Mazlin Mohideen1*, Nik Nur Syahidatul Jannah Mahadi1, Nur Aina Nabilah Suhaimi1, Nur Azzalia Kamaruzaman2 and Ahmad Yasser Hamdi Nor Azlan1
1Faculty of Pharmacy and Health Sciences, Universiti Kuala Lumpur Royal College of Medicine Perak, Ipoh, Perak, Malaysia
2National Poison Centre, Universiti Sains Malaysia, 11800 Minden, Pulau Pinang, Malaysia
Corresponding Author E-mail: mazlin.mohideen@unikl.edu.my
DOI : https://dx.doi.org/10.13005/bpj/2353
Abstract
Background: Citrus hystrix (C. hystrix), locally known as ‘limau purut,’ is Malaysia’s major commercial fruit harvest. Besides being a common ingredient in Asian cuisines, the extracted essential oil has diverse applications. The essential oil can be extracted from peels or leaves, which could give strong aromatic properties. Objective: The main purpose of this study is to evaluate the antibacterial activity of C. hystrix essential oil by using disc diffusion method. Materials and Methods: C. hystrix essential oil was extracted from the peels using the Clevenger apparatus of hydro-distillation method. The antibacterial activity of essential oil was evaluated by agar disc diffusion method against four strains of bacteria: two Gram-positive bacteria (Staphylococcus aureus ATCC 25923 and Staphylococcus epidermidis ATCC 12228) and two Gram-negative bacteria (Escherichia coli ATCC 25922 and Shigella dysenteriae ATCC 13313). Each assay was done in triplicates. In this research, positive controls gentamicin and streptomycin were used as indicators to prove the research’s validity. Results: The Gram-positive bacteria were more susceptible to the essential oil (average zone of inhibition diameter; S. aureus = 19.3 ± 1.5 mm and S. epidermidis = 19.3.0 ± 0.6 mm) as compared to the Gram-negative bacteria (average zone of inhibition diameter; E. coli = 8.3 ± 0.6 mm and S. dysenteriae = 11.7 ± 0.6 mm). Gentamicin was recorded to be most effective against all tested bacteria (more than 15 mm zone of inhibition diameter). However, only S. epidermidis showed resistance to streptomycin. Conclusion: C. hystrix essential oil was found to possess antibacterial activity. Thus, these findings indicated that C. hystrix essential oil could be developed as an antibacterial agent in various applications.
Keywords
Antibacterial; Citrus Hystrix Peel; Disc Diffusion, Essential Oil, Hydro-Distillation
Download this article as:| Copy the following to cite this article: Mohideen M. Mahadi N. N. S. J, Suhaimi N. A. N, Kamaruzaman N. A, Azlan A. Y. H. N. Antibacterial Properties of Essential Oil Extracted from Kaffir Lime (Citrus Hystrix) Peel. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Mohideen M. Mahadi N. N. S. J, Suhaimi N. A. N, Kamaruzaman N. A, Azlan A. Y. H. N. Antibacterial Properties of Essential Oil Extracted from Kaffir Lime (Citrus Hystrix) Peel. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3qQLU7g |
Introduction
Antibacterial activity of any substance can be defined as its ability to either inhibit (bacteriostatic) or kill (bactericidal) the bacteria. It is highly beneficial to the human body in counteracting skin infection and related diseases (Chaudhari, 2016). Recently, microbial infections have become one of the most severe health problems at the international level, as the established conventional antibiotics have been reported to lose their effectiveness against pathogens (Das et al., 2013). Arise with the situation, plants have attracted great interest as part of pharmaceutical development and medication since they display wide therapeutic actions (Dulay & De Castro, 2016). The plant components, especially fruits and leaves, have antibacterial components that reduce bacterial growth (Agudo et al., 2007). In addition, the presence of active secondary metabolites from plants has been beneficial as antibacterial and antioxidant agents, which can contribute significantly to the pharmaceutical industry (Dulay & De Castro, 2016).
