Janti Qar1* , Bahaa Al-Trad1, Alaa khmaiseh1, Riyadh Muhaidat1
, Bahaa Al-Trad1, Alaa khmaiseh1, Riyadh Muhaidat1 , Sahar Omari1
, Sahar Omari1 , Ghada Al-Omari1 and Mazhar Al Zoubi2
, Ghada Al-Omari1 and Mazhar Al Zoubi2
1Department of Biological Sciences, Faculty of Science, Yarmouk University, Irbid 211-63, Jordan.
2Department of Basic Medical Sciences, Faculty of Medicine, Yarmouk University, Irbid 211-63, Jordan.
Corresponding Author E-mail: jqar@yu.edu.jo
DOI : https://dx.doi.org/10.13005/bpj/2401
Abstract
Cardiovascular diseases account for most of the morbidity and mortality associated with diabetes. Diabetic cardiomyopathy (DCM) is associated with heart failure in diabetic patients without relation to other cardiovascular diseases such as hypertension or coronary artery disorders. Eugenol is a phenolic compound extracted from the clove tree and exhibits effective mitigation of hyperglycemic conditions in diabetic animals. Thus, in the current study, we aimed to explore the effect of eugenol treatment on rats with DCM. The experimental animals included 30 Sprague Dawley male rats which are divided into three experimental groups (10 rats each) as the following: the non-diabetic control group (ND), diabetic group (D), and a treated-diabetic group (20mg/kg/day of eugenol) (D+E). Diabetes was induced by streptozotocin (STZ) injection (60 mg/ kg). After 6 weeks, blood samples and left ventricles were collected for analysis. Serum glucose levels, heart weight/body weight ratio, and the myocardial mRNA expression of transforming growth factor β1 (TGF-β1), tumor necrosis factor-α (TNF-α), caspase 3 (casp3), vascular endothelial growth factor-A (VEGF-A), and collagen IV were evaluated. Furthermore, the myocardial superoxide dismutase (SOD) activity was measured. Diabetic rats showed a significant appearance of hyperglycemia and increased expression of myocardial TNF-α, TGF-β1, caspase 3, and VEGF-A compared to the control group (P < 0.05), and a tendency to increase collagen IV (P < 0.1). On the other hand, the eugenol treatment mitigates diabetic-associated hyperglycemia and the increased mRNA expression levels of myocardial TGF-β1, VEGF-A, caspase 3, and TNF-α (P < 0.05). In addition, the overexpression of collagen IV was inhibited, and the myocardial SOD activity was improved in the diabetic rats treated with eugenol. The study provided evidence that eugenol may have a potential therapeutic effect in the experimental models of DCM by reducing the expression of pro-inflammatory, pro-fibrotic, angiogenic, and pro-apoptotic factors (TNF-α, TGF-β, collagen IV, VEGF-A, and caspase 3 respectively). It is recommended for further studies investigate the exact molecular processes by which eugenol may ameliorate the DCM phenotype.
Keywords
Diabetes Mellitus; Diabetic Cardiomyopathy; eugenol; Rats
Download this article as:| Copy the following to cite this article: Qar J, Al-Trad B, khmaiseh A, Muhaidat R, Omari S, Al-Omari G, Al-Zoubi M. The Effect of Eugenol Treatment on Diabetic Cardiomyopathy in Streptozotocin-Induced Diabetic Rats. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Qar J, Al-Trad B, khmaiseh A, Muhaidat R, Omari S, Al-Omari G, Al-Zoubi M. The Effect of Eugenol Treatment on Diabetic Cardiomyopathy in Streptozotocin-Induced Diabetic Rats. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3ne6L2h |
Introduction
Despite the development of preventive measures, diagnostic procedures, and treatment options, the diabetic disease is still one of the commonest metabolic disorders among adults. Unfortunately, by 2030, the prevalence of diabetes among individuals aged between 20 to 79 years will reach 7.7% worldwide which will affect more than 400 million adults 1,2. Type 1 Diabetes Mellitus (DM-1) is characterized by its association with the chronic autoimmune features related to the distinctive destruction of insulin-producing pancreatic β-cells. On the other hand, type 2 Diabetes Mellitus (DM-2) is a heterogeneous disease related to the complicated interaction between genetic and environmental factors leading to diminished insulin function 3-5. Besides many other conditions, diabetes mellitus is associated with cardiovascular conditions. For instance, cardiovascular complications affect more than 30 % of DM-2 patients which makes heart failure conditions the major cause of high morbidity and mortality 6-9. Diabetic cardiomyopathy (DCM) is a cardiac disorder that is related to DM disease and distinguished by abnormal structural and function features of the myocardium 10,11. The early stage of DCM is clinically asymptomatic and characterized by diastolic dysfunction and increased fibrosis and stiffness 8,12. As the DCM proceeds, diastolic and systolic dysfunction may coexist, leading to a reduced ejection fraction and heart failure 8,12-14.
