Manuscript accepted on :28-03-2022
Published online on: 06-05-2022
Plagiarism Check: Yes
Reviewed by: Dr. Amit Kumar Tripathi
Second Review by: Dr. Salman Ahmed Pharmacognosy
Final Approval by: Dr. Ian James Martin
Sarvan Kumar Guguloth1, 2 , Narender Malothu1*
, Narender Malothu1* , Sunil Kumar Kadiri 3
, Sunil Kumar Kadiri 3 and Sowjanya Kunuru4
and Sowjanya Kunuru4
1Department of Pharmaceutical Chemistry, KL College of Pharmacy, Koneru Lakshmaiah Education Foundation, Vaddeswaram, Guntur, AP; India
2Department of Pharmacology, Sri Venkateswara College of Pharmacy, 86, Hi-tech City road, Madhapur, Hyderabad, India.
3Department of Pharmacology, Marri Laxman Reddy Institute of Pharmacy, Dundigal, Hyderabad, India
4Department of Pharmacology, Vijaya College of Pharmacy, Hyathnagar, Hyderabad, India.
Corresponding Author E-mail: narendermalothu@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2420
Abstract
Since, ancient time medicinal plants have been using to treat various human ailments. Blood clotting causes various heart associated diseases like myocardial infarction, deep vein thrombosis and renal vein thrombosis. Thrombolytic drugs are being employed to lyse the thrombus (blood clot) formed in the arteries. The modern systems of medicines have certain limitations and serious consequences which can alter the normal hemostasis process. Herbal medicines are known for their safety and efficacy in treating diseases effectively without producing any untoward effects. The present review provides the medicinal importance of some plants as thrombolytic agents. The source for the present review was taken from literature survey carried through the findings from suitable keywords in databases, PubMed, Google Scholar and Web of Science and Scopus, etc. This review provides the detailed information on medicinal plants and phytochemical compounds as thrombolytic agents.
Keywords
Efficacy; Herbal Plants; Hemostasis; Thrombolytic Activity; Thrombosis
Download this article as:| Copy the following to cite this article: Guguloth S. K, Malothu N, Kadiri S. K, Kunuru S. Thrombolytic Property of Herbal Plants: A Short Review. Biomed Pharmacol J 2022;15(2). |
| Copy the following to cite this URL: Guguloth S. K, Malothu N, Kadiri S. K, Kunuru S. Thrombolytic Property of Herbal Plants: A Short Review. Biomed Pharmacol J 2022;15(2). Available from: https://bit.ly/3LRQFpO |
Introduction
Hemostasis means regulating the blood loss consequent to bleeding. It should not confuse with similar word homeostasis which means maintenance of the body’s internal environment within physiological limits. In the blood vessels, the blood normally circulates in a fluid state. But if it is drawn from the body, it thickens and forms a gel called a clot. The process is called clotting or coagulation. Natural human circulatory or blood vessel systems are presented with two contending qualification to confirm the feasibility of the entity or organism: tissue intromission must be conserve at any movement, and the bleeding must be rapidly ceased at sites of blood vessel injury1. Cardiovascular illness caused by formation of thrombus is one of the most dangerous conditions which is rapidly increasing at an alarming rate in current times2. Hemostasis and fibrinolysis represent the important dynamics for stopaging the free flow of blood at sites of damage or injury and reinstate blood vessel capacity or potency during process of recovery of wound or healing correspondingly. The term hemostasis or coagulation refers to the transformation of dispersible fibrinogen into insoluble fibrin. This technique occurs by step by step process of enhancing the enzymatic activation in which the formation of an inactive state of plasma proteins into an active protease (serine) product3. Thrombin serves as the central serine protease of the coagulation cascade4. Blood clot formation is a censorious step in the vascular related diseases which includes myocardial infarction, hypoxia (anoxia), hypertension5, sudden heart blockage and infarction disorders that stand for a considerable number of mortality in Worldwide6.Intense research activity in the antithrombotic field was devoted to compounds showing antiaggregatory potency during the last three decades of the 20th century. Among the huge number of synthetic molecules tested, only very few of them found in clinical use (Dupinet al., 2002). Intravenous tissue plasminogen activator infusion is the fastest way to initiate thrombolytic therapy; however, poor recovery can be expected in up to 50% of patients (Alexandrov et al., 2001). Tissue plasminogen activator delivery to the thrombus is dependent on the residual flow to and around the arterial obstruction when given intravenously (Alexandrov, 2004). Though, thrombolytics which includes aspirin, warfarin, antiplatelet (Clopidogrel) and heparin have shown efficacy and are widely dispensed medications in many cardiovascular and cerebrovascular diseases, the main adverse effects of use of these medications were an increased incidence of over bleeding(Bond et al., 2005). The recent epidemiological status from WHO has given the statement that ischemic related heart disease and sudden heart blockage alone caused 9.8 million deaths Worldwide. Cerebrovascular stroke is the stoppage of blood to some areas of the brain leads to brain dysfunction.