Kaffir lime (Citrus hystrix), which belongs to the family Rutaceae, is a tropical plant with reported antibacterial activity. Locally in Malaysia, C. hystrix is known as ‘limau purut’. Several parts of C. hystrix, such as peel, leaf, and fruit, may contain medicinal properties (Srisukh et al., 2012). The essential oil extracted from C. hystrix was a concentrated aromatic colourless, and strong odour (Giacoma & Giacoma 2002). According to Sreepian (2019), chemical composition from C. hystrix’s fruit was reported to possess antibacterial activity after being screened for various pharmacological activities. Due to its great potential in commercial applications is also commonly used as an essential ingredient in aromatherapy (Anuja et al., 2020). Furthermore, the essential oil obtained from the plant’s fruits and leaves are widely used in cosmetics and pharmaceutical industries due to is numerous bioactivities, such as antibacterial, antioxidant, and anti-inflammatory (Tsai et al., 2011).
Until recently, there has been limited research on the antibacterial activity of C. hystrix extracts on a broad range of pathogenic bacteria. Previous research from Srisukh (2012) found that C. hystrix peel essential oil (CHPEO) has shown great antibacterial activity against multi-resistant bacteria, including the respiratory pathogen. Therefore, this research focused on investigating the antibacterial activity of C. hystrix peel extract’s essential oil on Gram-positive and Gram-negative bacteria strains. According to Sabulal (2016), the in vitro antibacterial activity can be performed using the modified Kirby-Bauer disc diffusion method as it is the easiest standard reference method.
Materials and Methods
Plant Materials
The fresh fruits of C. hystrix were purchased from a market located in Ipoh, Perak, Malaysia. The fruits were washed under running tap water. The peel was separated and cut into 4-5 mm size dice, dried in an oven for at least 5 days at 50 °C, grinded to a fine powder, and then processed through an extraction step using the hydro-distillation method.
Extraction of Essential Oil
About 100 g of the C. hystrix peels were soaked in the distilling flask containing 600 ml of distilled water. A Clevenger-type apparatus was used for the hydro-distillation method. The hydro-distillation process ran for 3 hours, and temperature was controlled below 100 °C to avoid excess evaporation. After the distillation process, the oily layer was separated using dried anhydrous sodium sulphate from the distillate. In this study, the percentage yield of extracted CHPEO was 8.34% (w/v). Extracted CHPEO was stored at 4 °C and protected from light until used (Abdallah, 2016). A stock solution of CHPEO was prepared at a 400 mg/mL (v/v) concentration in DMSO before being used.
Proximate Analysis
The parameters used for proximate analysis included total ash value, acid-insoluble ash, water-soluble extractives, alcohol-soluble extractives, and loss on drying (Bhargava et al., 2013).
Total Ash
Two grams (2 g) of C. hystrix peel powder was cremated at 450 °C. The ash was left in a desiccator for 20 minutes to let it cool. The total ash had been calculated by measuring the differences between crucible containing ash and empty crucible.
Acid-Insoluble Ash
The ash is mixed with 25 ml of dilute HCl, covered with a watch-glass, and boiled for 5 minutes. The insoluble matter was collected using an ashless filter paper and washed with hot water until the filtrate was neutral to collect all residue. The solution was ignited until it reached a constant weight.
Water-soluble Extractive Value
Four grams (4 g) of C. hystrix peel powder was mixed with 100 ml of distilled water. The mixture was regularly shaken for 24 hours. After 24 hours, the solution was filtered. About 25 ml of the collected filtrate was heated in the evaporating dish until a damp mass formed. Continuously, the evaporating dish was directly cooled and weighed. The upper part, which was the solid content, was discarded.
Alcohol-soluble Extractive
Four grams (4 g) of C. hystrix peel powder was mixed with 100 ml of methanol. The mixture was regularly shaken for 24 hours intervals. After 24 hours, the solution was filtered. About 25 ml of the collected filtrate was heated in the evaporating dish until a damp mass formed. Continuously, the evaporating dish was cooled and weighed. The upper part, which was the solid content, was discarded.
Loss on Drying
Two grams (2 g) of C. hystrix peel powder was dried in the oven at 105 °C for 3 hours. The weight was obtained every 1 hour until it reached a constant weight. After 3 hours, the sample was cooled in a desiccator for 15 minutes.
