Ravi Shankar Prasad Sawan1 , Sridevi N S1*
, Sridevi N S1* and Shashidhar K N2
and Shashidhar K N2
1Department of Anatomy, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India.
2Department of Biochemistry, Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India.
Corresponding Author E-mail: sridevins26@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2454
Abstract
Nicotine present in the tobacco leaves are activated through nicotinic acetylcholine receptors and are involved in neurobehavioral activity. Despite creating awareness, there is an increasing trend in the consumption of tobacco. Few plant products have been documented to protect the pathological consequences of nicotine. The present study is aimed to find the beneficial effects of Bacopa-Monnieri (BM) against nicotine induced physical, psychological, neurobehavioral and biochemical changes in cerebellum. Thirty-two male Sprague-Dawley rats (220-235g) were equally randomized into four groups: Group I: Control; received normal Saline. Group II: received Nicotine (5mg/kg Body-Weight) for 90 days. Group III: received nicotine (5mg/kg Body-Weight) for initial 90 days followed by Bacopa-Monnieri (100mg/kg Body-Weight) for next 90 days. Group IV: received Bacopa-Monnieri (100 mg/kg Body-Weight) for 90 days. All compounds were administered through oral gavage. Rats were subjected to Open Field Test, Elevated Plus Maze and Beam Walking Test. Following behavioral tests, rats were anesthetized with ketamine (80 ml/kg Body-Weight) and xylocaine (100 ml/kg Body-Weight), brain was dissected and cerebellum was separated. Concentration of Malondialdehyde, Nitric Oxide and activity of Glutathione Peroxidase were measured spectrophotometrically in the supernatant of cerebellum tissue homogenates. Nicotine increases the Malondialdehyde (MDA) and Nitric-Oxide (NO) level in cerebellar tissue compared to control. Nicotine induced increase in Malondialdehyde and Nitric-Oxide level were prevented by Bacopa-Monnieri. The Glutathione Peroxidase (GPx) activity was lower in nicotine treated rats whereas oral supplementation of Bacopa-Monnieri significantly increases the activity of Gluathione-Peroxidase. Bacopa-Monnieri supplementations significantly reverse the Nicotine induced reduction in locomotion activity, exploratory behavior, anxiety, motor impairment and balance. Bacopa-Monnieri confers the protective effects against nicotine induced neurobehavioral alteration and oxidative stress in rats.
Keywords
Bacopa-Monnieri; Behavioral activities; Cerebellum; Nicotine; Oxidative Stress Markers
Download this article as:| Copy the following to cite this article: Sawan R. S. P, Sridevi N. S, Shashidhar K. N. Ameliorating Effect of Bacopa-Monnieri against Nicotine Induced Cerebellar Toxicity in Male Sprague-Dawley Rats. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Sawan R. S. P, Sridevi N. S, Shashidhar K. N. Ameliorating Effect of Bacopa-Monnieri against Nicotine Induced Cerebellar Toxicity in Male Sprague-Dawley Rats. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3FX6edN |
Introduction
Cigarette smoking; a major source of tobacco consumption; is an addictive agent which is increasing across the younger generation and middle-aged population 1. Once, a person gets the taste of tobacco, the active ingredients help addiction and it is hard to withdraw from it. Although, tobacco smoking is recognized as a risk factor for adverse health outcomes, the daily smoking of tobacco has been increasing rapidly across the world 2. It has been observed that chronic consumption of tobacco products has been linked with wide spectrum of diseases especially the disease involving nervous system such as cognition-related or neurodegeneration related pathological changes and also alters the brain morphology and neurochemistry 3,4.
Nicotine; an important constituent of tobacco; is a natural alkaloid with chemical name C10H14N2, obtained from the leaves and stems of Nicotiana tabacum and Nicotiana rustica 5. The major form of nicotine consumption is by cigarette smoking. During cigarette smoke or oral consumption as tobacco chewing, nicotine enters the body which is quickly absorbed into the circulatory system and then reaches the brain within 10-20 seconds and gets concentrated in the brain showing alterations in multiple brain regions, such as thalamus, prefrontal cortex and cerebellum 6. Nicotine might also cause a cerebellar deficit which has many effects on its function, cognition and behavior 7,8. In rodents, nicotine exposure shows several neurobehavioral deficits such as attention, learning and memory sensory processing defects and also causes analgesia, depression or anxiety-like behavior 9. Presently, the information available regarding the scientific correlation linking cigarette smoke with the cerebellar dysfunction is limited. Though, nicotine causes postural imbalance, showing that nicotine might be the circuitry involving the cerebellum in smokers 10. Nicotine causes significant loss of white matter and reduction in number of Purkinje cells of cerebellum, with possible predisposition to progressive impairment in the structural integrity and function of the cerebellum 11,12 Therefore, chronic exposure to nicotine negatively impacted on the cerebellum, leading to impaired behavior and cognitive function. The excessive production of free radicals and reactive oxygen species by nicotine induced brain can produce a condition of oxidative stress which may cause structural alterations in various regions of the brain and also neurobehavioral impairments 13,14.
In Indian traditional medicine, Bacopa-Monnieri (BM) is considered as a magical medicinal plant, known to have a brain tonic adaptogenic effect15. BM has a vast number of active compounds including several alkaloids and saponins. Saponins, Bacosides A and B; the principle components of BM, are mainly attributed for cognitive effect and learning ability 16. Bacosides and bacopasaponins constituents of BM also showed anti-oxidant properties 17. Crude plant extract and bacosides have also shown neuropharmacological activities such as anticonvulsant, neuroprotection, antidepressant, anxiolytic effect and antioxidant activity 18.
Although, numerous studies have acknowledged the various pharmacological activities of BM intoto, very little is known about its interaction with the Nicotine induced cerebellar neurotoxicity. Keeping in view, the ameliorative effect of Bacopa-Monnieri which is studied less and is a dilapidated molecule, the present study addressed to investigate the neuroprotective effect of the crude form of Bacopa-Monnieri against nicotine induced oxidative stress in cerebellum of rats and also on locomotor and exploratory behavior, motor coordination and balance in rats.