Chronic hyperglycemia increases advanced glycation end-products (AGEs), contributing therefore to the development of diastolic dysfunction via activation of AGE receptors (RAGE) 13,15,16. Activation of RAGE consequently activates the transforming growth factor β (TGF-β) pathway, causing an increased inter-myofibril and perivascular collagen deposition which leads to fibrosis 15,17,18. Activation of inflammatory cytokines, such as interleukin (IL) IL1β, IL6, nuclear factor-κB (NF-ΚB), and tumor necrosis factor-α (TNFα), also has been related to the pathophysiology of DCM 13,19,20. The increased fibrosis and inflammation, along with hyperglycemia lead to the triggering of the cytochrome c–activated caspase-3 apoptotic route, suggesting induction of death of the myocardial cells and decreasing the performance of the myocardium 17,21. Furthermore, an abnormal angiogenic process has been described in the pathogenesis of DCM which has been shown through the impaired expression of vascular endothelial growth factor (VEGF) 22-24.
Eugenol is a clove tree extract characterized by its phenolic property. Previously, eugenol possesses potent anti-hyperglycemic, anti-inflammatory, and anti-oxidative effects in diabetic animals 25-27. In addition, the cardioprotective effect of eugenol was observed in doxorubicin and arsenic-induced cardiotoxicity 28,29, and isoproterenol-induced myocardial infarction 30. Moreover, a study showed the protective effect of eugenol against ischemia/reperfusion injury in the transplanted heart in rats. The potential protective effect of eugenol in the heart transplantation model has been attributed to the down-regulation of inflammatory and apoptotic markers 31. Therefore, in the current study, we aimed to investigate the potential cardio-protective effect of eugenol on the development of DCM in diabetic rats.
Material and methods
Before the performance of animal experimental procedures, ethical approval was gained by the Institutional Animal Care and Use Committee at Yarmouk University (IACUC/2021/3). The experimental animals included thirty male rats (Sprague-Dawley) which were supplied by the local institutional animal house. Normalization conditions were achieved by maintaining animals on a 12:12 h light-dark cycle at 24°C and standard rat chow-fed status. The experimental animals included thirty rats that weighed 200±50g which then were randomly assigned into three equal groups. The first group is assigned as a non-diabetic control (ND), the second group is assigned as a diabetic induced rat (D), while the third group is representing the diabetic rats receiving eugenol treatment by intraperitoneal injection at 20mg/kg/day dose (D+E) for 6 weeks. Diabetes induction was performed by a single intraperitoneal STZ injection (Sigma-Aldrich, USA) (60 mg/kg prepared in 0.9 % normal saline) after an overnight fast. Rats were provided for 24 h with 10% sucrose ad libitum water to avoid hypoglycemia. After that, blood glucose was measured from the tip of the tail 48 h post the STZ treatment to select the diabetic group that showed glucose levels of more than 200 mg/dl.
Tissue and blood samples collection
For tissue and blood sample collection, all rats were decapitated after six weeks where the hearts were quickly removed, rinsed with phosphate-buffered saline (PBS), weighed, and dissected. Serum was isolated from blood samples after centrifugation at 4,500 rpm/5 min and stored at -80°C for further analysis. Dissected left ventricles were immediately frozen in liquid nitrogen and stored at -80°C.
Total mRNA Isolation and Quantification
Total mRNA was isolated from left ventricle tissues using a commercially available kit (TRI Reagent from Zymo, USA) following the manufacturer’s instructions. RNA quantification was performed using QuantiFluor-RNA based on Quantus Fluorometer System provided by Promega (Madison, USA). cDNA synthesis was performed using Reverted First Strand cDNA Synthesis Kit provided by (Molecular, Lithuania (EU)) which is based on oligo-(DT) 15 primers and follows the manufacturer’s instructions. The yielded cDNA was stored at −20 ° C until the next use.