Phytochemical Screening
Qualitative Analysis
The essential oils of the C. hystrix peel were subjected to qualitative phytochemical analysis for flavonoids, alkaloids, tannins, phenols, and terpenoids by following standard procedures.
Tannin Test
In a small centrifuge tube, about 0.5 ml of essential oil of C. hystrix peel was mixed with 0.1 ml 5% FeCl3. The appearance of dark blue colour indicated the presence of tannins.
Flavonoid Test
0.1 ml essential oil was mixed with NaOH in a small centrifuge tube. The appearance of yellow fluorescence showed the presence of flavonoids.
Alkaloid Test
In Mayer’s test, 0.1 ml of essential oil was mixed with 0.1 ml HCl. Then, two drops of Mayer’s reagents were added to the solution. A creamy white precipitate confirmed the presence of alkaloids.
Quinone Test
In a small centrifuge tube, 0.1 ml of essential oil was mixed with 0.1 ml H2SO4. The appearance of a reddish colour indicated the presence of quinone.
Phenol Test
The test was performed by adding a few drops of 10% FeCl3 in a tube containing 0.5 ml distilled water. Then, 0.1 ml of essential oil was mixed into the solution. The formation of blue or green colour showed the presence of phenolic compounds.
Saponins Test
In a small centrifuge tube, 0.3 ml of essential oil was mixed with 0.6 ml of distilled water. The mixture was shaken for at least 15 minutes. A formation of foam at 1 cm layer indicated the presence of saponins.
Terpenoids Test
In this test, 0.1 ml essential oil was mixed with 0.5 ml H2SO4. A few drops of acetic anhydride were added to the mixture. An appearance of a reddish-brown colour indicated the presence of terpenoids.
Quantitative Analysis
The essential oils of the C. hystrix peel were subjected to the determination of Total Phenolic Contents and Total Flavonoid Contents by following standard procedures (Biswash, 2020).
Total Phenolic Content
Total Phenolic Content (TPC) was determined using the Folin-Ciocalteu (FC) method. About 0.5 ml of dilute Folin-Cioca reagent (FCR) solution was added to 1 ml CHPEO. After five minutes of incubation, 1.5 ml of prepared Na2CO3 solution was added and incubated for one and half hours in the dark at room temperature. The absorbance was measured at 725 nm using a UV spectrophotometer. TPC was expressed as µg of Gallic acid equivalent per mg (GAE/mg) of plant extract. Each assay has been performed in triplicates.
Total Flavonoid Content
AlCl3 colourimetric method was used to determine Total Flavonoid Content (TFC). 1 ml of CHPEO was added to a test tube containing 0.3 ml of 5% NaNO3 solution. The mixture was allowed to remain for five minutes. After that, about 0.5 ml of 10% of AlCl3 solution was mixed with the solution, followed by the addition of 0.5 ml of NaOH solution. The absorbance was taken at 510 nm using the UV-Visible spectrophotometer. TFC was expressed as µg of Quercetin equivalent per mg (QE/mg) of plant extract. Each assay was performed in triplicates.
Screening of Antibacterial Properties
Bacteria
The bacterial organisms determined in this study consisted of four bacterial strains from American Type Culture Collection (ATCC). The bacterial strains were composed of Gram-positive bacteria (Staphylococcus aureus ATCC 25923 and Staphylococcus epidermidis ATCC 12228) and two Gram-negative bacteria (Escherichia coli ATCC 25922 and Shigella dysenteriae ATCC 13313). These bacteria were obtained from the laboratory of UniKL-RCMP. The bacteria were cultivated in a solid medium of Nutrient Agar (NA) plates by streaking method and stored at 4 °C.
Preparation of Culture Media (Agar)
Mueller Hinton (MH) agar was used for the antibacterial test. 13.3 g of MH powder was dissolved in 350 ml of distilled water and autoclaved at 121 °C for 20 minutes. Once the agar cooled to 45 °C, the medium was poured into a sterile disposable petri dish. The pouring plate process was operated in the laminar airflow and left at room temperature until solidified. The prepared plates were put in upside-down positions and kept in the refrigerator.