Materials and Methods
Chemical
Study was conducted by using nicotine commercially available molecule as Nicotine Bi-L-tartrate Dihydrate supplied by TCI chemical India.
Experimental Animals and housing
The present study was approved Institutional Animal Ethics Committee (IAEC) with a reference No. IAEC/PHARMA/SDUMC/2018-19/12a. Experimental Animals were humanely cared with guidelines of Committee for the Purpose of Control and Supervision of Experiment on Animals (CPCSEA). Male Sprague-Dawley rats aged 10-12 weeks weighing 220 to 235 grams were obtained from Biogen Bangalore, Karnataka, India. The rats were kept in well-maintained temperature ranging from 200C-260C and preserved in polypropylene cages confirming 2-rats per cage. The cage was bedded with dust free paddy husk. Rats were provided with free access to standard feed pellets and drinking water Ad-libitum.
Selections of Animals
Inclusion criteria
Healthy male Sprague-Dawley rats aged 10-12 weeks with 220-235 gram in weight were included.
Exclusion criteria
Females rats and rats with any sign/symptoms of illness were excluded from the study.
Study Design
The study was an experimental study conducted in Central Animal House of Sri Devaraj Urs Academy of Higher Education and Research (SDUAHER). After 2-weeks of acclimatization, the study rats were equally distributed into 4-groups.
Group-I (N=8): Control rats; received only normal saline
Group-II (N=8): rats received nicotine (5mg/kg BW) orally through oral gavage once in a day for 90 days
Group-III (N=8): rats received nicotine (5mg/kg BW) for 90-days followed by Bacopa-Monnieri (100mg/kg BW) for next 90 days
Group-IV (N=8): rats received only Bacopa-Monnieri for 90 days
Preparation and administration of drugs
Nicotine
Nicotine Bi-L-Tartarate dihydrate 5mg/kg Body-Weight was freshly prepared on day of experiment. The salt form of the nicotine was dissolved in RO/UV-treated water and administered to rats. The pH of nicotine solution was adjusted to 7.0 and administered orally through oral gavage once in a day for 90 days.
Bacopa-Monnieri (BM)
The aerial part of BM was collected. The identification and authentication of BM was done by qualified Botanist of University of Agriculture Sciences, Bengaluru. The specimen was deposited in the herbarium with a voucher specimen number UASB4608.
The plant was washed with normal tap water followed by double distilled water and finally with ethanol to eliminate the bacterial contamination from the plant. The washed plants were air-dried at room temperature. The plants were powdered; crude form of extract was used for the study. Freshly prepared BM, 100 mg/kg Body-Weight was given daily throughout the study period.
Neurobehavioral study
After the last dose of treatment, all rats were subjected to behavioral test in the room where the temperature was maintained between 200C-260C with all requisite precautions against noise and dust. The experiment was carried out between 09:00 am to 03:00 PM. Rats were acclimatized in the test room for 30-minutes before the conduct of behavioral test.
Open field test (OFT)
Rats were tested to evaluate locomotion, anxiety and behavior activities in the OFT as per the study conducted by Arika WM et.al. 19. [Figure-1]
Rats were picked by tail from the home cage and dropped at the center of arena, allowed to explore the open field for 300 seconds; video recording was done to confirm the activities of rats.
The following parameters were recorded:
Motor activities were confirmed by locomotion across the lines drawn in the OFT box. Results were considered only if the rats had moved across with all four paws
Behavioral activities was confirmed by grooming, rearing, wall-rearing, frequency of entries on the central part of arena and freezing
 |
Figure 1: Open Field Test (OFT). |
Elevated Plus Maze (EPM)
This is an animal model for measuring anxiety as proposed by Donatti AF et. al. 20. The test was done during light phase of light-dark cycle. For testing, rats were individually placed in the central square of the maze with head facing open arm and were allowed free exploration for 300 sec. The behavioral activities were recorded by a video camera. [Figure-2]
The following parameters were recorded:
Open and Closed arm entry
Time spent in each arm
Freezing time
Number of entry in center and time spent in it.
Frequency and duration of rearing and grooming were also assessed.
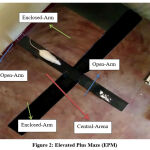 |
Figure 2: Elevated Plus Maze (EPM). |
Beam walking test (BWT)
Ataxia and dystonic movements were evaluated by BWT and confirmed by motor coordination and balance similar to the study conducted by Silveira EMS et. al. 21. [Figure-3]
The parameters evaluated were confirmed by video recordings and monitored for
Time taken to complete the beam
Number of foot slips
Number of near fall
Actual fall.
 |
Figure 3: Beam Walking Test (BWT). |
Biochemical Analysis
Collection of sample
Following behavioral tests, all rats were fasted overnight except water.
All rats were anesthetized by injecting ketamine and xylocaine intraperitoneally.
Rats were sacrificed by cervical decapitation.
Brains were immediately taken out and washed with ice cold saline and Phosphate buffer solutions (PBS) to remove blood.
The cerebellum was rapidly dissected from the intact brain carefully.
The cerebellum was frozen quickly into liquid nitrogen and stored at –800
Estimation of Oxidative stress markers in cerebellum tissue
Tissue homogenates were obtained from a 150-200mg piece of cerebellar tissue, homogenized by glass Teflon homogenizer in PBS with pH-7.4, in the ratio of 1:10 (W/V) on the ice for 10-15 minutes.
The homogenates were centrifuged to separate nuclear debris at 10,000g X 20 minutes at 40C and supernatant was collected and stored at -800C until use. Supernatant was used to estimate levels of Malondialdehyde, Nitric-Oxide and the activity of Gluathione-Peroxidase.
Estimation of Malondialdehyde (MDA)
The MDA level was estimated by thiobarbituric acid reactive substances assay (TBARs) methods 22. Briefly, 0.25ml of supernatant was mixed with 0.75% thiobarbituric acid (TBA) and 25% trichloroacetic acid (TCA) solution and kept in boiling water bath for 15 minutes. After cooling, the supernatant was taken and measured the absorbance of developed pink color at 530nm. The concentration of MDA was expressed as nmol/mg of protein in brain tissue according to a standard graphic, which was prepared with serial dilutions of standards 1,1,3,3-tetramethoxypropane.