Quantitative real-time PCR for TGF-β1, caspase3, VEGF-A, TNF- α, and collagen IV expressions, was determined using Line-Gen 9600 Thermal cycler (Bioer Technology, Bingjiang, China). The amplification conditions were performed as the following: activation at 95 ° C for 3 min followed by 40 cycles of 95 ° C for 5 sec and extension and quenching at 60 ° C for 30 sec. As a housekeeping gene, Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was amplified in triplicate for each target gene. For all target and reference genes, the specific primers were designed using the Primer3 software. The stock primers at a concentration of 100mmol were provided by IDT (Integrated DNA Technologies, INC., IA, USA) as shown in Table 1. The specificity of the target sequences agarose gel electrophoresis was performed. The quantitative RT-PCR reactions were performed in triplicates in 20 ul total volume using Premix Ex TaqII PCR master mixture (Takara, USA) where 10 μl of SYBR green master mix was mixed with 1 μl of each primer set, 6 μl nuclease-free water, and 2 μl cDNA). The expression level of each gene was calculated based on the 2−ΔΔCT value followed by normalized relative to the housekeeping gene (GAPDH) level.
Table 1: The sequence of primers used for qRT-PCR.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
| GAPDH
TGF-β1 TNF- α VEGF- A Caspase-3 Collagen IV |
ATGGTGAAGGTCGGT
GTGGAGCAACACGTAGAAC TTCGGAACTCACTGGATCCC CGAACAGAGAGAGGGACAGG GTGGAACTGACGATGATATGGC TTGGCTTTCCTGGTAGTCGT |
GAACTTGCCGTGGGTAGA
TTGGTTCAGCCACT GGAACAGTCTGGGAAGCTCT GTCTGTCTGTCTGTCCGTCA CGCAAAGTGACTGGATGAACC CAACCTTTCCTGCTTGACCC |
GAPDH: Glyceraldehyde 3-phosphate dehydrogenase; TGF-β1: Transforming growth factor-β1; TNF- α: Tumor necrosis factor α; VEGF-A: Vascular Endothelial Growth Factor-A
Serum Glucose Measurement
Glucose levels from the serum samples were determined by the glucose oxidase method using the GOD-PAP colorimetric method, according to the manufacturer’s protocol (Fortress diagnostics, UK).
Superoxide dismutase (SOD) activity
Tissue homogenates were prepared by homogenizing the left ventricular tissue samples in the ice-cold homogenizing buffer and centrifuged at 8000g/10min/4oC. Enzymatic activity of the superoxide dismutase (SOD) was determined in the homogenate utilizing a commercially available kit (Sigma-Aldrich, USA). The assay procedure of the enzyme activity was performed following the manufacturer’s instructions. For protein quantification, the Bicinchoninic Acid (SMART-BCA) protein assay kit was used (Intron Biotechnology, Korea).
Statistical Analysis
For all statistical tests, SPSS V23 software was used (SPSS Inc., Chicago, IL). Specifically, our data were analyzed using the One-way analysis of variance (ANOVA) test. When the P value is less than 0.05 the association was considered significant.
Results
Effect of eugenol on glucose level and heart weight
As shown in Table 2, the results did not show any significant difference in the cardiac weight/body weight ratio between the non-diabetic, diabetic, and diabetic treated groups. On the other hand, post six weeks of treatment, the results showed a significant change in the glucose level between the experimental groups (P < 0.05). Particularly, there was a significant increase in the glucose level in diabetic rats compared to the normal control. However, eugenol treatment showed a significant reduction in blood glucose levels compared to the diabetic rats (P < 0.05).
Table 2: Effect of eugenol treatment on the heart weight/body weight ratio and blood glucose level.
| Parameter | ND | D | D+E |
| Heart weight/body weight ratio | 0.32 ± 0.015 | 0.33 ± 0.017 | 0.34 ± 0.015 |
| Blood glucose (mg\dl) | 117.51 ± 4.75 | 361.84 ± 16.50* | 193.02 ±15*# |
| *P < 0.05 compared to the ND group. #P < 0.05 compared to D group. Data represent the mean ± SEM. Abbreviations: ND: non-diabetic; D: Diabetic; D+E: Diabetic rats treated with 20mg/kg eugenol. | |||
Effect of eugenol treatment on cardiac mRNA gene expressions of TGF-β1, VEGF-A, TNF- α, caspase 3, collagen IV, and the myocardial SOD activity.
Diabetic rats showed increased myocardial mRNA levels of TGF-β1, VEGF-A, TNF-α, and caspase-3 compared to the ND group (P < 0.05). A tendency to increase the myocardial collagen IV expression levels was observed in the diabetic rats (P < 0.1). On the other hand, eugenol treatment showed a significant attenuation in diabetic-associated blood glycemic levels and myocardial mRNA levels of TGF-β1, VEGF-A, TNF-α, and caspase 3 (Fig 1-4, P < 0.05). Furthermore, the overexpression of collagen IV was inhibited, and the myocardial SOD enzymatic activity showed an improvement in the eugenol-treated rats (Fig 5 and 6).
