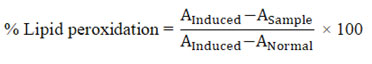Kartikey Jagtap1 , Anuradha Mulik1
, Anuradha Mulik1 , E. A. Singh2 and Suresh Jagtap1*
, E. A. Singh2 and Suresh Jagtap1*
1Department of Herbal Medicine, Interactive Research School for Health Affairs (IRSHA), Bharati Vidyapeeth Deemed to be University, Pune-Satara Road, Pune, Maharashtra, India
2Department of Plant Biotechnology and Environmental Biotechnology, Rajiv Gandhi Institute of Information Technology and Biotechnology, Bharati Vidyapeeth Deemed to be University, Pune-Satara Road, Pune, Maharashtra, India
Corresponding Author E-mail: chiritatml@rediffmail.com
DOI : https://dx.doi.org/10.13005/bpj/2352
Abstract
In Ayurveda, ‘Vidanga’ is one such species high in demand for its various uses. All the species of genus Embelia and Maesa belonging to the family Myrsinaceae are reported as ‘Vidanga’. Considering the availability of plant material in the market there is an ambiguity in supplying the authentic species as ‘Vidanga’. In the present study, a comparative analysis was carried out to determine the efficacy of different 'Vidanga’ spp. in terms of their phyto-constituents, antioxidant potential, and free radical scavenging activity. The highest total phenolic contents (TPCs) and total flavonoid contents (TFCs) were found to be in ethanolic and ethyl acetate extract. Quantitative measurements also showed that abundance of phenolic and flavonoid phytoconstituents was significantly (P<0.001) greater in ethanolic extract of all the ‘Vidanga’ fractions (1.773±0.01 to 137.17±0.19 mg/g GAE and 4.84±0.001 to 302.29±0.07 mg/g of quercetin respectively) than in ethyl acetate extract (1.15±0.003 to 15.12±0.01 mg/g GAE and 7.94±0.05 to 25.20±0.001 mg/g of quercetin respectively). Ethanolic extract of Embelia ribes had significant activity in terms of IC50 than ethyl acetate extracts in the case of 2,2-diphenyl,1- picryl hydrazyl (DPPH), ferric reducing antioxidant power (FRAP) and anti-lipid peroxidation (ALP) (9.53, 9.73 and 6.21 µg/mL respectively) indicates Embelia ribes found to be most effective species as ‘Vidanga’. Pearson’s correlation (r2) analysis also suggests a significant correlation between different antioxidant parameters and bioactive constituents. This study may helpful to draw attention of researchers to characterize the various bioactive compounds from the Embelia ribes in terms of their antioxidant prospective.
Keywords
Ambiguity; Comparative antioxidant potential; Embelia spp; Phyto-constituents; Vidanga
Download this article as:| Copy the following to cite this article: Jagtap K, Mulik A, Singh E. A, Jagtap S. Comparative Study to Evaluate Ethanol and Ethyl Acetate Extracts of Different 'Vidanga' Species for Antioxidant Efficacy and Phyto-Constituents Screening . Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Jagtap K, Mulik A, Singh E. A, Jagtap S. Comparative Study to Evaluate Ethanol and Ethyl Acetate Extracts of Different 'Vidanga' Species for Antioxidant Efficacy and Phyto-Constituents Screening . Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3D8KOcb |
Introduction
Herbal medicines are attainment towards popularization in global health debates due to its natural origin and lesser side effects. However, nowadays world has been gaining importance of Herbal and Ayurveda drugs1. ‘Vidanga’ is one of the medicinal herbs commonly used in Ayurveda, also has a strong traditional as well as an experimental base for its use.
‘Vidanga’ having species such as Embelia ribes Burm. f., Embelia drupacea (Dennst.) M.R. Almeida & S.M. Almeida, Embelia tsjeriam-cottam (Roem. & Schult) A. DC. and Maesa indica (Roxb.) A. DC. etc. belongs to the family Myrsinaceae2. These species are known for their medicinal use since thousands of years3. Embelia drupacea is woody climber growing in semi-evergreen to deciduous forests up to an altitude of 1600 m4. Embelia tsjeriam-cottom is distributed in the mountains of the Western Ghats of Karnataka, Kerala and Maharashtra5. Maesa indica is a species belonging to the same family which is distributed in Western Ghats, Eastern Himalayas and North East India6. Embelia ribes is an Indo Malayan species, distributed in India, Sri Lanka, Singapore, Malaysia, and S. China. Embelia ribes, popularly known as ‘Vidanga’ or ‘Vavding’ in Ayurveda while it yields embelin, and other highly valued secondary metabolites7.