Estimation of Nitric-Oxide (NO)
Modified Griess’s method was used to assess the levels of Nitric-Oxide 23. Briefly, 0.25 ml of supernatant was added to 25 µl of sulphosalicylic acid (70%) and mixed it every 5 minutes for 30 minutes then centrifuged it at 3000 rpm for 20 minutes. The supernatant was collected. 50 µl of supernatant was added to 7.5 µl of NAOH (10%) and 75 µl of tris-HCl buffer (pH-9) and then added 132.5µl of Griess reagent. All the reagents were kept in dark place for 15 minutes and the absorbance was read at 540nm against blank.
Griess reagent was prepared freshly by mixing 5 ml each of Sulphanilamide (3%) and N-(Naphtheline)-Ethylene Diamine Dihydrochloride (0.3%). The results were expressed as nmoles/mg protein.
Estimation of Glutathione Peroxidase (GPx)
Glutathione peroxidase (GPx) activity was measured following the procedure of Rotruck et.al. 24. 1 ml of the reaction mixture was prepared which contained 0.2 ml each of phosphate buffer (0.4 M, PH 7), 0.1 ml of sodium azide (10mM), 0.2 ml of EDTA (0.4 mM), 0.1 ml of H2O2 (0.2 mM), 0.2 ml of GSH (2 Mm) and 0.2 ml of samples were mixed and incubated at 370c for 10 minutes. The reaction was terminated by addition of 0.5 ml of 10% TCA. The tubes were centrifuged and the supernatant was collected. 1.8 ml of Ellman’s reagent (DTNB) was added to 0.2 ml of reaction supernatant. After mixing, the absorbance was read (within 2-3 minutes after the addition of DTNB) at 412 nm. The specific activity of GPx was expressed as mmoles of GSH oxidized/min/mg of protein for tissue.
Statistical Analysis
Licensed version of SPSS-20 developed by IBM was used for data analysis. Parametric variables were represented as Mean±SD and non-parametric variables were indicated as median (25th-75th Percentile). p-Value for parametric variables were calculated by ANOVA. Mann-Whitney U test was applied for non-parametric variables. p-Value<0.05 considered significant.
Results
Physical and physiological Observations
Physical and physiological observations are presented in the Table 1. Group II and III rats reduced their food and water intake for 5-15 days of exposure and between 16-42 days, they were normal when compared to Group I and IV rats. Rats showed reduction in food and water intake between 43-90 days of exposure in Group II and III rats. Further, we also noted that there was a reduction in food and water intake from 91-110 days and it was found normal after 110 days.
Table 1: Physical and physiological observations in comparison with Group I.
| Group | Parameters | 0-4 Days | 5-10
Days |
11-15
Days |
16-42
Days |
43-90 days | 91-110
Days |
111-180
Days |
|
Group II |
Food | Normal | Reduction | Reduction | Normal | Reduction | Rats were sacrificed | |
| Water | Normal | Reduction | Reduction | Normal | Reduction | |||
| Defecation | Normal | Semi-solid | Semi-solid | Normal | Normal | |||
| Hair-gloss | Normal | Normal | Normal | Normal | Shedding | |||
|
Group III |
Food | Normal | Reduction | Reduction | Normal | Reduction | Reduction | Normal |
| Water | Normal | Reduction | Reduction | Normal | Reduction | Reduction | Normal | |
| Defecation | Normal | Semi-solid | Semi-solid | Normal | Normal | Normal | Normal | |
| Hair-gloss | Normal | Normal | Normal | Normal | Shedding | Shedding | Shedding | |
| Group IV | Food | Normal | Normal | Normal | Normal | Normal | Rats were sacrificed | |
| Water | Normal | Normal | Normal | Normal | Normal | |||
| Defecation | Normal | Normal | Normal | Normal | Normal | |||
| Hair-gloss | Normal | Normal | Normal | Normal | Normal |
We found that rats showed alteration in the fecal texture in group II and III for 5-15 days of exposure and was found to be normal after 15 days.
Hair shedding was observed in Group II and III after 42 days of exposure and continued to shed till their decapitation. Group I and IV showed no changes in any of the observations.
Effect of Bacopa-Monnieri on body-weight and brain weight
Body weight and Brain weight are presented in the Table 2A & 2B. We have observed a good body weight gain of 91.87 grams in group I compared to other groups. The net weight gain in group II and Group III in the first half (0- 90days) was almost the same as in the second half of the Group III (91-180 days). This clearly indicates that rats treated with combination of nicotine and BM has improved the body weight to an extent of 28 grams. To our surprise, rats that were only given BM showed a net weight gain of 70.63 grams. However, the brain weight in all the four groups was consistent between 2.13 to 2.23 grams; which was observed after decapitation. This gives an idea that the gross brain weight is not altered in any of the four groups, irrespective of permutation and combination of nicotine and Bacopa-Monnieri. This does not stop thinking of the morphological or intracellular changes that is happening within the brain and also the molecular mechanisms need to be addressed to construe the decision. With the results that we have observed with respect to body weight gain, hypothetically, we comment that BM has got an antagonistic effect on the nicotine.
Table 2A: Comparison of weight of Rats following exposure to different duration. [Data were expressed as Mean±SD].
| Group | Initial body weight
(0-days) (Gram) |
Body Weight After
90-days (Gram) |
Body Weight After
(180-days) (Gram) |
Net Body Weight gain
(Gram) |
| Group I | 225.38±6.78 | 317.25±6.985 | – | 91.87±0.204 |
| Group II | 234.88±5.12 | 291.63±6.664 | – | 56.75±1.539 |
| Group III | 230.50±5.26 | 284.00±6.477 | 365.5±7.03 | 53.5±1.213(0-90 days)
81.5±0.554(90-180 days) |
| Group IV | 224.00±9.05 | 294.63±10.3 | – | 70.63±1.246 |
Table 2B: Brain-weight; Data were Mean±SD.
| Parameters | Group-I | Group-II | Group-III | Group-IV | p-Value |
| Brain-Weight | 2.17±0.19 | 2.21±0.13 | 2.23±0.09 | 2.13±0.15 | 1 |
p>0.05 considered no significant difference. The present results were not significant between any of the groups. [Group I=Control, Group II= Nicotine, Group III= Nicotine+Bacopa-Monnieri, Group IV= Bacopa-Monnieri]
Effect of Bacopa-Monnieri (BM) on Nicotine induced Oxidative Stress Parameters
Lipid peroxidation (MDA), Nitric Oxide (NO) and Glutathione Peroxidase (GPx) were assessed to evaluate the protective role of BM against nicotine-induced oxidative stress in the cerebellum of rats. (Table 3).