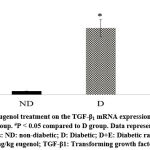 |
Figure 1: Effect of eugenol treatment on the TGF-β1 mRNA expression. *P < 0.05 compared to the ND group. #P < 0.05 compared to D group. Data represent the mean ± SEM. |
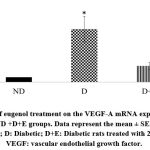 |
Figure 2: Effect of eugenol treatment on the VEGF-A mRNA expression. *P < 0.05 compared to the ND +D+E groups. Data represent the mean ± SEM. |
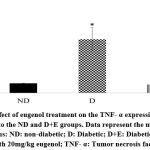 |
Figure 3: Effect of eugenol treatment on the TNF- α expression. *P < 0.05 compared to the ND and D+E groups. Data represent the mean ± SEM. |
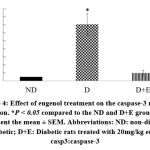 |
Figure 4: Effect of eugenol treatment on the caspase-3 mRNA expression. *P < 0.05 compared to the ND and D+E groups. Data represent the mean ± SEM. |
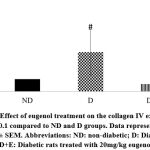 |
Figure 5: Effect of eugenol treatment on the collagen IV expression. #P < 0.1 compared to ND and D groups. Data represent the mean ± SEM. |
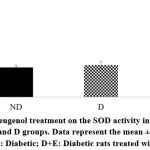 |
Figure 6: Effect of eugenol treatment on the SOD activity in the heart. *P < 0.05 compared to the ND and D groups. Data represent the mean ± SEM. |
Discussion
Diabetes is a highly prevalent metabolic disorder worldwide, and the prevalence of DCM is increasing in parallel with the increase in diabetes mellitus 8. DCM which affects approximately 12% of diabetic patients is a cardiac dysfunction in the lack of coronary artery disease, valvular disease, or other cardiac diseases including hypertension 24,32,33. Eugenol is the main active component of essential oil isolated from Syzygium aromaticum, commonly known as clove 34,35. Recent studies reported that eugenol has the potential for an anti-diabetic effect in vivo 36,37. Consequently, we designed the current study to investigate the potential impact of eugenol treatment in the mitigation of the development of DCM in STZ-induced diabetes mellitus in rats. We demonstrate an effective impact of eugenol on different molecular markers in STZ-induced diabetic rats. In particular, treatment with 20mg/kg/day of eugenol showed a cardio-protective effect as evident from the reduction in the mRNA expression of inflammatory and profibrotic factors (collagen-IV, TNF-α TGF-β), apoptosis, (caspase3), and the angiogenic factor (VEGF-A) in the treated diabetic rats.
Cardiac fibrosis, the hallmark feature in the pathology of DCM, led to distinctive pathophysiological features involving left ventricular hypertrophy, perivascular fibrosis, increased thickness of the capillary basement membrane, and diastolic/systolic dysfunction 13,38,39. Extracellular matrix (ECM) remodeling due to the discrepancy between ECM creation and deterioration is crucial for the progression of cardiac fibrosis. TGF–β pathway is one of the most-studied mediators that are related to alteration of the ECM 40-43. Of the three isoforms of the TGF-β superfamily of cytokines, fibrosis is mediated primarily by the TGF-β1 isoform. During diabetes disease, hyperglycemia showed a significant enhancement of TGF-β expression, as well as TGF-β receptors as a nuclear for the cardiac fibrosis, ending up with cardiac hypertrophy 44,45. TGF-β1 induced ECM remodeling and fibrosis in diabetes have been associated with the promotion of collagen expression combined with metalloproteinase inhibition 46-49. Our study showed that the myocardial TGF-β1 expression levels were higher in STZ-diabetic rats relative to the normal rats and the eugenol treatment attenuated this increase. Hence, our data suggest that eugenol could alleviate the development of DCM by preventing the overexpression of TGF-β1 in experimental models of DCM.
Cardiac inflammation is an initial response to diabetes and is implicated in the pathogenesis and development of cardiac hypertrophy, fibrosis, and DCM 50,51. Hyperglycemia and dyslipidemia directly induce the up-regulation and secretion of several inflammatory markers including TNF-α, IL-1 and L-6 contribute to cardiac inflammation and can directly induce cardiomyocyte hypertrophy. Besides cardiac inflammation induction, TNF-α is associated with the development of hypertrophy, and apoptosis 50,52. Previous work showed that anti-TNF-α monoclonal antibody attenuates the development of experimental DCM which was attributed to the lessening in intra-myocardial inflammation and cardiac fibrosis 19,51. In this study, TNF-α mRNA showed significant overexpression in the STZ-induced diabetic rats which was mitigated by eugenol treatment. The current findings highlight the molecular mechanism by which eugenol might prevent the development of DCM in diabetic rats by inhibiting the myocardial inflammatory process by targeting TNF-α. The current findings are supporting other studies that demonstrated a cardioprotective and anti-inflammatory effect of eugenol as a mediator in different animals disease models such as ischemia/reperfusion injury in the transplanted heart 31 and isoproterenol-induced myocardial infarction 30.