Clotting (coagulation) occurs through a process of a series of reactions and thrombin is the last enzyme that plays a vital role in the coagulation cascade which converts fibrinogen into insoluble fibrin (Fig 1)7. There are thirteen factors are involving in the coagulation and thrombin activates factor-XIII, enhance the formation of factor-V, which increases the thrombin formation and stimulates thrombocytes (platelets), and cause platelets aggregation8. Platelets respond to injury of blood vessels by first adhering to the vascular endothelial matrix as a single layer of cells. Throughout of lifetime in blood circulation, were exposed to a variety of external stimuli that control and regulate their state of activation9. The adhesion of platelets to the matrix is mediated by von Willebrand factor, a plasma glycoprotein that links platelets through their specific proteins (called surface glycoprotein) IX/lb receptors to collagen, by direct association with collagen through their surface glycoprotein Ia/IIa receptors. The primary extracellular molecule that increases platelet cAMP is prostaglandin I210.
Animal survivallance on this earth has been made acceptable only because of the important role played by plants. Medicinal plants are the plants or their parts used for the protecting the health care of an individual as they probably contains a large number of functional groups which shows therapeutic activity against various dysfunctions of organs or tissues or systems in the body. The future of the plants as sources of medicinal agents for use in investigation, prevention, and treatment of diseases is very promising. Herbal preparations contain a wide variety of chemical constituents like tannins, salicylate, and coumarins and proved as clot lysis or thrombolytic agents. Therefore herbal preparations would be used in the place of synthetic drugs to avoid unwanted effects.
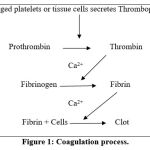 |
Figure 1: Coagulation process. |
The present scenario in thrombolytic therapy
To get cleanse of problems developed due to the formation of a clot in the blood or thrombus to remodify function to the affected area, fibrinolytic therapy is required11. The agent which is having the thrombolytic activity also known as clot busters which shows long term benefits for survivors, who have just a 5 % death rate at one year12. Fibrinolytic substance is being used in blood vessels or venous thrombosis, pulmonary (lung) embolism, myocardial infarction formation of clot in the arteries (arterial thromboembolism), and acute ischemic strokeetc13.
Contraindications for thrombolytic therapy
Thrombolytics were predominantly safe and effective in correcting thrombosis, but excessbleeding (hemorrhage) is the major adverse effect. The current epidemiological data stated that 11% of all diseased persons who were administered thrombolytics have presented adverse effects like hemorrhage. About 0.3-1.3% had significant adverse drug reactions like intracranial hemorrhage. These drugs are contraindicated in vascular lesions, severe uncontrolled hypertension, brain tumor, peptic ulcer and pregnancy.
Methodology
Published articles in connection with thrombolytic activity of various herbal plants were retrieved from PubMed, Science Direct, Taylor and Francis, BMC, ACS, Google scholar, Web of Science, Scopus and other literature database. Some articles were found by tracking citations from publications. The research key words used were thrombolytic activity and herbal plants. In this review briefly discussed the recent scientific findings regarding the thrombolytic activities of various herbal plants and indicatedtheareas where further study is required. The literature collections were restricted to publications in English language.
Medicinal Plants Having Thrombolytic Activity
Various studies have been reported the thrombolytic properties of plant parts and their isolated forms of few plants (Table 1). In the present review the scientific data of each reported medicinal plants were presented hereunder.
Table 1: List of medicinal plants having active constituents responsible for thrombolytic activity.
| S. No | Plant name | Family | Active constituents | Reference |
| 1 | Cyamopsistetragonoloba | Fabaceae, | Gum residues, saponins and polyphenol | 14 |
| 2 | Pulmonaria officinalis | Boraginaceae | Flavonoids, vitamins like C and B, Al+, Mn+, nickel and Fe+2 | 15 |
| 3 | Petroselinum
crispum |
Apiaceae | Flavonoids, dihydroxycoumarin, apiin, and apigenin | 15 |
| 4 | Tridax procumbens L. | Asteraceae | Taraxasterylacetate, lupeol, oleonic acids. | 15 |
| 5 | Paeonia anomala | Paeoniaceae | Acid like ellagic, Acetophenone, t-butylhydroperoxide, methyl and ethyl gallate, quercetin, fischeroside B | 15 |
| 6 | Ferula communisL. | Apiaceae | Ferulenol, fertdin,anisate, oxajaeskeanadioyl and costic acid | 15 |
| 7
|
Panax notoginseng | Araliaceae
|
Ginsenoside Rh4, Rh1, notoginsenoside S, notoginsenoside T ginsenoside Re | 15
|
| 8
|
Cinnamomum cassia
|
Lauraceae.