Table 3: Oxidative Stress Parameters, Data were Median [25th-75th Percentile].
| Parameter | Group I (N=8)
Median [25th-75th percentile] |
Group II (N=8)
Median [25th-75th percentile] |
Group III (N=8)
Median [25th-75th percentile] |
Group IV (N=8)
Median [25th-75th percentile] |
p-Value |
| MDA [nmol/mg of protein] | 3.02[2.35-3.61] | 6.65 [6.37-8.18]a | 3.58 [2.74-4.20]b | 3.3 [2.6-4.41]b | 0.001 |
| NO [nmol/mg of protein] | 13.93[11.01-16.16] | 23.5[20.74-25.29]a | 11.61 [10.4-15.17]b | 16 [15.14-18.86]bc | 0.005 |
| GPx [mmoles of GSH oxidized/min/mg of protein] | 7.63[7.08-9.19] | 3.95 [2.71-4.92]a | 5.7 [5.1-6.62]ab | 11.86 [10.34-3.37]abc | 0.005 |
Groups with values that have different superscript small-letter (a,b,c) differ significantly from other groups [a=compared to Group-I, b=compared to Group-II and c=compared with Group-III]. Mann-Whitney U test was used to compare the between groups. p<0.05 was considered significant.
MDA level in Group III and Group IV rats showed no any marked changes and are comparable to that of Group I. There was a significant increase in MDA level in Group II [6.65 (6.37-8.18)] rats compared to Group I [3.02(2.35-3.61), Group III [3.58 (2.74-4.20)] and Group IV [3.3(2.6-4.41)]. The observed lower concentration of MDA in Group III rats showed the protective effects of BM against Nicotine induced degenerative changes.
The cerebellar Nitric Oxide (NO) level showed a significant increase in the rats of Group II [23.5(20.74-25.29)] compared to Group I [13.93(11.01-16.16)], Group III [11.61 (10.4-15.17)] and Group IV [16 (15.14-18.86)]. We also found that rats that Group III had a significantly lower value than Group IV with a p-value of <0.018. There was no significance difference between the Group I [13.93(11.01-16.16)], Group III [11.61 (10.4-15.17)] and Group IV [16 (15.14-18.86)] rats. This suggests that BM may have the protective effect of preventing nicotine-induced excess nitric oxide.
The activity of Glutathione Peroxidase (GPx) showed a significantly lower value in rats of Group II [3.95 (2.71-4.92)] than Group I [7.63(7.08-9.19)], Group III [5.7 (5.1-6.62)] and Group IV [11.86 (10.34-13.37)]. On the other hand, we also found a significant increase in Group IV [11.86 (10.34-13.37)] rats when compared with Group I [7.63(7.08-9.19)] and Group III [5.7 (5.1-6.62)].
Effect of Bacopa-Monnieri on Nicotine induced neurobehavioral changes
Effect of Bacopa-Monnieri on OFT parameters
Nicotine induced rats showed significant decrease in the frequency of square line crossed, rearing, wall-rearing and grooming compared to Group I with a p-value of <0.001. The oral ingestion of BM significantly improved in all the elements of the OFT in nicotine treated rats. High frequency of square line crossed and rearing and reduction in freezing time indicates high level of locomotion and low level of anxiety. It was observed that nicotine treated rats remained inactive for more duration, which leads into reduction of activities in OFT. BM treated rats showed no marked changes and resembled the control group in rearing, wall rearing, time spent in center and grooming. [Table-4].
Table 4: Open field test values, Data were presented as Median [25th-75th Percentile].
| Parameters | Group I (N=8)
Median [25th-75th percentile] |
Group II (N=8)
Median [25th-75th percentile] |
Group III (N=8)
Median [25th-75th percentile] |
Group IV (N=8)
Median [25th-75th percentile] |
p-Value |
| Number of Square Line Crossed | 128 [121.7-141.7] | 35 [34.4-36.25]a | 71.3 [68.4-74.4]ab | 102[89.8-105.3]abc | 0.001 |
| Number of Rearing | 13.7 [11.8-16.2] | 3.3 [2.7-5.5]a | 12.2 [11.3-12.9]b | 13.2 [12-17]b | 0.001 |
| Number of Wall-Rearing | 25.2 [22.3-27.3] | 9.3 [7.8-10.6]a | 19 [16.9-23.2]ab | 18.5 [17.3-22.3]b | 0.05 |
| Time Spent in Center (Seconds) | 5.3 [3.5-13.75] | 0.67 [0-1.92]a | 1.5 [0-2.25]a | 3.5 [1.5-7.5]b | 0.05 |
| Freezing Time (Seconds) | 47.84 [39.5-62] | 216.5 [194-231.4]a | 76.3 [73.1-82.25]ab | 75.17 [67-81.08]ab | 0.006 |
| Number of Grooming | 10.5 [9.7-11.9] | 4.5 [4-5.25]a | 8.17 [7-8.3]ab | 8.3 [7.5-8.3]ab | 0.006 |
| Time Spent in Grooming (Seconds) | 26.3 [21.4-28.8] | 26.7 [24.1-28.8] | 35.2 [32.5-38.9]ab | 32.2 [20.7-34.3] | 0.006 |
Groups with values that have different superscript small-letter (a,b,c) differ significantly from other groups [a=compared to Group-I, b=compared to Group-II and c=compared with Group-III]. Mann-Whitney U test was used to compare the between groups. p<0.05 was considered statistically significant.