Increased reactive oxygen species (ROS) production in diabetic myocardium by hyperglycemia and/or hyperlipidemia is supposed to be an initial step in the development of DCM 53,54. ROS accumulation stimulates cellular lipids, proteins, or DNA damage and modulates diverse intracellular signaling pathways that, in turn, contribute to the DCM development and progression 54,55. Myocardial cell death is predominantly driven by hyperglycemia-induced oxidative stress which is partly driven through TNF-α 11,56. Therefore, enhancing cardiac antioxidants might help prevent DCM. Innate cellular mechanisms have been developed to compensate for the consequences of ROS induction which include enzymatic and no-enzymatic reactions. For instance, SOD is a crucial antioxidant enzyme that converts superoxide anion to oxygen and hydrogen peroxide later, catalase and glutathione peroxidase enzymes hydrogen peroxide is converted to oxygen and water 57-59. In transgenic animal models for type 1 diabetes, Mn-SOD overexpression was able to provide overall protection to the diabetic heart as shown by repealing cardiac tissue structure and improving the cardiac contraction function 60. In different in vitro and in vivo studies, the researchers showed the ability of eugenol to display antioxidative activity 61-63. Increased myocardial SOD activity in the eugenol treatment in our study suggests that eugenol protects the diabetic heart via, at least in part, enhancing mitochondrial antioxidant defense systems.
The incidence of myocardial apoptotic cell death is increased in diabetic patients 34,64, as well as in diabetic animal models generated by STZ-induction 32. The diabetic myocardial apoptotic process is associated with impaired contractile activity, cardiac hypertrophy, and eventual fibrosis development 21,60,65. These cardiac deterioration processes are attributed to the generation of ROS which is induced by hyperglycemia and leads to cardiac cell apoptosis likely through the activation of the caspase-3 mechanism 21,64. Eugenol treatment significantly reduced myocardial cell apoptosis in a transplanted heart 31. In isoproterenol-induced apoptosis in neonatal cardiomyocytes 66. Also, prevented the doxorubicin-induced activation of cardiac caspase-3 in acute doxorubicin cardiotoxicity 29. In the current study, eugenol treatment significantly downregulated caspase3 expression at the mRNA level in the diabetic heart, therefore, we suggested a potential cardio-protective effect of eugenol by decreasing in myocardial apoptosis.
The angiogenesis process relies on many factors including a major regulator vascular endothelial growth factor (VEGF). Mainly, during the angiogenesis process, the VEGF factor in inducing endothelial cell growth 67,68. Different studies showed variable results about the effect of diabetes on the myocardial VEGF expression. For instance, a four-week follow-up study showed a decline in the VEGF expression and VEGF-R in diabetic animals 69. On the contrary, using the diabetic rat model, the researchers showed a significant increase in the VEGF mRNA level 22,70 which was attributed to the reduction in nitric oxide (NO) under hyperglycemic conditions that were also associated with the generation of ROS 22. In this report, the results showed significant overexpression of VEGF-A in the left ventricle of induced diabetic rats. Nevertheless, an effective reduction in the angiogenesis process was exhibited after eugenol treatment as shown by the downregulation of VEGF-A mRNA levels in the eugenol treated diabetic rats.
Conclusion
In experimentally induced DCM, 20mg/kg/day of eugenol can attenuate cardiac damage through inhibition of different pathological processes initiated by inflammation and proceeding through angiogenesis, and eventually approaching cardiac fibrosis and apoptosis. In the current experimental model, the eugenol exhibited an anti-diabetic effect as well as mitigation in the DCM development which provides a sight for future therapeutic application of eugenol in diabetes mellitus.
Acknowledgment
The Deanship of Scientific Research and Graduate Studies at the University of Yarmouk funded this work (Grant Number 10/2020).
Conflict of interest
The authors declare there are no conflicts of interest regarding the publication of this article.
References
- Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes research and clinical practice. 2010;87(1):4-14.
- Filipska A, Bohdan B, Wieczorek PP, Hudz N. Chronic kidney disease and dialysis therapy: Incidence and prevalence in the world. Pharmacia. 2021;68:463.