Embelia ribes possesses close similarities especially in terms of active ingredient, viz., embelin with Embelia tsjeriam-cottam. According to a literature survey, the Embelia tsjeriam-cottam is used as substitute of Embelia ribes8,9,10,11. Data show that over 95 percent of the traded species is Embelia tsjeriam-cottam3. Therefore, there is a timely need to find out potent Embelia species as ‘Vidanga’. Embelia ribes has been used since ages in traditional medicine and it possesses drenching activity in humans. Root of the Embelia ribes is effective against chest pains and the fruits also have various properties such as anthelmintic, carminative, antibacterial, hypoglycemic, antifertility etc2. Moreover, leaves pasted with honey is consumed to treat mouth ulcers12. While with lime, juice roots are grounded to make a paste and taken orally with honey against cough13. It is considered to keep the digestive system healthy; also used in skin ailments like acne and pimple, in constipation, in piles, as a brain tonic, etc14. Embelia drupacea are used to treat toothache, laxative, anthelmintic, carminative, hypoglycemic, antifertility properties, antiseptic etc4. Embelia tsjeriam-cottam are used for their anti-inflammatory, analgesic, wound healing, antiproliferative, hepatoprotective, antimicrobial, antidiabetic, cardio-protective properties etc15. Fruits of Maesa indica are consumed raw by Irula tribal. It is good blood purifier and also used in anthelminthic ailments, nutritional, anti-diabetic etc16.
Oxidative stress has been known to be the basis for development and evolution of many diseases14. Hence, the present study is designed to evaluate the potential of authenticate ‘Vidanga’ species by using different biochemical assays to check their efficacies against reactive oxygen species. Present work is designed to validate the ‘Vidanga’ species by in vitro biochemical, phyto-constituents analysis and comparative investigation among all the four species of ‘Vidanga‘. The total phenolic contents (TPCs) and total flavonoid contents (TFCs) in both the polar and non-polar solvent will be studied and positively correlated with their antioxidant properties.
Materials and Methods
Sample collection and extraction
‘Vidanga’ fruits were collected from different locations Western Ghats in Maharashtra, India. Using mechanical blender, fruits were crushed to fine powders. Both polar and non-polar solvents, viz., ethyl acetate and ethanol (90%) were used. Extracts were prepared by Soxhlet apparatus (Rotamantal, Remi) with slight modification at 60-800C for 16-24 h19. Solvents were evaporated under reduced pressure at 45°C in a rotary evaporator (IKA RV 10) leaving small yields of dry plant extracts. The dry extract was dissolved in methanol for obtaining sample of final concentration 1mg/ml for further experiments.
Chemicals and reagents
All the chemicals and reagents used in the experiments were of analytical grades. Ascorbic acid, sodium acetate trihydrate, 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ), ascorbic acid, gallic acid, Folin–Ciocalteau reagent, methanol purchased from Sigma Aldrich (USA). 2,2- Diphenyl,1-picryl hydrazyl (DPPH), thiobarbituric acid reactive substance (TBARS), KCl, FeCl3, thiobarbituric acid (TBA), trichloro acetic acid (TCA), butylated hydroxy toluene (BHT) was prepared in methanol.
Estimation of TPCs and TFCs
TPCs of different ‘Vidanga’ samples were determined by the colorimetric Folin-Ciocalteu method. A calibration curve was prepared using standard gallic acid solutions at different concentrations every time analysis was run. TPCs concentrations in the samples were calculated from the standard curve and the results were expressed as gallic acid equivalents per gram (mg GAE/g) dry weight of the extract. TFCs of the different ‘Vidanga’ samples were also determined using the aluminium chloride colorimetric assay and expressed as quercetin per gram (mg RE/g) of dry weight. All determinations were done in triplicates and averaged.