Effect of Bacopa-Monnieri on Elevated Plus Maze parameters
Group II rats showed a significant reduction in number and time spent in open arm, center, rearing, grooming compared to Group I with a p-value <0.001. On other hand, freezing time, time spent in center, significantly increased in Group II rats compared to Group I with a p-value of <0.001. The oral administration of BM prevented this degenerative changes that was treated with both nicotine and BM, as it was observed that the frequency and duration of open arm, center square, rearing and grooming were significantly increased than those of the Group II with a p-value of <0.001. (Table-5).
Table 5: Elevated Plus Maze values, Data were expressed as Median [25th-75th Percentile].
| Parameters | Group I (N=8)
Median [25th-75th percentile] |
Group II (N=8)
Median [25th-75th percentile] |
Group III (N=8)
Median [25th-75th percentile] |
Group IV (N=8)
Median [25th-75th percentile] |
p-Value |
| Number Of Entry in Closed Arm | 11.8 [10.7-14.2] | 5.2 [4.7-6.3]a | 5.3 [5.1-6.8]a | 10.5 [8.5-11.8]bc | 0.001 |
| Time Spent in Closed Arm (Sec) | 35.5 [29-39.2] | 228.8[227.4-232.3]a | 82.3[75.8-98.8]ab | 33.3 [21.9-35.4]bc | 0.001 |
| Number of Entry in Open Arm | 26.7[25.2-28.2] | 7.7[6.7-8.2]a | 22.2 [20-27.1]b | 29.3 [28.2-31.5]abc | 0.001 |
| Time Spent in Open Arm (Sec) | 212 [204.5-216] | 50.3 [44.7-52.7]a | 170.5 [156.4-174.4]ab | 215.3 [213.7-230.7]bc | 0.001 |
| Freezing Time (Sec) | 38.7[34.9-43.4] | 208.3 [198-211.7]a | 80.8 [78.5-86.4]ab | 36 [29.8-41.4]bc | 0.001 |
| Number of Entry in Center | 39.2 [37.7-41.4] | 12.8 [12.1-13.3]a | 27.7 [25.8-32.3]ab | 40 [39.7-41.1]bc | 0.001 |
| Time Spent in Center (Sec) | 57.8 [55.7-64.2] | 20 [19.4-21.3]a | 46.5 [41.8-50.2]ab | 50.34[47.8-52.4]ab | 0.001 |
| Number of Rearing | 18.7 [18.1-21.8] | 6.5 [6.1-7]a | 16.5 [15.2-17.2]ab | 27 [25.3-29.9]abc | 0.001 |
| Time Spent in Rearing (Sec) | 33.5 [31.7-36.8] | 13 [12.7-13.9]a | 27.8 [24.4-28.6]ab | 53.2 [52.4-55]abc | 0.001 |
| Number of Grooming | 14.5 [13.1-16.6] | 4.8 [4.2-5.3]a | 9.5 [9-10.6]ab | 14.7[12.7-16.2]bc | 0.001 |
| Time Spent in Grooming (Sec) | 27.3[25.4-32.2] | 7.3 [6.8-7.9]a | 20.7 [18.4-23.1]ab | 24.7[23.9-25.2]abc | 0.001 |
Groups with values that have different superscript small letter (a,b,c) differ significantly from the other groups. [a=compared with Group-I, b=Compared with Group-II, c=compared with Group-III]. p<0.05 was considered significance difference.
Effect of Bacopa-Monnieri on BWT parameters
The nicotine treated rats took significantly longer time to traverse the beam when compared with Group I with a p-value of <0.001. Oral administration of BM significantly reduces the time to traverse the beam in Group II with a p-value of <0.008. We also noted that Group II rats significantly increased the number of foot slips and near fall compared with Group I (<0.001), Group III (<0.001), Group IV (<0.002). Number of foot slips and near fall in Group I and Group III rats were nil. There were no rats which had a fall from the beam in any of the groups. (Table-6).
Table 6: Beam Walking Test values, Data were Median [25th-75th Percentile].
| Parameters | Group I (N=8)
Median [25th-75th percentile] |
Group II (N=8)
Median [25th-75th percentile] |
Group III (N=8)
Median [25th-75th percentile] |
Group IV (N=8)
Median [25th-75th percentile] |
p-Value |
| Time taken to cross the beam (Sec) | 9.0[7.25-10.0] | 33.33[28.42-47.34]a | 25.0[22.08-27.67]ab | 14.83[11.42-22.09]abc | 0.05 |
| Number of foot slips (No) | 0 | 6.33[5.00-7.67]a | 0b | 2.67[2.33-3.59]abc | 0.001 |
| Number of near fall (No) | 0 | 2.33[1.42-2.33]a | 0b | 0b | 0.001 |
Discussion
The present study highlights that the daily administration of nicotine for 90 days induced excessive oxidative stress, which can be observed by the high significant increase in the MDA and NO levels. Also, a significant inhibition of GPx activity was observed. Nicotine exposure also showed impairment in motor coordination and balance (by increasing number of foot-slips and near-fall as observed in BWT), reduction in locomotory and exploratory behavior (as assessed in OFT by reducing number of square-line crossed, rearing, wall-rearing and grooming) and more anxious (by increased freezing time and time spent in closed arm as evaluated in EPM). On the contrary, oral supplementation of Bacopa-Monnieri (100 mg/kg BW) ameliorates the oxidative stress markers and neurobehavioral activities against nicotine treated rats.
We found that the nicotine treated rats reduced their body weight compared to the control group. This may be due to reduction in food intake. Our observation findings have shown that the weight gain in control group is almost double to those rats that were administered only nicotine. Further, we observed that the reduction was consistent and similar to those rats which were treated with a combination of Nicotine and Bacopa Monnieri, during the initial 90-days. However, the weight gain is similar to that of the control group of those rats which was treated with combined exposure of Nicotine and Bacopa Monnieri after 180 days. The values that we have observed in the second half of those rats treated with Nicotine and Bacopa-Monnieri may be because of the antagonistic effect of Bacopa-Monnieri verses Nicotine. This indicates that the compounds of Bacopa-Monnieri shall mask the adverse effects of Nicotine. These results are in agreement with the rats which have been treated with Bacopa-Monnieri depriving them with Nicotine and the difference we could observe is around 32%. This experimental data were supported by the findings where rats exposed to nicotine showed decrease in body weight 25,26. This could be due to increased lipolysis, energy expenditure and metabolic rate or loss of appetite or reduction in insulin sensitivity 26. However, the oral administration of BM prevented the decrease in body weight against nicotine induced rats which can occur as a result of increased food consumption.