- Ozougwu J, Obimba K, Belonwu C, Unakalamba C. The pathogenesis and pathophysiology of type 1 and type 2 diabetes mellitus. Journal of physiology and pathophysiology. 2013;4(4):46-57.
- Kumar A. Characterization of Latent autoimmune diabetes in adults in a region of India: Universitat Autònoma de Barcelona; 2018.
- Lovic D, Piperidou A, Zografou I, Grassos H, Pittaras A, Manolis A. The growing epidemic of diabetes mellitus. Current vascular pharmacology. 2020;18(2):104-109.
- Einarson TR, Acs A, Ludwig C, Panton UH. Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovascular diabetology. 2018;17(1):83.
- Gulsin GS, Athithan L, McCann GP. Diabetic cardiomyopathy: prevalence, determinants and potential treatments. Therapeutic advances in endocrinology and metabolism. 2019;10:2042018819834869.
- Jia G, Hill MA, Sowers JR. Diabetic cardiomyopathy: an update of mechanisms contributing to this clinical entity. Circulation research. 2018;122(4):624-638.
- del Olmo-Garcia MI, Merino-Torres JF. GLP-1 receptor agonists and cardiovascular disease in patients with type 2 diabetes. Journal of diabetes research. 2018;2018.
- Aneja A, Tang WW, Bansilal S, Garcia MJ, Farkouh ME. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. The American journal of medicine. 2008;121(9):748-757.
- Chen Y, Hua Y, Li X, Arslan IM, Zhang W, Meng G. Distinct types of cell death and the implication in diabetic cardiomyopathy. Frontiers in pharmacology. 2020;11:42.
- Evangelista I, Nuti R, Picchioni T, Dotta F, Palazzuoli A. Molecular dysfunction and phenotypic derangement in diabetic cardiomyopathy. International journal of molecular sciences. 2019;20(13):3264.
- Murtaza G, Virk HUH, Khalid M, et al. Diabetic cardiomyopathy-A comprehensive updated review. Progress in Cardiovascular Diseases. 2019;62(4):315-326.
- Shah AM, Uno H, Køber L, et al. The inter‐relationship of diabetes and left ventricular systolic function on outcome after high‐risk myocardial infarction. European journal of heart failure. 2010;12(11):1229-1237.
- Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. The Korean Journal of Physiology & Pharmacology. 2014;18(1):1-14.
- Bansal S, Kare PK, Tripathi AK, Madhu SV. Advanced glycation end products: A potential contributor of oxidative stress for cardio-vascular problems in diabetes. Oxidative stress in heart diseases: Springer; 2019:437-459.
- Ghosh N, Katare R. Molecular mechanism of diabetic cardiomyopathy and modulation of microRNA function by synthetic oligonucleotides. Cardiovascular diabetology. 2018;17(1):1-25.
- Ghosh N. Therapeutic modulation of miRNA-320a to prevent diabetic cardiomyopathy, University of Otago; 2021.
- Westermann D, Van Linthout S, Dhayat S, et al. Tumor necrosis factor-alpha antagonism protects from myocardial inflammation and fibrosis in experimental diabetic cardiomyopathy. Basic research in cardiology. 2007;102(6):500.
- Imanaka‐Yoshida K. Inflammation in myocardial disease: From myocarditis to dilated cardiomyopathy. Pathology international. 2020;70(1):1-11.
- Cai L, Li W, Wang G, Guo L, Jiang Y, Kang YJ. Hyperglycemia-induced apoptosis in mouse myocardium: mitochondrial cytochrome C–mediated caspase-3 activation pathway. Diabetes. 2002;51(6):1938-1948.
- Sasso FC, Carbonara O, Persico E, et al. Increased vascular endothelial growth factor mRNA expression in the heart of streptozotocin-induced diabetic rats. Metabolism. 2003;52(6):675-678.
- Sasso FC, Torella D, Carbonara O, et al. Increased vascular endothelial growth factor expression but impaired vascular endothelial growth factor receptor signaling in the myocardium of type 2 diabetic patients with chronic coronary heart disease. Journal of the American College of Cardiology. 2005;46(5):827-834.
- Wang M, Li Y, Li S, Lv J. Endothelial Dysfunction and Diabetic Cardiomyopathy. Frontiers in Endocrinology. 2022;13:851941.
- Al-Trad B, Alkhateeb H, Alsmadi W, Al-Zoubi M. Eugenol ameliorates insulin resistance, oxidative stress and inflammation in high fat-diet/streptozotocin-induced diabetic rat. Life sciences. 2019;216:183-188.
- Bhattacharjya D, Adhikari S, Biswas A, Bhuimali A, Ghosh P, Saha S. Ocimum phytochemicals and their potential impact on human health. Phytochemicals in Human Health. 2019.