|
Derivatives of coumarin, cinnamyl acetate, hydroxyl cinnamaldehyde, and N-acetyl-l-cysteine | 16
|
| 9
|
Filipendula
ulmaria |
Rosaceae | Flavonoids, tannins and vitamins, tannins, polyphenol, ellagitannins, phenolic acids, methyl gallate | 17 |
Camellia sinensis
C. sinensis belongs to the Theaceae family. C. sinensis is commonly known as green tea18. The herb is resident of the Asian and Southeast region for many years. C. sinensis is being widely consuming in countries like India, North Africa, North East Asia and China and Pakistan19. C. sinensis has been proved to have a wide variety of useful pharmacological and physiological actions20. Leaves were used as probiotic activities21. Warfarin effect is reduces in persons taking an excessive concentration of C. sinensis and also found hemorrhage development. In vitro thrombolytic potential C. sinensis reported that about 95.24% and 90.34% of clot lysis observed for methanolic extract and aqueous extract, respectively at 800 µg/mL concentration in dose dependent manner22.
Allium sativum
A. sativum (garlic) represent the Amaryllidaceae family. A.sativum bulb is used in the conditions like hyperlipidaemia and heart related diseases. It blocks thromboxane formation and counteracts with thrombocytes functions and also reduces platelet aggregation. In addition, the plant has fewer complications like postoperative bleeding and epidural hematoma. Scientific studies, advises the avoidance of the supplements of garlic preparation with warfarin therapy, as this combination will causes serious complications23. Anti-coagulant property of garlic extract was achieved by preventing or prolonging the time required for clot formation thereby alters the coagulation mechanism24. Ethanolic extract at 10 µg/mL dose displayed the potential fibrinolytic activity and time factors exposure was influenced25.
Terminalia bellerica
It belongs to the Combretaceae family. It is one of the compositions of well-established preparation known as “Triphala” which is being using in a wide variety of pharmacological and physiological actions like in hair care, as a laxative, leucorrhoea, liver diseases and gastrointestinal disorders. Fruits are being reported as anticoagulant and thrombolytic activity26. In addition it also acts as anthelmintic27, 28 antidepressants29, antianxiety30 and antiplasmodialactivity31. In vitro thrombolytic activity of methanolic leaf extract showed moderate amount of (32.95%) of clot lysis when compared with standard streptokinase.
Typha angustifolia
T. angustifolia belongs to the family of Typhaceae and it is commonly known as elephant grass or cattail. Various parts of the plant are edible; including dormant sprouts on the roots and bases of the leaves, ripen pollens, the stem, and the starchy roots32, 33. The medicinal use of the plant includes treatment of renal calculi34, uncontrolled bleeding in uterus, abscesses, infection with tapeworm, diarrohea, and dysentery. Modern research on pollen grains of angustifolia showed that presence of sterols, terpinoids, flavonoids and glycosides35 and these secondary active metabolites possess various pharmacological activities like immune suppression36, antiplatelet aggregation37, anti microbial38 and lipid lowering property39. In vitro fibrinolytic activity of methanolic leaf extract T. angustifolia showed 58±2.32 % of clot lysis 40.
Zingiber officinale
Z. officinale belongs to the family of Zingiberaceae. Roots used in the treatment of motion sickness disorder and rheumatism. It inhibits the thromboxane synthetase and thereby reduces the platelets aggregation. Excess intake of ginger in the diet may cause bleeding with warfarin41. The methanolic extracts of Z. officinale showed moderate thrombolytic activity (30.13% clot lysis) at test concentration of 10 mg/mL 42.
Salvia miltiorrhiza
This S. miltiorrhiza belongs to the Lamiaceae family. Rhizomes possess variouspharmacological and physiological actions. It shows beneficial pharmacological uses in the various circulatory disorders. The complications are gastric cancer, bleeding and pleural hemorrhage with warfarin. Previous studies suggested that blood clotting mechanism is altered by various factors like enhancement of fibrinolytic efficacy, promotion of antithrombin III like action and inhibition of platelet aggregation43. A purified extract of this plant containing its major constituents, cryptotanshinone, tanshinone I, and tanshinone IIA can protect against liver toxicity in vivo and in vitro44. S. Miltiorrhiza was shown to possess unique efficacy in treating thromboangiitis obliterans45.
Wedelia trilobata L.