As the nicotine pass through BBB, it binds to nicotinic acetylcholine receptor and disturbs the cholinergic systems and disrupts brain development, neuronal migration and synaptogenesis and neurotransmitter release 27. These adverse effects may produce oxidative stress and neurobehavioral impairments. Oxidative stress was evaluated on the basis of changes in the activity of GPx and concentration of MDA and NO in cerebellar tissues of rats.
Nicotine promotes the over production of free radicals such as reactive oxygen species (ROS) both in-vivo and in-vitro conditions and alter the brain tissue. Many experimental data from the animal study have shown that nicotine potentiated the oxidative stress indices including MDA in different organs such as brain, testes and liver 2,28.
Our results disclosed that nicotine elevates the lipid peroxidation in the cerebellum tissue; observed as an increase in MDA levels and elevated MDA level may lead to cell disruption, oxidative damage to cell membrane and hence increase the susceptibility to lipid-peroxidation 29. Our results are similar with the finding of Omotoso GO et al. and Das S et al. who documented that the nicotine treated group has a higher MDA expression in cerebellar tissue compared with other groups 7,30. Notably, the oral supplementation of BM significantly neutralizes the free radical generation and thus protects the biomembranes damage caused by lipid-peroxidation due to nicotine.
Nitric-oxide is a signaling molecule that plays a pivotal role in the pathogenesis of inflammation. The data from our study showed that nicotine treated rats produced a marked increase in NO level in the cerebellum which may be due to upregulation of induced NOS (iNOS) expression induced by nicotine 31. The increase in NO level may specify the initiation of neuro-inflammation due to nicotine. Interestingly, treatment with BM supplementation has significantly brought down the NO level to the normal suggesting that the beneficial effect of BM may either involve suppression of iNOS or attenuation of pro-inflammatory cytokine expression 32.
The present study also clarifies that nicotine treated rats significantly decreased the GPx activity in cerebellar tissue. The lowered GPx activity may impair protection against ROS, causing damage to fatty acids, proteins or DNA and can suggest an inhibitory action of nicotine on anti-oxidant enzyme in brain and brain neurons which are sensitive to oxidative stress 33. This oxidative stress leads to an imbalance between oxidation/reduction reactions (redox state) within the cells, which cause a progressive loss in cellular functions. In the present study, alterations in the GPx activity in cerebellum tissue due to Nicotine were restored to a great extent on oral administration of BM. It is traditionally well-known anti-oxidant and controls the expression of enzymes involved in generation and scavenging of reactive oxygen species 34.
To substantiate the protective effect of BM in motor co-ordination, balance, locomotion and anxiety related behavior in nicotine treated rats, Elevated Plus maze (EPM), Open Field test (OFT) and Beam Walking Test (BWT) was performed.
The EPM is relatively simple behavioral assessment method which was developed for the investigation of quantifying anxiety related behaviors in rodents 20. Many of the available data indicate that nicotine increases the neuropsychiatric symptoms such as anxiety 29,35.
The results of EPM experiment showed that the frequency and duration in open arm and center in nicotine treated rats were lower than those of the other groups, whereas freezing time and time spent in enclosed arm were higher in nicotine treated rats than those of the other groups. These alterations in nicotine treated rats (i.e. Decreased frequency and duration in open arm and center and increased freezing time and time spent in enclosed arm) indicate anxiogenic like effect for the drug in EPM. The pathogenesis of anxiety may be associated either with alteration in free radicals or activation of nicotinic-acetylcholine receptors by nicotine which enhances the release of many neurotransmitters such as dopamine, noradrenalin, glutamate and 5-HT involved in anxiety 36. The obtained results are in line with the previous report showing that nicotine exerted an anxiogenic response 29,35,37. Our study is contradictory to the study done by Budzynska B.et al. and Benwell ME et.al. 133,38. It is interesting to note that BM abolished these alterations caused by nicotine treatment; pointing anxiolytic impact of BM against nicotine treated rats. The study demonstrates for the first time the anxiolytic effect of BM in nicotine induced rats.
To assess the locomotion, exploratory and also anxiety related behavior, we performed OFT. Animal behaviors such as frequency of line crosses, frequency of rearing, central square entries and latency in the central square are used as measures of locomotion and exploration and vice-versa 39.
In the present study, frequency of square line crossed, rearing and wall rearing and also time spent in center were significantly reduced in nicotine-treated rats and also inactive for longer duration of time, which illustrates nicotine exposed rats decreases the locomotion. The decrease in locomotion could be due to alteration in neuronal motor function or anxiety or reduction in body-weight which may decrease the muscle strength or inactiveness 40. However, our study agrees with the results obtained by Omotoso GO et.al. 7. On contrary to the studies conducted by Clarke and Kumar where they stated that chronic exposure of Nicotine increases the locomotor activity 41. This non-consistent observation may be because of the doses and/or duration of nicotine exposure.
In addition, we also observed nicotine induced rats significantly reduces the rearing frequency and duration in EPM and OFT, which could be due to impairing motor coordination and balance 42. The present study is in agreement with Iñiguez SD et. al. He described intraperitoneal administration of nicotine decreased the frequency of rearing in adult rats 43. Furthermore, we evaluated the grooming activity and found that number and duration of grooming was significantly decreased in nicotine treated rats. This alteration in grooming might be related to dysfunction in serotonergic, glutamatergic and dopaminergic neurotransmission within corticostriatal circuitry 42. Interestingly, co-exposure of BM along with nicotine significantly improves the behavioral activities altered due to nicotine administration as evidenced in EPM and OFT, establishing its neuropharmacological impact on the nicotine.