Preparation of Bacterial Suspension
A single colony of each bacteria culture in the plate for Gram-positive and Gram-negative were transferred in 10 ml of MH using a sterile loop. The prepared bacterial suspension was incubated for 3 hours at 37 °C to achieve the exponential phase of 0.5 McFarland Standard (Abdallah, 2016). Each bacterial suspension was subjected to turbidity check using a single-beam spectrophotometer at 600 nm. A 0.5 McFarland Standard has an absorbance reading of 0.08 to 0.1. All bacterial suspensions were directly used in the antibacterial activity assay.
Agar Disc Diffusion
Agar disk diffusion was performed to screen for in vitro antibacterial activity of CHPEO as previously described (Sabulal et al., 2016) with some modifications. The bacterial suspension was spread homogeneously onto the surface of MH agar using a sterile cotton swab. Sterilized disks (6 mm) impregnated with 20 µl of CHPEO were placed on the surface of each plate. In addition, gentamycin and streptomycin disks (10 µg and 120 µg respectively) were included as positive controls, and 20 µl of 1% DMSO impregnated disc was used as a negative control. The plates were incubated for 24 hours at 37 °C. The inhibition zone diameter (IZD) of CHPEO was measured and interpreted using the following criteria: no activity, IZD = 6 mm; weak activity, 6 mm < IZD < 12 mm; moderate activity, 12 mm < IZD < 20 mm; and strong activity, IZD > 20 mm (Lv et al., 2011).
Results and Discussion
Proximate Analysis
The proximate analysis provided valuable information on the plant composition that helped to assess the CHPEO sample (Table 1).
Table 1: Proximate Analysis
| No. | Experimental studies | Experimental value (%) |
| 1 | Total ash value | 8% |
| 2 | Acid insoluble ash value | 6.25% |
| 3 | Water soluble extractive value | 32% |
| 4 | Alcohol soluble extractive value | 21% |
| 5 | Loss on drying | 14.5% |
Ash value designates the presence of remaining inorganic residues after heating. The value provides the minerals’ amount as they cannot be destroyed by heating (Vidita et al., 2013). Less extractive values indicate that there is an addition of exhaustive materials due to incorrect processing during the drying process. Alcohol soluble extractive value is also indicated for the same purpose as the water-soluble extractive value. This study showed that the constituents in C. hystrix peel were more extracted and soluble in water than alcohol with 32% and 21%, respectively.
Phytochemical Analysis
Qualitative and quantitative analyses are essential to identify phytochemical constituents present in the herbal plant. Therefore, phytochemical screening was conducted to evaluate the active phytoconstituents in CHPEO.
Qualitative Analysis
Phytochemicals are biologically active, where the secondary metabolites may exert antimicrobial properties (Aziman et al., 2012). The overall results of the phytochemical analysis are shown in Table 2.
Table 2: Phytochemical Analysis of CHPEO
| No | Test | Extracted Sample | ||
| 1 | 2 | 3 | ||
| 1 | Tannin | – | – | – |
| 2 | Flavonoid | – | – | – |
| 3 | Alkaloid | – | – | – |
| 4 | Quinone | + | + | + |
| 5 | Phenols | – | – | – |
| 6 | Saponins | + | + | + |
| 7 | Terpenoid | + | + | + |
| (+) indicates positive results, (-) indicates negative results | ||||
The presence or absence of phytochemicals was confirmed by observing colour intensities when reacting with different reagents. According to Barile et al. (2007), saponins are active constituents which are known for their antimicrobial activity, a trait which is desirable for disease resistance in plants.
Quantitative Analysis
TPC and TFC are listed in Tables 3 and 4, respectively. All determinations were carried out in triplicates, and the values were expressed as mean (Biswash, 2020).