- Przeor M. Some Common Medicinal Plants with Antidiabetic Activity, Known and Available in Europe (A Mini-Review). Pharmaceuticals. 2022;15(1):65.
- Binu P, Priya N, Abhilash S, Vineetha RC, Nair RH. Studies on curative efficacy of monoterpene eugenol on anti-leukemic drug arsenic trioxide induced cardiotoxicity. Biomedicine & Pharmacotherapy. 2017;91:559-566.
- Fouad AA, Yacoubi MT. Mechanisms underlying the protective effect of eugenol in rats with acute doxorubicin cardiotoxicity. Archives of pharmacal research. 2011;34(5):821.
- Mnafgui K, Hajji R, Derbali F, et al. Anti-inflammatory, antithrombotic and cardiac remodeling preventive effects of eugenol in isoproterenol-induced myocardial infarction in Wistar rat. Cardiovascular toxicology. 2016;16(4):336-344.
- Fen W, Jin L, Xie Q, et al. Eugenol protects the transplanted heart against ischemia/reperfusion injury in rats by inhibiting the inflammatory response and apoptosis. Experimental and Therapeutic Medicine. 2018;16(4):3464-3470.
- Lorenzo-Almoros A, Tunon J, Orejas M, Cortés M, Egido J, Lorenzo Ó. Diagnostic approaches for diabetic cardiomyopathy. Cardiovascular diabetology. 2017;16(1):1-14.
- Ge Z-D, Lian Q, Mao X, Xia Z. Current status and challenges of NRF2 as a potential therapeutic target for diabetic cardiomyopathy. International heart journal. 2019;60(3):512-520.
- Cortés-Rojas DF, de Souza CRF, Oliveira WP. Clove (Syzygium aromaticum): a precious spice. Asian Pacific journal of tropical biomedicine. 2014;4(2):90-96.
- El Ghallab Y, Al Jahid A, Jamal Eddine J, Ait Haj Said A, Zarayby L, Derfoufi S. Syzygium aromaticum L.: phytochemical investigation and comparison of the scavenging activity of essential oil, extracts and eugenol. Advances in Traditional Medicine. 2020;20(2):153-158.
- Singh P, Jayaramaiah RH, Agawane SB, et al. Potential dual role of eugenol in inhibiting advanced glycation end products in diabetes: proteomic and mechanistic insights. Scientific reports. 2016;6(1):1-13.
- Antora RA, Salleh RM. Antihyperglycemic effect of Ocimum plants: A short review. Asian Pacific Journal of Tropical Biomedicine. 2017;7(8):755-759.
- Lee W-S, Kim J. Diabetic cardiomyopathy: where we are and where we are going. The Korean Journal of internal medicine. 2017;32(3):404.
- Lin H, Guan L, Meng L, Uzui H, Guo H. SGLT1 knockdown attenuates cardiac fibroblast activation in diabetic cardiac fibrosis. Frontiers in Pharmacology. 2021;12.
- Yue Y, Meng K, Pu Y, Zhang X. Transforming growth factor beta (TGF-β) mediates cardiac fibrosis and induces diabetic cardiomyopathy. Diabetes research and clinical practice. 2017;133:124-130.
- Yoncheva ID, Biserov DE, Negreva MN. Changes in profibrotic activity in cardiovascular diseases. World Journal of Advanced Research and Reviews. 2021;11(2):093-099.
- Reese-Petersen AL, Olesen MS, Karsdal MA, Svendsen JH, Genovese F. Atrial fibrillation and cardiac fibrosis: A review on the potential of extracellular matrix proteins as biomarkers. Matrix biology. 2020;91:188-203.
- Li L, Zhao Q, Kong W. Extracellular matrix remodeling and cardiac fibrosis. Matrix biology. 2018;68:490-506.
- Wu L, Derynck R. Essential role of TGF-β signaling in glucose-induced cell hypertrophy. Developmental cell. 2009;17(1):35-48.
- Frangogiannis NG. Transforming growth factor–β in tissue fibrosis. Journal of Experimental Medicine. 2020;217(3).
- Blyszczuk P, Müller-Edenborn B, Valenta T, et al. Transforming growth factor-β-dependent Wnt secretion controls myofibroblast formation and myocardial fibrosis progression in experimental autoimmune myocarditis. European heart journal. 2017;38(18):1413-1425.
- Fan D, Takawale A, Lee J, Kassiri Z. Cardiac fibroblasts, fibrosis and extracellular matrix remodeling in heart disease. Fibrogenesis & tissue repair. 2012;5(1):15.