Is a flowering plant belongs to the family of Asteraceae. It is used to treat hepatitis infections and to reduce the menstrual pain and unspecified female complaints46.The plant has various medicinal activities, such as antidiabetic, antimicrobial, antitumor, liver protectant, and CNS depressant47.The reports indicated the maximum blood clot lysis (57.89%) effects of leaf extracts of W. trilobata 48.
Triclisia dictyophylla
T. dictyophylla belongs to the family of Menispermaceae. The aqueous root extract of T. dictyophylla showed anti-platelet activity. The aqueous root extract of T. dictyophylla prolonged the in vitro whole human blood clotting time at concentrations of 50 and 100 mg/mL, complete anticoagulation effect was achieved 49, 50.
Aporosa wallichii Hook. f
This plant belongs to Th Phyllanthaceae family. This study showed that this plant has an acceptable level of the free radical scavenging property and thrombolytic property along with cytotoxic activity. In a thrombolytic model the methanolic extract exhibited 24.5% of clot lysis at 100 µL51.
Melissa officinalis
It is belongs to the family of Lamiaceae is a perennial aromatic herb, it is generally applied for the preparations of food and cosmetics related products. An in vitro thrombolytic model of methanolic extracts of leaves and stem showed 25.87±1.089, 41.482 ± 0.948 % clot lysis, respectively 52.
Enhydra fluctuans Lour (Leaves)
E. fluctuans Lour belongs to the family Asteraceae is a small genus of marsh herb, available in tropical and subtropical regions. Leaves of the plant are used as a laxative and also used in the treatment of the skin and nervous system. The phytochemical analysis displayed that the crude extract contains tannin, alkaloid, and saponins; it could be predicted that these phytochemicals may be responsible for its clot lysis activity. An in vitro thrombolytic model of methanolic extract showed 31.25% of clot lysis 53.
Luffa cylindrica linn
L. cylindrica Linn belongs to the family of Cucurbitaceae. Originally the literature report revealed the folk claims of L. cylindrica. The plant is having a potent thrombolytic property which is beneficial in treating heart attacks and pulmonary embolism and atherothrombosis. In an in vitro study the ethanolic extract showed 45% of clot lysis54.
Angelica sinensis
A. sinensis is herbal plant belongs to family Apiaceae. Primarily it is used in menses in females. The plant also exhibits a fibrinolytic activity by blocking the activation and aggregation of platelets.A study on rabbits revealed that prothrombin time was increased after its consumption55.
Wedelia chinensis (Osbeck) Merr
The family of W. chinensis (Osbeck) Merr is Asteraceae. It is also known as ChineseWedelia and herb of sunflower. Fruits, stems and leaves possess medicinal importance 56. The plant leaves possess pharmacological activities in the conditions of kidney dysfunction, cold, wounds, and amenorrhea. The other medicinal uses are treatment of wounds, seminal weakness, astringent, bitter, acrid, anti-inflammatory and cardio protection and hepatitis57. The methanolic leaf extract of W.chinensis (osbeck) Merr showed significant clot lysis 24.48 % at 100 µg/mL concentration with reference to streptokinase as standard 58.
Vigna unguiculata Linn (seed)
V. unguiculata Linn is a herbal plant of Fabaceae family. It possesses a wide variety of physiological and pharmacological properties. Few reports suggest that methanolic extract of V. unguiculata Linn (seed) showed significant thrombolytic activity with 40.33% of clot lysis at 10 mg/mL concentration59.
Punica granatum
Punica granatum belongs to the family of Lythraceae. Pomegranate fruits has been considered as a healing food owing to its vast advantageous biological effects against several diseases since ancient times. It is widely used in the preparation of ayurvedic blood tonic 60. Ethanolic and aqueous extract of fruit and peel of plant was used for in vitro thrombolytic activity by using streptokinase as standard. Ethanolic extract of fruit and peel showed moderate amount of in vitro clot lysis (59%) at 100 µg/mL concentration.
Murraya koenigii
It belongs to the family of Rutaceae. Leaves and roots are medicinally useful. Leaves possess promising thrombolytic property. In an in vitro thrombolytic model of an aqueous extract exhibited the remarkable clot lysis (22.14 %) effect at a concentration of 10 mg/mL61.
On extensive literature search, the reports suggest majorly the plant constituents have possessed their antithrombotic properties through prolonging the prothrombin time or by preventing the platelet aggregations. As per literature information’s few other antithrombotic plant sources with their probable mode of actions are depicted in the Table 2.