Table 3: Total Phenolic Content Determination
| Absorbance | Total Phenolic Content (ug GAE/mg) | Observation |
| 0.211 | 23.904 | Blue |
Table 4: Total Flavonoid Content Determination
| Absorbance | Total Flavonoid Content (ug QAE/mg) | Observation |
| 2.332 | 145.767 | Light blue |
TPC was quantified for essential oil extraction by Folin-Ciocalteu (FC) method using Gallic acid as standard. TPC for essential oil was equivalent to 23.904 ug/mg Gallic acid equivalent. The reaction between the Folin-Ciocalteu reagent and the CHPEO resulted in blue colour formation, which absorbed the radiation and allowed quantification.
TFC was quantified for essential oil extraction by the AlCl3 colourimetric method using Quercetin as standard. TFC content for essential oil was equivalent to 145.767 ug/mg Quercetin equivalent. The colour formation was light blue with a small layer of separation. According to a study by Francisco (1995), it was emphasized that the conversion from yellow colour to orange colour was regarded as a marker for flavonoid content. The current results indicated that CHPEO extraction did not contain flavonoid contents.
Antibacterial Activity of CHPEO by Agar Disk Diffusion
Antibacterial activity of CHPEO against the bacterial strains, clinically isolated Gram-positive bacteria and Gram-negative bacteria are shown in Table 5. The results demonstrated various antibacterial activities of CHPEO against four selected bacterial strains. Overall, moderate to the strong antibacterial activity of CHPEO was observed in S. aureus and S. epidermidis, while weak antibacterial activity was observed in E. coli and S. dysenteriae.
Table 5: Antibacterial activity of CHPEO against bacterial strains
| Bacteria | Agar disk diffusion; IZD (mm) | |||
| CHPEOa | Gentamicin | Streptomycin | 1% DMSO | |
| Staphylococcus aureus | 19.3 ± 1.5 | 15 ± 0.0 | 13 ± 1.0 | NDb |
| Staphylococcus epidermidis | 19.3 ± 0.6 | 50.7 ± 0.6 | NDb | NDb |
| Escherichia coli | 8.3 ± 0.6 | 23.7 ± 0.6 | 17.7 ± 0.6 | NDb |
| Shigella dysenteriae | 11.7 ± 0.6 | 27.3 ± 0.6 | 24.7 ± 0.6 | NDb |
Values are expressed as mean±SD of triplicate experiments.
aInterpreted criteria of antibacterial activities: IZD = 6 mm is no activity (N), 6 mm < IZD < 12 mm is weak activity (W), 12 mm < IZD < 20 mm is moderate activity (M), and IZD > 20 mm is strong activity (S) (Lv et al., 2011)
bND – not determined. Negative control (1% DMSO) indexes were not determined when the inhibition zone was not presented (IZD = 6 mm).
The results demonstrated that CHPEO displayed moderate to strong antibacterial activities against Gram-positive bacteria [S. epidermidis (Figure 1) and S. aureus (Figure 2)]. This is an interesting finding as compared to the Gram-negative bacteria, these Gram-positive bacteria have a thicker peptidoglycan layer in their cell walls which usually makes them more resistant towards antibacterial agents (Katas et al., 2019; Venkatpurwar and Pokharkar, 2011). In addition, the highest potency of CHPEO could be observed for S. aureus compared to the positive controls (gentamicin and streptomycin). The susceptibility of the bacteria towards CHPEO could be attributed to the presence of several phytochemical constituents such as terpenoid, quinone and saponins. The results obtained were also in line with the previous study, which stated that the essential oil containing terpenoids and having phenol and alcohol functionalities showed higher bactericidal activity against Gram-positive and Gram-negative bacteria (Lo Cantore et al., 2009). Both gentamicin and streptomycin, which are positive controls, generated moderate inhibition for bacterial growth. For S. epidermidis, the bacteria were more susceptible to only one positive control, which was gentamicin. However, the bacteria showed resistance against streptomycin antibiotics. Thus, this proved that CHPEO has antibacterial activity against the genus Staphylococcus.