- Schultz JEJ, Witt SA, Glascock BJ, et al. TGF-β1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin II. The Journal of clinical investigation. 2002;109(6):787-796.
- Su C, Wang Q, Luo H, et al. Si-Miao-Yong-An decoction attenuates cardiac fibrosis via suppressing TGF-β1 pathway and interfering with MMP-TIMPs expression. Biomedicine & Pharmacotherapy. 2020;127:110132.
- Frati G, Schirone L, Chimenti I, et al. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovascular Research. 2017;113(4):378-388.
- Wang L, Wu H, Deng Y, et al. FTZ Ameliorates Diabetic Cardiomyopathy by Inhibiting Inflammation and Cardiac Fibrosis in the Streptozotocin-Induced Model. Evidence-Based Complementary and Alternative Medicine. 2021;2021.
- Rolski F, Błyszczuk P. Complexity of TNF-α signaling in heart disease. Journal of clinical medicine. 2020;9(10):3267.
- Wold LE, Ceylan‐Isik AF, Ren J. Oxidative stress and stress signaling: menace of diabetic cardiomyopathy. Acta Pharmacologica Sinica. 2005;26(8):908-917.
- Liu J-W, Liu D, Cui K-Z, et al. Recent advances in understanding the biochemical and molecular mechanism of diabetic cardiomyopathy. Biochemical and biophysical research communications. 2012;427(3):441-443.
- Al Hroob AM, Abukhalil MH, Hussein OE, Mahmoud AM. Pathophysiological mechanisms of diabetic cardiomyopathy and the therapeutic potential of epigallocatechin-3-gallate. Biomedicine & Pharmacotherapy. 2019;109:2155-2172.
- Aikawa R, Nitta-Komatsubara Y, Kudoh S, et al. Reactive oxygen species induce cardiomyocyte apoptosis partly through TNF-α. Cytokine. 2002;18(4):179-183.
- Ighodaro O, Akinloye O. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria journal of medicine. 2018;54(4):287-293.
- Weydert CJ, Cullen JJ. Measurement of superoxide dismutase, catalase and glutathione peroxidase in cultured cells and tissue. Nature protocols. 2010;5(1):51-66.
- Byrne NJ, Rajasekaran NS, Abel ED, Bugger H. Therapeutic potential of targeting oxidative stress in diabetic cardiomyopathy. Free Radical Biology and Medicine. 2021;169:317-342.
- Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55(3):798-805.
- Nagababu E, Rifkind JM, Boindala S, Nakka L. Assessment of antioxidant activity of eugenol in vitro and in vivo. Free Radicals and Antioxidant Protocols: Springer; 2010:165-180.
- Gülçin İ. Antioxidant activity of eugenol: A structure–activity relationship study. Journal of medicinal food. 2011;14(9):975-985.
- Makuch E, Nowak A, Günther A, et al. Enhancement of the antioxidant and skin permeation properties of eugenol by the esterification of eugenol to new derivatives. AMB Express. 2020;10(1):1-15.
- Volpe CMO, Villar-Delfino PH, Dos Anjos PMF, Nogueira-Machado JA. Cellular death, reactive oxygen species (ROS) and diabetic complications. Cell death & disease. 2018;9(2):1-9.
- Jubaidi FF, Zainalabidin S, Mariappan V, Budin SB. Mitochondrial dysfunction in diabetic cardiomyopathy: the possible therapeutic roles of phenolic acids. International journal of molecular sciences. 2020;21(17):6043.
- Choudhary R, Mishra KP, Subramanyam C. Interrelations between oxidative stress and calcineurin in the attenuation of cardiac apoptosis by eugenol. Molecular and cellular biochemistry. 2006;283(1-2):115-122.
- Tham E, Wang J, Piehl F, Weber G. Upregulation of VEGF-A without angiogenesis in a mouse model of dilated cardiomyopathy caused by mitochondrial dysfunction. Journal of Histochemistry & Cytochemistry. 2002;50(7):935-944.
- Melincovici CS, Boşca AB, Şuşman S, et al. Vascular endothelial growth factor (VEGF)-key factor in normal and pathological angiogenesis. Rom J Morphol Embryol. 2018;59(2):455-467.
- Chou E, Suzuma I, Way KJ, et al. Decreased cardiac expression of vascular endothelial growth factor and its receptors in insulin-resistant and diabetic States: a possible explanation for impaired collateral formation in cardiac tissue. Circulation. 2002;105(3):373-379.
- Yan F, Sun X, Xu C. Protective effects of resveratrol improve cardiovascular function in rats with diabetes. Experimental and therapeutic medicine. 2018;15(2):1728-1734.