Table 2: Plants proved as thrombolytic agents and their mode of action
| Plant name | Family | Part used | Functions | Mode of action | Reference |
| Cyamopsis tetragonoloba | Fabaceae, | Seeds | Thrombolytics | Increases prothrombin time | 62 |
| Panax notoginseng | Araliaceae | Roots | Thrombolytics | Increases prothrombin time | 63 |
| Ferula communisL. | Apiaceae | Leaves | Thrombolytics | Increasing the clotting time | 64 |
| Paeonia anomala | Paeoniaceae | Fruits and roots | Anti coagulants | Antithrombotic and thrombolytic | 65 |
| Filipendula ulmaria | Rosaceae | Flowers | Thrombolytics | Antithrombotic and thrombolytic | 66 |
| Pulmonaria officinalis | Boraginaceae | Aerial parts | Anticoagulants | Antithrombotic and thrombolytic | 67 |
| Cinnamomum cassia | Lauraceae. | Bark | Thrombolytics | Antithrombotic and thrombolytic | 68 |
| Petroselinum crispum | Apiaceae | Aerial parts | Thrombolytics | Antithrombotic and thrombolytic | 69 |
| Careya arborea | Lecythidaceae | Bark | Thrombolytic | Prolongs the thrombin time by decreasing the clotting factors | 70, 71 |
| Terminalia bellerica | Combretaceae | Fruit | Thrombolytic | Antithrombotic and thrombolytic | 72 |
| Allium cepa | Amaryllidaceae | Bulb | Thrombolytic | Prolongs prothrombin time | 73 |
| Molineria recurpata | Hypoxidaceae | Leaves | Antithrombotic | Increases the prothrombin time | 74 |
| Curcuma longa | Zingiberaceae | Root and rhizome | Antithrombotic | Suppresses the ability of platelet adhesion | 75 |
Conclusion
This review helps to understand the effect of various medicinal plants having thrombolytic activity. The accessibility of treating the thrombotic diseases with herbal medicine or plant derived compounds and not necessity of laborious pharmaceuticals synthesis seems that the approach is highly attractive. As per the presented data, many literature studies offered the thrombolytic importance of crude and/or solvent extracts of the various plants parts. Though the thrombolytic property was proved in these investigations, further much attention and an extensive study is required in exploring the phytochemical and pharmacological profile of the active principles. It will be helpful to the pharmacologists, scientists and health professionals to develop new safer pharmaceutical products with thrombolytic properties.
Acknowledgements
The authors are thankful to Sri Venkateswara College of Pharmacy, 86-high tech city road, Madhapur, and KL College of Pharmacy, KoneruLakshmaiah Education Foundation, Vaddeswaram, Guntur, Andhra Pradesh for providing facilities and support.
Conflict of interest
The authors declare that there is no conflict of interest.
Funding Sources
There is no funding source.
References
- Rajeswari S, Vidhya R. Evaluation of in vitro thrombolytic and antiproteinase activities of Wedelia Trilobata (Linn.). Innov J Life Sci.2017; 5(3): 6-9.
- Sandeep YS, Panigrahi M, Divya GC, Beena DB. Evaluation of in vitro thrombolytic activity of phytochemicals in Bacopamonnieri J Pharm Res. 2012; 5(1): 100-10.
- Furie B, FurieBC. Molecular and cellular biology of blood coagulation. N Engl J Med.1992; 326(12): 800-6.
CrossRef - Coughlin SR. How the protease thrombin talks to cells. Proc Natl AcadSci. USA.1999; 28(96):11023-7.
CrossRef - Sultana I, Noor MA, Barua J, Mahmood A, Das MC, Ibrahim MM et al. In vitro anti-atherothrombosis activity of four Bangladesh plants. Int J Green Pharm. 2012; 6(1): 5-8.
CrossRef - Furie B and Furie BC. Mechanisms of thrombus formation. N Engl J Med. 2008; 359(9): 938-49.
CrossRef - Kishore K. In vitro and in vivo screening methods for antithrombotic agents. Am J Phytomed ClinTher.2013; 1(5): 497-506.
CrossRef - Sherwani SK, Bashir A, Haider SS, Shah MA, Kazmi SU. The thrombolytic potential of aqueous and methanolic crude extracts of Camellia sinensis (Green tea): In vitro study. J Pharmacogn Phytochem.2013; 2(1): 125-29.
- Fatima T, Chadni SH, Mou KN, Imrul Hasif KM, Ahmed T, Akter M et al. In vitro thrombolytic activity and phytochemical evaluation of leaf extracts of four medicinal plants of Asteraceae J Pharmacogn phytochem.2017; 6(4): 1166-69.
- Vaseem AA, Siddiqui HH, Singh SS. Antithrombotic and thrombolytic activity of Terminalia belerica fruit extracts. ResJPharm Biol Sci. 2012; 3(2): 471-78.
- Perler B. Thrombolytic therapies: the current state of affairs. J 2005; 2(12): 224–32.
CrossRef - Delude C, Jackson C. Clot Busters!! Discovery of thrombolytic therapy for treating heart attack and stroke. FASEB J.2005; 6(19):3885–96.