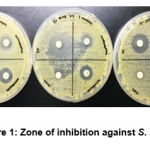 |
Figure 1: Zone of inhibition against S. aureus |
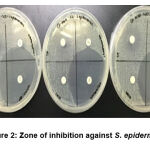 |
Figure 2: Zone of inhibition against S. epidermidis |
For Gram-negative bacteria, CHPEO displayed weak antibacterial activity against E. coli (Figure 3) and moderate antibacterial activity against S. dysenteriae (Figure 4). The results obtained were probably due to the presence of a cell wall in Gram-negative bacteria, which prevented passage of hydrophobic molecules, thus increasing resistance to biological activities imposed by the essential oils and their components (Nazzaro et al., 2013). Furthermore, it could be observed that the bacteria were more susceptible to the positive controls than the plant extracts as gentamicin and streptomycin generated potent inhibition by having greater diameter sizes.
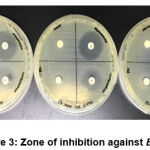 |
Figure 3: Zone of inhibition against E. coli |
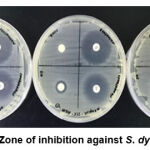 |
Figure 4: Zone of inhibition against S. dysenteriae. |
Data obtained in this study were similar to the previous study by Sreepian et al. (2019). In their study, CHPEO displayed moderate antibacterial activity against S. aureus and weak antibacterial activity against E. coli. In the present study, Gram-positive bacteria showed higher sensitivity to the plant extracts compared to Gram-negative bacteria. This could be explained as Gram-negative bacteria contain complex components in their cell wall compared to Gram-positive bacteria (Nazzaro et al., 2013). The presence of the lipopolysaccharide layer in Gram-negative bacteria could limit the permeability of essential oil in penetrating the bacterial cell (Chimmnoi et al., 2018).
Apart from that, all bacteria tested were affected by the positive control, gentamicin (S. aureus; IZD 15 ± 0.0, S. epidemidis; IZD 50.6 ± 0.6, E. coli; IZD 23.7 ± 0.6, and S. dysenteriae; IZD 27.3 ± 0.6). A standard susceptible zone diameter of inhibition for gentamicin is ≥15 mm, and this study reported equal and higher values for all bacterial strains (Table 5). Meanwhile, only S. epidermidis were resistant against streptomycin antibiotic, in which no antibacterial activity was observed in all three replicates. Collectively, CHPEO displayed promising results as a potent antibacterial agent, with its ability to inhibit both Gram-positive and Gram-negative bacteria.
Conclusion
Our findings demonstrated that CHPEO exerted antibacterial activities against a broad range of bacterial organisms, thus implying that CHPEO has the potential to be developed as an effective antibacterial agent. Therefore, CHPEO, which is less toxic to humans than other existing antibiotics, would be able to reduce unnecessary antibiotic use and subsequently decrease the probability of antibiotic resistance development. Further evaluations on the mode of action of CHPEO, potential synergistic effects with essential oil from other herbal plants, as well as in vivo adverse effects, are needed further to develop CHPEO as a new alternative antibacterial agent.
Acknowledgment
The authors gratefully acknowledged the Universiti Kuala Lumpur – Royal College of Medicine Perak for Final Year Project financial support.
Conflict of Interest
We declare that we have no conflict of interest.
Funding Sources
There is no funding source.
References
- Abdallah E. M. Preliminary phytochemical and antibacterial screening methanolic leaf extract of Citrus aurantifolia. Pharm Biotechnology Current Research, 2016; 1(1): 1-5.
- Agudo A, Cabrera L, Amiano P, Ardanaz E, Barricarte A, Berenguer T, Chirlaque M. D, Dorronsoro M, Jakszyn P, Larrañaga N, Martínez C, Navarro C, Quirós J. R, Sánchez M. J, Tormo M. J and González C. A. Fruit and vegetable intakes, dietary antioxidant nutrients, and total mortality in Spanish adults: Findings from the Spanish cohort of the European Prospective Investigation into Cancer and nutrition (epic-spain). The American Journal of Clinical Nutrition, 2007; 85(6): 1634-1642.
CrossRef - Anuja S, Sangeetha V, Sudha A and Menaha R. Techniques for essential oil extraction from kaffir lime and its application in health care products-A review. Flavour Fragrant Journal, 2020; 1-17.
