each compound. The results are presented in Table 1:
Table 1: Solubility of Reagents in Various Solvents.
| Reagents | Methanol | Ethanol | DMSO | DMF | H2O | Acetone |
| Reagent {1} | + | + | + | + | – | – |
| Reagent {2} | + | + | + | + | – | – |
| Reagent {3} | + | + | + | + | – | – |
| Reagent {4} | + | + | + | + | – | – |
Physical-Chemical Analyses
Physical-chemical properties were analysed. Data regarding colour and percentage yield are presented in Table 2:
Table 2: Other Chemical-Physical Properties.
| Reagents | Yield % |
Colour |
| Reagent {1} | 80 | Orange |
| Reagent {2} | 76 | Yellowish Red |
| Reagent {3} | 70 | Yellowish Orange |
| Reagent {4} | 76 | Yellow |
Biological Study Against Bacteria
The study of microbiology has revealed bacteria to be the cause of most diseases that affect humans and animals, and the discovery of therapeutic agents has an important effect in their control and prevention. These chemical agents are prepared by chemists, such as the world-famous Ehrlich Paul who discovered (1915) many useful drugs that added a new branch to medicine called ‘chemotherapy’, which includes treating bacterial diseases by means of chemical agents that destroy microorganisms without affecting the receptor tissue. Among these treatments are Anil-Azo reagents,7, 16 which have a high efficacy in inhibiting many different types of pathogenic bacteria. The reason for this is their ability to dissolve the outer cell wall, which leads to the exudation of cell fluids. The biologically reactive agent synthesises monovalent potassium ions needed by the bacterial cell and thus leads to cell death due to its loss of these elements. One of the most important characteristics that any biological or chemical treatment must have been selective toxicity, which means that the drug is harmful to pathogens and harmless to the host, and selectivity that is relative and not absolute, which requires that the drug be in a specific state in order to eliminate the bacteria that cause inflammation in a way that is effective. As a result of the increase in bacterial resistance to antibiotics and the development19 of bacteria of increased virulence, new chemical compounds were tested for their effectiveness in inhibiting bacterial growth of Gram-positive bacteria and Gram-negative bacteria (such as Escherichia coli) that cause many common diseases affecting humans, animals and plants.
 |
Figure 13: Staphylococcus aureus. |
 |
Figure 14: Streptococcus pneumonia. |
 |
Figure 15: Escherichia coli. |
Antibacterial Assessment
The assessment of the formulated reagents against various kinds of bacteria was carried out using a medium (agar) via numerous processes. 7, 16 Microbial inhibition was tested at three concentrations: 30, 50 and 70 micrograms, with a blank solvent (DMSO), for bacteria Staphylococcus aureus, E. coli and Streptococcus pneumonia with an incubation period of 24 hrs at 37℃. The assessment of the reagents on the bacteria showed more significant data with reagents {2} and {3} than with reagents {1} and {4}, due to the imidazole ring and the benzothizole ring in those reagents, which increase the inhibition of bacteria. The results are shown in Table 3:
Table 3: Inhibition test of reagents at a concentration of 50 micrograms.
| Reagents | Staphylococcus aureus | Streptococcus pneumonia | Escherichia coli |
| Reagent {1} | + | + | + |
| Reagent {2} | +++ | +++ | ++ |
| Reagent {3} | ++ | ++ | ++ |
| Reagent {4} | ++ | ++ | + |
(+): inhibition (2-6) mm (++): inhibition (8-12) mm (+++): inhibition (13-16) mm
Conclusion
This research has produced significant data on the chemical performance of the formulated new Anil-Azo reagents. In addition, methods were used to estimate the stability of the reagents and the influence of fluctuating temperatures. All reagents showed good chromatogram separation data, according to the polarity of groups in the reagents, in addition to good results as inhibitors of bacteria.
Acknowledgment
we would like to express our heartfelt thanks to the Chemical Research Lab at Tehran University for providing measurements.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Source
There is no funding Source.
References
- Pradhan,; Xu, L.; Chen, S. Janus nanoparticles by interfacial engineering. Adv. Funct. Mater., 2007; 17 (14), pp. 2385-2392.
CrossRef - Bravo-Díaz, C.D. “Diazohydroxides, Diazoethers and Related Species”, in Rappoport, Zvi (ed.), PATai’s Chemistry of Functional Groups, John Wiley & Sons, Ltd, 2010; Doi:1002/9780470682531.pat0511, ISBN 9780470682531
CrossRef - Carey, F.A. Advanced organic chemistry. Sundberg, Richard J. (5th ed.). New York: 2007; ISBN 9780387448978. OCLC 154040953.