CrossRef - Thripathi KD. Essentials of Medical Pharmacology.8th Jaypee brothers medical publishers.2018.
- Mestechkina NM, Shcherbukhin VD, Bannikova GE, Varlamo VP, Drozd NN. Anticoagulant activity of low-molecular-weight sulfated derivatives of galactomannanfrom Cyamopsis tetragonoloba (L.) Seeds. Appl Biochem Microbiol.2008; 44(1):111-6.
CrossRef - Akram MD, Rashid A. anti-coagulants of plants mini review. J Thromb Thrombolysis. 2017; 44(3): 406-11.
CrossRef - Rahman M, Khatun A, Islam M, Akter N , Afreen SC, Alikhan A, Shahid MS, Rahman AA. Evaluation of antimicrobial, cytotoxic, thrombolytic, diuretic properties and total phenolic content of Cinnamomum cassia. Int J Green Pharm. 2013; 7(3):236-43.
CrossRef - Naqash SY, Abdool NR. Anticoagulant, antiherpetic and antibacterial activities of sulphated polysaccharide from Indian medicinal plant Tridax procumbens (Asteraceae). Appl Biochem Biotechnol.2011; 165(3-4):902-12.
CrossRef - Sherwani SK, Khan MM, Munir S, Shah MA, Kazmi SU. The anthelmintic potential of crude extract of Camellia sinensis (Green tea).Int Res J Pharm. 2013; 4 (3):94-6.
CrossRef - Katiyar S, Mukhtar H. Tea in chemoprevention of cancer: epidemiologic and experimental studies. Int J Oncol. 1996; 8(2):221–38.
CrossRef - Hamilton JM, Miller T. Antimicrobial Properties of Tea (Camellia sinensis). Antimicrob AgentsChemother1995; 39(11): 2375–77.
CrossRef - Choi YB, Kim YI, Lee KS, Kim BS, Kim DJ. Protective effect of epigallocatechingallate on brain damage after transient middle cerebral artery occlusion in rats. Brain Res 2004; 1019(1–2):47– 54.
CrossRef - Taylor JR, Wilt VM. Probable antagonism of warfarin by green tea. Ann Pharmacother 1999; 33(4):426-8.
CrossRef - Rose KD, Croissant PD, Parliament CF, Levin MB. Spontaneous spinal epidural hematoma with associated platelet dysfunction from excessive garlic ingestion: a case report. J Neurosurg.1990; 26(5):880-2.
CrossRef - Reddy ANR, Srividya L, Swamy TP , Prasad VB. Effect of Allium sativum (Garlic) Extract on Blood Coagulation and Fibrinolysis. Adv Pharmacol Clin Trials. 2017; 2(1): 1-3.
CrossRef - Ansari F, Mohammad S, Naderi G, Sabet S, Karimi A. Study of garlic effect on fibrinolytic activity of the blood clot in vitro. Iran J Pediatr Hematol Oncol. 2021; 1(2): 48-52.
- Ansari V, Siddiqui H, Singh S. Antithrombotic and thrombolytic activity of Terminalia belerica fruit extracts. Res J Pharma Bio Chem Sci.2012; 3(2):471–78.
- Kumar B, Kalyani D, Hawal LM, Singh S. In vitro anthelmintic activity of ethanolic and aqueous fruit extract of Terminalia belerica. J Pharm Res.2010; 3(5): 1061-62.
- Kulkarni CG, Lohar BN, Jadhav ST, Salunkhe SS. Evaluation of Anti helmintic Activity of Indian Herbs. Int J of Pharmacy. 2014; 4(1): 357-62.
- Dhingra D, Valecha R. Evaluation of antidepressant-like activity of aqueous and ethanolic extracts of Terminalia bellirica Fruits in mice. Indian J Exp Biol. 2007; 45(7): 610-6.
- Chandrasekhar R, Manohar VR, Bharathi PR, Mohandas R. Attenuation of anxiety on acute administration of aqueous extract of Terminalia belerica fruit pulp in Swiss albino mice. Int J Basic Clin Pharmacol.2017; 6(2): 303-7.
CrossRef - Pinmai K, Hiriote W, Soonthornchareonnon N, Jongsakul K, Sireeratawong S, Udom TS. In vitro and in vivo anti plasmodial activity and cytotoxicity of water extracts of Phyllanthus emblica, Terminalia chebula, and Terminaliabellerica. J Med Assoc Thai. 2010; 93(12):120-6.
- Elias T. Dykeman PA. Edible Wild Plants. Sterling Publishing Co Inc. New York NY 2009.
- Hirschmann SG, Razmilic I, Gutierrez MI, Loyola JI. Proximate composition and biological activity of food plants gathered by Chilean Amerindians. Econ Botany.1999; 53:177 -87.