The BWT requires non-paretic paws and unimpaired inter-limb organization for maintaining the balance and motor coordination to traverse a narrow beam 21. The present result showed that nicotine exposed rat took longer time to traverse the beam and also increased number of foot-slips and near-falls than all other groups. The abnormal performances (longer duration to cross the beam and increased frequency of near-fall and actual fall) in BWT indicate impaired motor coordination and balance in nicotine treated rats signifying cerebellar dysfunction due to their disability to integrate sensory input with appropriate motor action to balance posture and at the same time adjust the limb movements on the beam 43. Further, inability to judge distance and timing, loss of motor movement is also an indicative of cerebellar impairment 44.
Conversely, the treatment with BM considerably reduced the time of crossing and number of foot-slips and near-fall against nicotine treated rats. This strongly suggests that BM improved motor coordination and balance possibly due to improving oxidative stress and oxidative stress and neurotransmitters. This is the first study exhibited on the effect of BM against nicotine in motor coordination and balance in BWT.
Conclusion
The current study investigated that BM-supplementation prevents body weight loss caused by nicotine exposure. The findings of our study showed that BM treatment possesses a potential to control levels of oxidative stress markers and thus prevent the oxidative damage and neurotoxicity caused by nicotine administration. The data also indicate that impairment in motor coordination and balance, reduction in locomotor activities and high level of anxiety was observed in nicotine treated rats which were improved by oral administration of crude form of BM. Therefore, based on the present evidence, we conclude that BM has protective effect against nicotine on the cerebellum of rat brain. Further to draw a line in conclusion, Molecular studies may confirm whether BM administration prevents the structural degeneration of cerebellum in nicotine treated rats.
Acknowledgment
Our sincere thanks to Dr. Sarala N. Prof., Department of Pharmacology and animal house facility In-charge, Sri Devaraj Urs Medical College, Kolar, Karnataka, India for providing animal house to perform the study. We also thank Mr. Ravi Shankar, Biostatistician for his help.
Conflict of interest
Authors have no conflicts of interest to declare.
Funding Source
There is no funding source.
References
- Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA et.al. Current Cigarette Smoking Among Adults – United States, 2016. MMWR Morb Mortal Wkly Rep. 2018; 67(2):53-59.
CrossRef - Jain A, Dwivedi N, Bhargava R, Flora SJ. Silymarin and naringenin protects nicotine induced oxidative stress in young rats. Oxidants and Antioxidants in Medical Science. 2012; 1(1):41-49.
CrossRef - Sonnen JA, Larson EB, Gray SL, Wilson A, Kohama SG, Crane PK et.al. Free radical damage to cerebral cortex in Alzheimer’s disease, microvascular brain injury and smoking. Ann Neurol. 2009; 65(2):226-229.
CrossRef - Maritz GS, Mutemwa M. Tobacco smoking: patterns, health consequences for adults and the long-term health of the offspring. Glob J Health Sci. 2012; 4(4):62-75.
CrossRef - Mosbah R, Yousef MI, Mantovani A. Nicotine-induced reproductive toxicity, oxidative damage, histological changes and haematotoxicity in male rats: the protective effects of green tea extract. Exp Toxicol Pathol. 2015; 67(3):253-259.
CrossRef - Sutherland MT, Riedel MC, Flannery JS, Yanes JA, Fox PT, Stein EA et.al. Chronic cigarette smoking is linked with structural alterations in brain regions showing acute nicotinic drug-induced functional modulations. Behav Brain Funct. 2016; 12(1):1-5.
CrossRef - Omotoso GO, Gbadamosi IT, Olajide OJ, Dada-Habeeb SO, Arogundade TT, Yawson EO. Moringa oleifera phytochemicals protect the brain against experimental nicotine-induced neurobehavioral disturbances and cerebellar degeneration. Pathophysiology. 2018; 25(1):57-62.
CrossRef - Shen Z, Huang P, Wang C, Qian W, Yang Y, Zhang M. Cerebellar Gray Matter Reductions Associate With Decreased Functional Connectivity in Nicotine-Dependent Individuals. Nicotine Tob Res. 2018; 20(4):440-447.
CrossRef - Hayase T. Differential effects of TRPV1 receptor ligands against nicotine-induced depression-like behaviors. BMC Pharmacol. 2011;11:1-11.
CrossRef - Pereira M, Strupp T, Holzleitner T. Smoking correlation of nicotine induced nystagmus & postural disbalances. Neuroreport. 2001; 8:1223-1226.
CrossRef - Chen WJ, Edwards RB, Romero RD, Parnell SE, Monk RJ. Long-term nicotine exposure reduces Purkinje cell number in the adult rat cerebellar vermis. Neurotoxicol Teratol. 2003; 25(3):329-334.
CrossRef - Tewari A, Hasan M, Sahai A, Sharma PK, Rani A, Agarwal AK. White core of cerebellum in nicotine treated rats-a histological study. Journal of Anatomical Society of India. 2010; 59(2):150-153.
CrossRef - Sudheer AR, Muthukumaran S, Devipriya N, Menon VP. Ellagic acid, a natural polyphenol protects rat peripheral blood lymphocytes against nicotine-induced cellular and DNA damage in vitro: with the comparison of N-acetylcysteine. Toxicology. 2007; 230(1):11-21.
CrossRef - Sirasanagandla SR, Rooben RK, Rajkumar, Narayanan SN, Jetti R. Ascorbic Acid ameliorates nicotine exposure induced impaired spatial memory performances in rats. West Indian Med J. 2014; 63(4):318-324.
CrossRef - Sukumaran NP, Amalraj A, Gopi S. Neuropharmacological and cognitive effects of Bacopa monnieri (L.) Wettst – A review on its mechanistic aspects. Complement Ther Med. 2019; 44:68-82.
CrossRef - Paulose CS, Chathu F, Khan SR, Krishnakumar A. Neuroprotective role of Bacopa monnieri extract in epilepsy and effect of glucose supplementation during hypoxia: glutamate receptor gene expression. Neurochem Res. 2008; 33(9):1663-1671.
CrossRef - Rastogi M, Ojha RP, Prabu PC, Devi BP, Agrawal A, Dubey GP. Prevention of age-associated neurodegeneration and promotion of healthy brain ageing in female Wistar rats by long term use of bacosides. 2012; 13(2):183-195.