In vitro free radical scavenging and antioxidant potential
DPPH (2, 2-diphenyl-1-picryl-hydrazyl-hydrate)
The radical scavenging ability of ‘Vidanga’ plant extract was measured by a method described previously20 with slight modifications. Briefly, 3ml of DPPH (33 mg/L in methanol) solution and diluted plant extracts in the range of (0.05–0.50 mg/mL) along with ascorbic acid as a standard (1 mg/mL). Samples were incubated in the dark for 30 min absorbance measured at 517 nm. The results were expressed as IC50 values which required 50% scavenging inhibition of DPPH radical.
Ferric reducing antioxidant power assay (FRAP)
Antioxidant capacity of ‘Vidanga’ plant extracts were estimated colorimetrically as per Benzie and Strain (1996)21. Antioxidant potential was determined on the basis of Fe3+ TPTZ complex (colorless complex) reduced to Fe2+ -tripyridyltriazine (blue-colored complex) due to the action of electron donating antioxidants at low pH, change in absorbance is measured at 593 nm. FRAP reagent was prepared by mixing 300 mM acetate buffer (pH 7.4), 10 mM TPTZ solution in 40 mM HCl and 20 mM FeCl3·6H2O were prepared and mixed in 10:1:1 proportion respectively. FRAP solution (2850 μl) was mixed with 150 μl of plant extract at different concentrations (0.05-0.50 mg/ml) and incubated for 2 h in dark at room temperature and absorbance measured at 593 nm. Ferric ion reducing activity of all the extracts was determined by standard FeSO4 (0.1-1 mM). Results were analyzed from the standard curve of ferrous sulphate and expressed in terms of µM Fe/g of dry mass. The results were expressed as IC50 values.
The percentage free radical scavenging activity was calculated by the formula:
where, AControl is absorbance of the control and ASample is absorbance of samples.
Anti-lipid peroxidation assay (ALP)
Previously reported protocol was adopted to determine the Fe3+/ascorbic acid-dependent non-enzymatic lipid peroxidation activity of ‘Vidanga’ plant extract22. The lipid peroxidation inhibition potential of extracts was determined by thiobarbituric acid reactive substance (TBARS). The reaction mixture, in the absence and presence of ‘Vidanga’ extract (0.05-0.50 mg/ml) or standard, containing 0.5 ml of goat liver as lipid source (5 mg/ml), 100 μl of 1 mM Iron (III) chloride (FeCl3) mixture was incubated at 37°C for 30 min while the reaction was terminated by using 2 ml ice-cold 0.25 N hydrochloric acid (HCl) containing 0.19% thiobarbituric acid (TBA) and 7.5% trichloroacetic acid (TCA). After adding 200 μl of 0.5% butylated hydroxytoluene (BHT) prepared in methanol mixture were incubated at 80°C in a water bath for 60 min and 1 mM ascorbic acid in 20 mM phosphate buffer used as a standard. Percent inhibition of free radicals was calculated and IC50 values were compared. All the experiments were carried out in triplicates.
Percentage lipid peroxidation was calculated using formula:
where, Ainduced is absorbance of induced; ASample is absorbance of sample and ANormal is absorbance of normal.
Preliminary phytochemical screening
Qualitative phytochemical screenings of ‘Vidanga’ plant extracts to identify the presence of vital phytoconstituents, such as total carbohydrates, alkaloids, tannins, saponins, flavonoids, steroids, terpenes, glycosides, proteins, amino acids, reducing sugars and phenol were carried out using standard biochemical procedures as described previously23.
Statistical Analysis
A curve was obtained by plotting the percent inhibition values versus extract concentrations and IC50 values were determined. Data were analyzed by one-way ANNOVA and expressed as mean ± standard error (SE) (where n>3). P value less than 0.05 is considered statistically significant. Karl Pearson’s correlation coefficient (r2) was determined to evaluate the correlation between the antioxidant activity and the TPCs and TFCs.