- Aziman N, Abdullah N, Noor Z. M, Zulkifli K. S and Syida W. S. Phytochemical constituents and in vitro bioactivity of ethanolic aromatic herb extracts. Sains Malaysiana, 2012; 41(11): 1437-1444.
- Barile E, Bonanomi G, Antignani V, Zolfaghari B, Ebrahim Sajjadi S, Scala F and Lanzotti V. Phytochemistry, 2007; 68: 596-603.
CrossRef - Bhargava V. B, Saluja A. K and Dholwani K. K. Detection of heavy metal contents and proximate analysis. Journal of Pharmacognosy and Phytochemistry, 2013; 1(6): 61-65.
- Biswash S. Preliminary phytochemical screening and quantitative analysis of Citrus maxima (Brum.) leave extract. International Journal of Resources in Pharmacology & Pharmacotherapeutic, 2020; 9(1): 100-106.
- Chaudhari V. M. Studies on antimicrobial activity of antiseptic soap and herbal soaps against selected human pathogens. Journal of Scientific and Innovative Research, 2016; 5(6): 201-204.
- Das S, Baroh M and Ahmed S. Antibacterial activity of the ethanolic extract of leaves of Citrus maxima (Berm.) Merr. on Escherichia coli and Pseudomonas aeruginosa. Asian Journal of Pharmaceutical and Clinical Research, 2013; 6(4): 136-139.
- Dulay R. R and De Castro M. G. Chemical constituents, antioxidant and antibacterial activity of Syzygium cumini (L.) Skeels (Myrtaceae).Der Pharma Chemica, 2016; 8(20): 317-321.
- Francisco J. B. Harborne (ed.) the flavonoids–advances in research since 1986 Chapman & Hall, London, U.K. 1994, £195.00, 676 pp. ISBN 0-412-48070-0. Phytochemical Analysis, 1995; 6(1): 55-55.
CrossRef - Giacoma A. D and Giacoma G. D. Essential oil production is Medicinal and Aromatic plants. New York: Taylor and Francis 26, 2002; 114-493.
- Katas H, Lim CS, Nor Azlan AYH, Mh Busra MH and Buang F. Antibacterial activity of biosynthesized gold nanoparticles using biomolecules from Lignosus rhinocerotic and chitosan. Saudi Pharmaceutical Journal, 2019; 27: 283-292.
CrossRef - Lo Cantore P, Shanmugaiah V and Iacobellis NS. antibacterial activity of essential oil components and their potential use in seed disinfection. Journal of Agricultural and Food Chemistry, 2009; 57: 9454–9461.
CrossRef - Lv F, Liang H, Yuan Q and Li C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Research International, 2011; 44: 3057-3064.
CrossRef - Nazzaro F, Fratianni F, De Martino L, Coppola R and De Feo V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals, 2013; 6: 1451-1474.
CrossRef - Sabulal B, Dan M, Pradeep N. S, Valsamma R. K and George V. Composition and antimicrobial activity of essential oil from Amomum cannicarpum. Acta Pharmaceutica, 2016; 56: 473-480.
- Sreepian A, Sreepian P. M, Chanthing C, Mingkhwancheeo T and Prathit P. Antibacterial activity if essential oil extracted from Citrus hystrix (Kaffir Lime) peels: An in vitro Tropical Biomedicine, 2019; 36(2): 531-541.
- Srisukh V, Tribuddharat C, Nukoolkarn V, Bunyapraphatsara N, Chokephaibulkit K, Phoomniyom S, Chuanphung S and Srifuengfung S. Antibacterial activity of essential oils from Citrus hystrix (makrut lime) against respiratory tract pathogens. ScienceAsia, 2012; 38(2): 212.
CrossRef - Tsai M. L, Lin C. C, Lin W. C and Yang C. H. Antimicrobial, antioxidant, and anti-inflammatory activities of essential oils from five selected herbs. Bioscience, Biotechnology, and Biochemistry, 2011; 75(10): 1977-1983.
CrossRef - Venkatpurwar V and Pokharkar V. Green synthesis of silver nanoparticles using marine polysaccharide: study of in-vitro antibacterial activity. Materials Letters, 2011; 65: 999-1002.
CrossRef