- Badawy, S.S.; Issa, Y.M.; Abdelfattah, H.M. Thermogravimetric studies on lanthanide complexes of new derivatives of 1,5-di aryl-3-acetylformazan. Thrmo Acta, 1999; 144, 249-255.
CrossRef - N.M. Inventing of Macrocyclic Formazan Compounds and Studying Them Against Breast Cancer for the first Time Globally, Annals of pharma research 2021; 9 ,7, Pp 525- 533., Available at:https://www.annalsofpharmaresearch.com/index.php?journal=apr&page=article&op=view&path% 5B%5D=38
- Berridge, M.V., Herst, P.M., and Tan, A.S. Tetrazolium dyes as tools in cell biology: new insights into their cellular Biotechnology Annual Review, 2015; 11: 127-152.
CrossRef - , N.M. 2015. Synthesis and Chemical Identification of Macro Compounds of (Thiazol and Imidazol)”., Research J. Pharm. and Tech, 2015; 8,1, 78-84., DOI: 10.5958/0974- 360X.2015.00016.5.
CrossRef - Stockert, J.C., Blázquez-Castro, A., Cañete, M., Horobin, R.W., and Villanueva, A. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochemica, 2012; 114: 785-796. Doi:10.1016/j.acthis.2012.01.006
CrossRef - Mosmann, T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity Journal of Immunological Methods. 1993; 65 (1–2): 55–63. Doi:10.1016/0022-1759(83)90303-4. ISSN 0022-1759. PMID 6606682
CrossRef - Aljamali, N.M. “Effect of Conditions and Catalysis on Products”.,1st Edition, Eliva Press , 2021; ISBN: 9781636482286
- Bernas, T. and Dobrucki, J. Reduction of a tetrazolium salt, CTC, by intact HepG2 human hepatoma cells: subcellular localisation of reducing Biochim Biophys Acta, 1999; 1451(1): p. 73-81.
CrossRef - Fadel, O., Gomes Rodrigues D., Girard L., Bauduin P., Rossignol-Castera A., L’Hermitte A., Gaillard J.C., Diat O. Separation and identification of polar polyphenols in oily formulation using high- performance thin-layer chromatography and mass spectroscopy techniques. OCL, 2018; https://doi.org/10.1051/ocl/2018039.
CrossRef - Sun S.Y., Jiang W.G., Zhao Y.P. Comparison of aromatic and phenolic compounds in cherry wines with different cherry cultivars by HS-SPME-GC-MS and HPLC. Int. Food Sci. Technol. 2012; 47:100–106. doi: 10.1111/j.1365-2621.2011.02812.x.
CrossRef - Karm, I.; Abdullabass, H.K.; Aljamali, N.M. Invention of (Gluta.Sulfazane-Cefixime) Compounds as Inhibitors of Cancerous Tumors., Journal of Cardiovascular Disease Research, 2020; 11, , 44-55 ., DOI: 10.31838/jcdr.2020.11.02.09 .
- Jawad, A.M.; Aljamali, N.M.; Jwad, S.M. Development and Preparation of ciprofloxacin Drug Derivatives for Treatment of Microbial Contamination in Hospitals and Environment, Indian Journal of Forensic Medicine & Toxicology, 2020;14, 2, p:1115-
- Abebe, A., Sendek, A., Ayalew, S., and Kibret, M. Copper (II) mixed-ligand complexes containing 1, 10-phenanthroline, adenine and thymine: Synthesis, characterization, and antibacterial Chemistry International, 2017; 3(3), 230-239
- Ren, J.; Yao, P.; Chen, J.; Jia, L. Salt-independent hydrophobic displacement chromatography for antibody purification using cyclodextrin as supermolecular displacer. J Chromatogr A. 2014; 1369:98-104. doi: 10.1016/j.chroma.2014.10.009. Epub 2014 Oct 12. PMID: 25441076.
CrossRef - Manish, T. “How does column chromatography work?”. Bright Mags. Archived from the original on 21 April Retrieved 7 April 2017.
- Milocco, F.; de Vries, F.; Siebe, H.S. Engbers, S.; Demeshko, S.; Meyer, F.; Otten. Widening the Window of Spin-Crossover Temperatures in Bis (formazanate) iron (II) Complexes via Steric and Noncovalent Interactions. Inorganic Chemistry 2021; 60 (30), 2045-2055. https://doi.org/10.1021/acs.inorgchem.0c03593.
CrossRef