CrossRef - Gupta A, Mishra AK, Bansal P, Kumar S, Sannd R, Gupta V et al. Antileptotic potential of Ethanomedicinal herbs: A Review. Drug Invent. 2010; 2(3):191-3.
- Tao WW, Yang N, Dekang Wu, Guo JM, Tang Y, Qian D et al. Simultaneous determination of eleven major flavonoids in the pollen of Typha angustifolia by HPLC-PDA-MS. Phytochem Anal. 2011; 22(5): 455–61.
CrossRef - Qin F, Sun HX. Immunosuppressive activitity of Pollen Typhae ethanol extract on the immune response in mice. Ethanopharmacol. 2005; 102(3):424-9.
CrossRef - Tao WW, Yang NY, Duan JA, Kang Wu, Shang EX, Qian DW. Two new nanocosanetriols from the pollen of Typha angustifolia. Chin Chem 2010; 14(2) :209-12
CrossRef - Alexeyena V, Usha G, Suja A, Parambi BGT, Asha SJ. Phytochemical screening and antimicrobial investigation of Typha angustifolia Int J ChemSci. 2009; 7(3):1905-10.
- Zhao J, Zhang CY, Xu DM, Huang GQ, Xu YL, Wang ZY et al. The anti atherogenic effects of components isolated from pollen typhae. Thrombosis Research. 1990; 57(6):957-66.
CrossRef - Umesh MK, Sanjeev kumar CB, Hanumantappa BN, Ramesh LL. Evaluation of in vitro anti-thrombolytic activity and cytotoxicity potential of Typha Angustifolia leaves extracts. Int J Pharm Sci. 2014; 6(5): 81-5.
- Backon J. Ginger: inhibition of thromboxane synthetase and stimulation of prostacyclin: relevance for medicine and psychiatry. Med Hypotheses.1986; 20(3):271–78.
CrossRef - Manju P, Pushpa A. A study on thrombolytic and cytotoxic activity of methanolic extract of Zingiber officinale.IntJ Life Sci Pharma Res. 2020;10 (5): 1-5.
CrossRef - Chan T. Interaction between warfarin and danshen (Salvia miltiorrhiza). Ann Pharmacother 2001; 35(4):501-4.
CrossRef - Park EJ, Zhao YZ, Kim YC, Sohn DH. Preventive effects of a purified extract isolated from Salvia miltiorrhiza enriched with tanshinone I, tanshinone IIA and cryptotanshinone on hepatocytes injury in vitro and in vivo. Food Chem Toxicol. 2009; 47(11):2742-48.
CrossRef - Zhang BM. Clinical efficacy observation of Salvia Miltiorrhiza Bge.f.alba on thromboangiitis obliterans in 113 cases. J Shandong Uni TCM. 1993; 3: 40-1.
- Lans C. Ethnomedicines used in Trinidad and Tobago for reproductive problems. J Ethnobiol Ethnomedicine. 2007; 15: 3-13.
CrossRef - Wu ML, Zhang DZ. Progress of researches on the invasive plant Wedelia trilobata. Pharm Today. 2008; 6:21-3.
- Rajeswari S, Vidhya R. Evaluation of Invitro thrombolytic and antiproteinase activities of Wedelia trilobata (Linn.). Innov J Life Sci. 2017; 5(3): 6-9.
- Azikiwe CC, Amazu LU, Unekwe PC, Chilaka KC, Afonne AJ, Nwose PJ et al. Anticoagulation and antithrombotic effects of Triclisia dictyophylla (moon seed). NigerJ Nat Prod Med. 2007; 11: 29-31.
CrossRef - Ajugw AO, Ezimah AC. In vivo studies of anticoagulation activity of Triclisia dictyophylla using albino wistar rats. IntJPharm Sci. Invent.2013; 2 (5): 37-40.
- Sharmin S, Kabir MT, Islam MN, Jamiruddin MR, Rahman I, Rahman A et al. Evaluation of antioxidant, thrombolytic and cytotoxic potentials of methanolic extract of AporosawallichiiHook.f. Leaves: An unexplored phytomedicine. J App Pharm Sci. 2018; 8(7):51-6.
CrossRef - Mutalib. Biochemical composition and in vitro thrombolytic activity of Melissa officinalis growing naturally in Kurdistan region Iraq. Int J Cur Res Rev.2015; 7(20) 17-21.
- Ali R MD. Preliminary phytochemical screening and in vitro thrombolytic potential of the methanolic extract of Enhydra Fluctuans Lour (Leaves). Int J Pharmamedix India. 2013; 1(2): 270-80.