Results and Discussion
Plant material and extraction yield
The fruits of ‘Vidanga’ species are globose, smooth succulent, tipped with persistent style. The drupes are green when young and become black with wrinkles on the surface (Figure 1).
 |
Figure 1: Fruits of ‘Vidanga’ species: A. Embelia ribes, B. Embelia tsjeriam-cottam, C. Embelia drupacea and D. Maesa indica |
The extraction yield of different extracts of ‘Vidanga’ species obtained by ethanolic and ethyl acetate extraction methods are shown in Table 1. Variation in the yield produced by different solvents during extraction indicated that it follows the polarity of solvents. Embelia tsjeriam-cottam provided the highest yield of 23% followed by Embelia ribes (12.01%), Maesa indica (7.3%) in ethanolic extract, and 1.5% in Embelia drupacea from ethyl acetate extract.
Table 1: The extraction yields of two solvent extracts
| Sr No. | ‘Vidanga’ species | Ethyl acetate Yield (%) | Ethanol Yield (%) |
| 1 | Embelia ribes | 0.44 | 12.1 |
| 2 | Embelia drupacea | 1.5 | 1.1 |
| 3 | Embelia tsjeriam-cottam | 0.85 | 23.0 |
| 4 | Maesa indica | 5.3 | 7.3 |
Phytochemical screening
Many researchers have reported that photochemical such as glycosides, phenols, steroids, saponins, alkaloids and terpenoids have enormous free radical scavenging and antioxidant activities24,25,26. Moreover, plants that are rich in phytoconstituents have been shown to exhibit free radical scavenging and antioxidant potentials27,28,29. In the present work, a preliminary phytochemical analysis was studied using polar and nonpolar solvents. Glycosides are present in all the fractions except ethyl acetate extracts of Embelia drupacea (Table 2). Glycosides, containing either terpenoid or steroids and sugar chains, possess several therapeutic activities like anti-inflamation30, immuno-modulatory activity31 and anti-cancer property32. Fixed oils are present in all the fraction of both ethyl acetate and ethanol, while saponins are present only in ethanolic fraction of Embelia ribes (Table 2). Saponins are a type of aglycone containing triterpenoids or steroids33, possesses health promoting properties in functional food34 and also exerts immune enhancement effect35,36. Amino acids play vital role in the synthesis of proteins and precursors in the formation of secondary metabolites37 that participates in hormone synthesis, protein phosphorylation and antioxidant capacity38. Proteins and amino acids are present in all the ‘Vidanga’ fractions (Table 2). All the constituents showed more appearance in ethanolic fractions. Results shown suggest that phytoconstituents extracted from all the plants are polarity dependent (Table 2).
Table 2: Preliminary phytochemical screening of ‘Vidanga’ fractions
| Tests | Ethyl acetate extract | Ethanolic extract | ||||||
| Embelia ribes | Embelia drupacea | Embelia tsjeriam-cottam | Maesa indica | Embelia ribes | Embelia drupacea | Embelia tsjeriam-cottam | Maesa indica | |
| Carbohydrates | ü | × | ü | ü | ü | × | ü | ü |
| Alkaloid’s | × | × | × | × | ü | × | ü | × |
| Test for Fixed oils | ü | ü | ü | ü | ü | ü | ü | ü |
| Glycosides | ü | × | ü | ü | ü | ü | ü | ü |
| Proteins and Amino acids | ü | ü | ü | ü | ü | ü | ü | ü |
| Saponins | × | × | × | × | ü | × | × | × |
| Gum and Mucilages | × | × | × | × | × | × | × | × |
| Terpenoids | ü | × | ü | × | ü | × | × | × |
| Steroids | ü | × | × | × | ü | × | × | × |
|
Tannins |
ü | ü | ü | ü | ü | ü | ü |
ü |
Quantitative analysis of phytoconstituents
Quantitative results of phytoconstituents in ethyl acetate and ethanolic extracts of ‘Vidanga’ species showed significant results. TFCs and TPCs present in both the extracts, i.e., ethanol and ethyl acetate showed non-significant amount, but ethanolic extract had higher contents (302.29±0.07 and 137.17±0.19 mg/g respectively) in Embelia ribes. According to literature survey, use of ethanol provided much better results for extraction process, as has previously been reported for various Embelia spp.39,40,41,42,43. According to Xu et al.44 yield for aqueous extraction is low due to the polyphenols present in the plant tissues are often associated with other molecules also via hydrogen and hydrophobic bond whereas, ethanol is better hydrogen bond donor and acceptor than water45. Our results show higher yield of polyphenolic contents in ethanolic extract (Table3). Phyto-constituents, a group of vital secondary metabolites have potent antioxidant properties that contribute to defense mechanisms of the plant46,47,48. Polyphenols are good electron donors with the ability to cease the free radical chain reaction by converting them into more stable compounds42. Among all the plant extracts Embelia ribes and Mesa indica showed maximum TFCs and TPCs in ethanolic extract while quite the opposite Embelia drupacea and Embelia tsjeriam-cottam showed significant result in ethyl acetate extract (Table 3). The differences between both groups were statistically significant (p <0.05). The difference between TFCs and TPCs observed may be due to the solvents having different polarities, or may be due to differences in the solubility of these compounds in polar and non-polar solvents.