CrossRef - Reas SK, Amee K, Paulose CS. Glutamate receptor gene expression and binding studies in pilocarpine induced epileptic rat: neuroprotective role of Bacopa monnieri extract. Epilep Behav. 2008; 12:54-60.
CrossRef - Arika WM, Kibiti CM, Njagi JM, Ngugi MP. Effects of DCM Leaf Extract of Gnidia glauca(Fresen) on Locomotor Activity, Anxiety, and Exploration-Like Behaviors in High-Fat Diet-Induced Obese Rats. Behav Neurol. 2019;2019:1-15
CrossRef - Donatti AF, Soriano RN, Leite-Panissi CR, Branco LG, de Souza AS. Anxiolytic-like effect of hydrogen sulfide (H2S) in rats exposed and re-exposed to the elevated plus-maze and open field tests. Neurosci Lett. 2017; 642:77-85.
CrossRef - Silveira EMS, Kroth A, Santos MCQ, Silva TCB, Silveira D, Riffel APK et.al. Age-related changes and effects of regular low-intensity exercise on gait, balance, and oxidative biomarkers in the spinal cord of Wistar rats. Braz J Med Biol Res. 2019; 52(7):1-13.
CrossRef - Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2):351-358.
CrossRef - Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem. 1982; 126(1):131-138.
CrossRef - Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973; 179(4073):588-590.
CrossRef - Grebenstein PE, Thompson IE, Rowland NE. The effects of extended intravenous nicotine administration on body weight and meal patterns in male Sprague-Dawley rats. Psychopharmacology (Berl). 2013; 228(3):359-366.
CrossRef - Omotoso GO, Babalola FA. Histological changes in the cerebelli of adult wistar rats exposed to cigarette smoke. Niger J Physiol Sci. 2014; 29(1):43-46.
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004; 198(2):132-151.
CrossRef - Saad AB, Rjeibi I, Brahmi N, Elaloui E, Zouari N. Nicotine-induced oxidative stress, testis injury, AChE inhibition and brain damage alleviated by Mentha spicata. Inflammopharmacology. 2020; 28(4):939-948.
CrossRef - Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Kurzepa J, Biala G. Mephedrone and nicotine: oxidative stress and behavioral interactions in animal models. Neurochem Res. 2015; 40(5):1083-1093.
CrossRef - Das S, Gautam N, Dey SK, Maiti T, Roy S. Oxidative stress in the brain of nicotine-induced toxicity: protective role of Andrographis paniculata Nees and vitamin E. Appl Physiol Nutr Metab. 2009; 34(2):124-135.
CrossRef - Chang WC, Lee YC, Liu CL, Hsu JD, Wang HC, Chen CC et.al. Increased expression of iNOS and c-fos via regulation of protein tyrosine phosphorylation and MEK1/ERK2 proteins in terminal bronchiole lesions in the lungs of rats exposed to cigarette smoke. Arch Toxicol. 2001;75:28–35.
CrossRef - Saini N, Singh D, Sandhir R. Bacopa monnieri prevents colchicine-induced dementia by anti-inflammatory action. Metab Brain Dis. 2019; 34(2):505-518.
CrossRef - Budzynska B, Boguszewska-Czubara A, Kruk-Slomka M, Skalicka-Wozniak K, Michalak A, Musik I et.al. Effects of imperatorin on nicotine-induced anxiety- and memory-related responses and oxidative stress in mice. Physiol Behav. 2013; 122:46-55.
CrossRef - Kapoor R, Srivastava S, Kakkar P. Bacopa monnieri modulates antioxidant responses in brain and kidney of diabetic rats. Environ Toxicol Pharmacol. 2009; 27(1):62-69.
CrossRef - Raeeszadeh M, Mortazavi P, Atashin-Sadafi R. The Antioxidant, Anti-Inflammatory, Pathological, and Behavioural Effects of Medicago sativa L. (Alfalfa) Extract on Brain Injury Caused by Nicotine in Male Rats. Evid Based Complement Alternat Med. 2021; 2021:1-9.
CrossRef - Wonnacott S. Presynaptic nicotinic ACh receptors. Trends Neurosci. 1997; 20(2):92-98.
CrossRef - Zarrindast MR, Aghamohammadi-Sereshki A, Rezayof A, Rostami P. Nicotine-induced anxiogenic-like behaviours of rats in the elevated plus-maze: possible role of NMDA receptors of the central amygdala. J Psychopharmacol. 2012; 26(4):555-563.
CrossRef - Benwell ME, Balfour DJ, Khadra LF. Studies on the influence of nicotine infusions on mesolimbic dopamine and locomotor responses to nicotine. Clin Investig. 1994; 72(3):233-239.
CrossRef - Davies KG, Ekpennyong C, Green O, Antai A, Osim E. Locomotor and exploratory behaviour in mice treated with oral artesunate. British Journal of Science. 2013; 8(1):47-57.
CrossRef - Venero C, Guadaño-Ferraz A, Herrero AI, Nordström K, Manzano J, de Escobar GM et.al. Anxiety, memory impairment, and locomotor dysfunction caused by a mutant thyroid hormone receptor alpha1 can be ameliorated by T3 treatment. Genes Dev. 2005; 19(18):2152-63.
CrossRef - Clarke PB, Kumar R. The effects of nicotine on locomotor activity in non-tolerant and tolerant rats. Br J Pharmacol. 1983; 78(2):329-337.
CrossRef - Bandiera S, Caletti G, Giustina CLD, Hansen AW, Deniz BF, Confortim HD et.al. Changes in behavioral and neuronal parameters by alcohol, cigarette, or their combined use in rats. Behav Pharmacol. 2019; 30(6):490-499.
CrossRef - Iñiguez SD, Warren BL, Parise EM, Alcantara LF, Schuh B, Maffeo ML et. al. Nicotine exposure during adolescence induces a depression-like state in adulthood. Neuropsychopharmacology. 2009; 34(6):1609-1624.
CrossRef - Krishnakumar A, Nandhu MS, Paulose CS. Upregulation of 5-HT2C receptors in hippocampus of pilocarpine-induced epileptic rats: antagonism by Bacopa monnieri. Epilepsy Behav. 2009; 16(2):225-230.
CrossRef