- Gandhamlla P, Buddola SG, Rachakonda P, Manga R, Boggula.N. Preliminary phytochemical analysis and thrombolytic screening of Luffa cylindrica linn fruits an in vitro Int J Innov. 2018;6(1): 61-74.
- Lo A, Chan K, Yeung J. Danggui. Angelica sinensis affects the pharmaco dynamics but not the pharmacokinetics of warfarin in rabbits. Eur J Drug Metab Pharmacokinet. 1995; 20(1):55–60.
CrossRef - Tsai CH, Lin Yang FM, Lee YC, Cha MT, TL et al. Herbal extract of Wedelia chinensis attenuates androgen receptor activity and orthotopic growth of prostate cancer in nude mice. Clin Cancer Res. 2009; 15(17):5435-44.
CrossRef - Banu HR, Nagrajam N. Antimicrobial activity of Wedelia chinensis leaves. J Pharm Res. 2012; 5(1):407-12.
- Tabassum F, Chadni SH, Mou KN, Hasif KMI, Ahmed T et al. In vitro thrombolytic activity and phytochemical evaluation of leaf extracts of four medicinal plants of Asteraceae J Pharmacogn Phytochem. 2017; 6(4): 1166-69.
- Hussain S MD, Hossain S MD, Amin T MD , Millat S MD. In vitro thrombolytic potentials of methanolic extract of Vigna unguiculata Linn (seed). J Pharmacogn Phytochem.2016; 5(3): 129-31.
- Martos VMJ, Lopez F, Alvarez P. Pomegranate and its many functional components as related to human health: Review. Compr RevFood Sci 2010; 9: 635-54.
CrossRef - Pappa AR, Pushpa A. Evaluation of thrombolytic activity of Murraya Koenigii and Spinacia Oleracea. In vitro and in vivo. Helix. 2014; 6: 622-30.
- Sharma P, Dubey G, Kaushik S. Chemical and Medico-biological profile of Cyamopsis tetragonoloba (L) Taub: An overview. J Appl Pharm Sci.2011; 1(2): 32-7.
- Lau A, Toh D, Chua T. Antiplatelet and anticoagulant effects of Panax notoginseng; comparison of raw and steamed Panax notoginseng with Panax ginseng and Panax quinquefolium. J Ethnopharmacol. 2009; 125(3):380–6.
CrossRef - Lamnaouer D. Anticoagulant activity of coumarins from Ferula communis L. Therapie 1999; 54(6):747–51.
- Liapina A, Kondashevskaia V, Ziadetdinova A .Comparative study of anticoagulants obtained from various extracts of Paeoniaanomala. Russ Chem Bull Biol.2000; 3:345–9.
- Kudriashov A, Liapina A, Azieva D. The content of a heparin-like anticoagulant in the flowers of Filipendula ulmaria. Farmakol Toksikol.1990; 53(4):39–41.
- Byshevskii S, Gerbert A, Dement’eva P. Nature, properties and the mechanism of the effect on blood coagulation of the preparation obtained from Pulmonaria officinalis. Gematol Transfuziol. 1990; 35(10):6–9.
- Kim Y, Koo K, Koo Y. Platelet anti-aggregation activities of compounds from Cinnamomum cassia. J Med Food.2010; 13:1069–74.
CrossRef - Gadi D, Bnouham M, Aziz M. Parsley extract inhibits in vitro and ex vivo platelet aggregation and prolongs bleeding time in rats. J Ethnopharmacol. 2009; 125(1):170–4.
CrossRef - Varadharajan S, Josey C, Kuppusamy A, Muthuswamy U, Thirumalaisamy A, Mugham S et al. Anticoagulant activity of methanolic extract of Careya arborea Roxb bark. Int J Pharm Sci Bio.2010; 1(2):93–5.
- Chan CK, Yin MC, Chao WJ. Effect of dial-lyl trisulfide-rich garlic oil on blood coagulation and plasma activity of anticoagulation factors in rats. Food Chem Toxicol. 2007; 45(3):502–7.
CrossRef - Ansari V, Siddiqui H, Singh S .Antithrombotic and thrombolytic activity of Terminalia belerica fruit extracts. Res J Pharma Bio Chem Sci 2012; 3(2):471–8.
- Lee SM, Moon J, Chung JH, Cha YJ, Shin MJ. Effect of quercetin- rich onion peel extracts on arterial thrombosis in rats. Food Chem Toxicol. 2013; 57: 99–105.
CrossRef - Quick AJ. Coagulation. Hemorrhagic diseases and thrombosis. Lea and Febiger. Philadelphia. 1996; 50(2): 460–64.
CrossRef - Kim D, Ku S, Bae J. Anticoagulant activities of curcumin and its derivative. BMB Rep. 2012; 45(4):221–6.
CrossRef