Table 3: Polyphenolic content of the ‘Vidanga’ samples
| ‘Vidanga’ species | Total phenolic content (50µg/ml)
(mg/g GAE equivalanta,b) |
Total flavonoid content (1mg/ml conc.)
(mg/g quercetin equivalanta,c) |
||
| Ethyl acetate extract | Ethanolic extract | Ethyl acetate extract | Ethanolic extract | |
| Embelia ribes | 15.12±0.01 | 137.17±0.19 | 25.20±0.001 | 302.29±0.07 |
| Embelia drupacea | 1.15±0.003 | 1.773±0.01 | 7.94±0.05 | 4.84±0.001 |
| Embelia tsjeriam-cottam | 7.88±0.008 | 4.176±0.01 | 16.21±0.005 | 15.64±0.01 |
| Maesa indica | 7.13±0.001 | 1.839±0.03 | 9.35±0.0005 | 8.69±0.03 |
aAll values are mean ± SE, n=3, b values are expressed as equivalent to gallic acid (mg/g of GAE), c values are expressed as equivalent to quercetin (mg/ g of quercetin).
Antioxidant prospective
DPPH scavenging activity
‘Vidanga’ species showed considerable antioxidant capacities (Figure 2). A positive linear correlation between DPPH scavenging capacity of ‘Vidanga’ fruit extracts and quantitative measurements was dependent on phytoconstituents (Table. 4). The highest correlation was observed between DPPH scavenging ability of ‘Vidanga’ fruit extract and both the phyto-constituents present in extract. Among these, the highest correlation (0.958) observed between phenol content and DPPH indicates that the scavenging ability of ‘Vidanga’ extract may be due to the presence of phytoconstituents. The report support those of other researchers, who have found positive correlation between polyphenolic contents and antioxidants activities49,50,51,52.
Table 4: Correlation analysis (r2) between antioxidant parameters and phytoconstituent of ‘Vidanga’ species plant extracts.
| ‘Vidanga’ species extract | Phytoconstituents | r2 value | ||
| DPPH assay | ALP assay | FRAP assay | ||
| Ethanolic extract | Phenol | 0.958 | 0.859 | 0.952 |
| Flavonoids | 0.965 | 0.870 | 0.958 | |
| Ethyl acetate extract | Phenol | 0.592 | 0.311 | 0.549 |
| Flavonoids | 0.349 | 0.683 | 0.458 | |
Maximum % inhibition for DPPH assay was observed in ethanolic fraction of Embelia ribes (91.41%) which is significantly higher than those of standard ascorbic acid (Figure 2). Various medicinal plant species shown DPPH-free radical scavenging activity53,54,55. Zebeaman and Gebeyehu56 have reported that, Embelia schimperi V exhibited 62.4% antioxidant inhibition while the standard vitamin C scored 97% of inhibition. However, ethanolic extract of Embelia basaal showed 97% of free radical scavenging activity52. Furthermore, the biological system contains several free radical and oxidant sources and they act by multiple mechanisms in a single system57. DPPH radical scavenging activities of the different ‘Vidanga’ sp. fractions showed a large variation of IC50 ranging from 9.53 to 56.55 μg/mL (Table 5).
Table 5: Antioxidant activities in the fractions of ‘Vidanga’ species by DPPH, ALP and FRAP
| Sr No. | Sample extracts | DPPH (IC50 µg/mL) | ALP (IC50 µg/mL) | FRAP (IC50 µg/mL) | |||
| Ethanolic | Ethyl acetate | Ethanolic | Ethyl acetate | Ethanolic | Ethyl acetate | ||
| 1 | Embelia ribes | 9.53 | 13.47 | 6.21 | 7.778
|
9.73 | 10.02 |
| 2 | Embelia drupacea | 48.15 | 56.55 | 23.29 | 26.80 | 44.61 | 47.94 |
| 3 | Embelia.tsjeriam-cottam | 29.22 | 44.81 | 16.24 | 17.83 | 34.28 | 36.38 |
| 4 | Maesa Indica | 40.72 | 55.06 | 17.83 | 21.31 | 36.78 | 38.13 |
*Highlighted numbers indicate highest 50% inhibitory concentration (IC50)
The highest DPPH scavenging activity in terms of their 50% inhibitory concentration (IC50) exhibited in ethanolic extract of Embelia ribes (9.53 µg/mL) whereas the lowest activity exhibited in ethyl acetate extract of Maesa Indica (56.55 µg/mL). The result showed that the ethanolic extract of Embelia ribes to be the most efficient free radical scavenger.
 |
Figure 2: Comparative DPPH scavenging activity of ‘Vidanga’ samples a. Ethanolic extract and b. Ethyl acetate extract. |
The order of major DPPH-scavenging activity observed in ethanolic extract of ‘Vidanga’ species was Embelia ribes (9.53µg/mL) followed by Embelia tsjeriam-cottam (29.22 µg/mL), Maesa indica (40.72 µg/mL) and Embelia drupacea (48.15 µg/mL) which was similar to their TFCs. Results indicate significant correlation (r2=0.965) between free radical scavenging activities exhibited by different fractions and their flavonoid contents (Table 4). It is noted that flavonoids possess various biochemical properties. One of the mainly imperative activities is their capacity to serve as antioxidants58. Several flavonoids such as baicalein and wogonin are reported for chronic inflammatory disorders,59 rutin for hepatoprotective activity,60 butein for adipocyte inflammation activities61 and quercetin for immunomodulatory activities32. ‘Vidanga’ species are rich in flavonoids, and hence could be used for different pharmacological activities. Among all the ‘Vidanga’ species, Embelia ribes having the highest capacity to scavenge free radicals.
Anti-lipid peroxidation assay (ALP)
The results showed that all the fractions of ‘Vidanga’ species inhibited lipid peroxidation in dose dependent manner (Figure 3). Significant inhibition was observed in both the fractions (ethanol and ethyl acetate) of ‘Vidanga’ species. Lipid peroxidation is a process in which, free radicals take electrons from the lipids, resulting in a loss of membrane fluidity with an increase in membrane permeability and a decrease in physiological activity resulting endanger cell viability43. The protective effect of ‘Vidanga’ fractions against FeSO4– induced lipid peroxidation was assessed in the goat liver. During this process, polyunsaturated fatty acids present in the lipid membrane undertake oxidation resulting in formation of the malonaldehyde (MDA) which reacts with molecules of thiobarbituric acid (TBA) to form TBARS62.
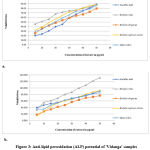 |
Figure 3: Anti-lipid peroxidation (ALP) potential of ‘Vidanga’ samples a. Ethanolic extract and b. Ethyle acetate extract |
Maximum inhibition in terms of IC50 was observed in ethanolic fraction of Embelia ribes (6.21µg/mL) whereas lowest activity exhibited in ethyl acetate fraction of Embelia drupacea (26.80 µg/mL). Significant lipid lowering ability of E. ribes in rats have been reported previously41,63. Protection against free radical lipid peroxidation is of great significance for their use against various disorders like inflammation 64 as well as several pathologies like aging, wound healing, oxygen toxicity etc.65. Observations indicated a significant correlation (r2=0.870) between lipid lowering ability of ‘Vidanga’ species and TFCs content (Table 4). Plant flavonoids have been reported for prevention of lipid peroxidation in microsomes and liposomes66.
Ferric reducing antioxidant power assay (FRAP)
Antioxidants can be explained as antioxidant inactivators and reductants, measures the reducing potential of the test sample67. The antioxidants donating electron or hydrogen atoms to the ferric complex which converts to ferrous complex (Fe3+ to Fe2+-TPTZ complex), thus breaking the radical chain reaction68. Results showed lower reducing activity in Embelia drupacea extract as compared to the other ‘Vidanga’ species extract. The reducing power of the plant extracts correlate with their antioxidant activities while this property of antioxidant depends upon its electron donating capacity. In the present study, all the fractions have reducing power and absorbance is increased, due to the formation of the Fe2+-TPTZ complex with an increase in concentration. The highest ferric reducing activity in terms of their 50% inhibitory concentration (IC50) exhibited in ethanolic extract of Embelia ribes (9.73µg/mL) whereas the lowest activity exhibited in ethyl acetate extract of Embelia drupacea (47.94µg/mL). The ethanolic fractions had slightly higher reducing activity in comparison to ethyl acetate extract.
 |
Figure 4: Ferric Reducing Antioxidant Power (FRAP) potential of ‘Vidanga’ samples a. Ethanolic extract and b. Ethyl acetate extract |
Maximum % reduction was observed in the ethanolic fraction of Embelia ribes (63.8%) which is significantly higher than those of other fractions (Figure 4). A positive linear correlation between Ferric reducing antioxidant power of ‘Vidanga’ plant extracts and phytoconstituents present in the extract were observed (Table. 4). The significant correlation was observed between Ferric reducing ability of ‘Vidanga’ plant extract and both the phytoconstituents present in ethanolic extract. While the highest correlation (0.958) was observed between flavonoids and ethanolic ‘Vidanga’ fractions. Today, antioxidant supplements may provide an important resource to conflict organ-related disorders, accumulation of harmful compounds, and metabolic dysfunctions69.
Embelia ribes contains different phyto-constituents, viz., potassium embelate, 2,5-dihydroxy, 3-undecyl-1,4-benzoquinone, embelin, quercitol, fatty ingredients, vilangin which are previously reported for different activities like antibacterial, antifertility, antiprotozoal, constipation, antifungal, mouth ulcer, sore throat, pneumonia, obesity, analgesic, anti-inflammatory, antioxidant, anthelminthic, antidiabetic, anticonvulsant, anticancer, antihyperlipidemic, wound healing and mollusicidal properties62. A comparative study for the presence of phenolic and flavonoid compounds in extracts of different ‘Vidanga’ species clearly indicates that ethanolic extract had the greatest phenol and flavonoid content than ethyl acetate extracts. The percent yield of the ‘Vidanga’ fractions was also found to be high in the ethanol extract than ethyl acetate. Among all the species, highest yield was observed in Embelia ribes (12.1%). However, the highest activity was also observed in Embelia ribes for antioxidant profile. Hence, positive relationship between these methods of antioxidant assays suggesting that Embelia ribes is more efficient species among all to assess the DPPH, FRAP and ALP antioxidant activities.
Conclusion
This study expanded the current knowledge of phytoconstituents, antioxidant potential, and free radical scavenging activity of both ethanolic and ethyl acetate fractions of ‘Vidanga. The results of the present study generally implies that the fruits of Embelia ribes could be potential natural source of bioactive compounds and may be greatly utilized as therapeutic agent to reducing or preventing the oxidative stress-related ailments. However, study indicates the affirmative way to researchers to characterize the various bioactive compounds from the Embelia ribes in terms of their antioxidant prospective. Further research on Embelia ribes may be helpful for the confirmation of most potent species as ‘Vidanga’ and in treating various health issues.
Acknowledgement
Authors are thankful to Dr. Suresh Jagtap, Taxonomist, IRSHA, Pune, Maharashtra and Interactive Research School for Health Affairs (IRSHA) and Bharati Vidyapeeth (Deemed to be University) for their overall support.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding Sources
There is no funding source.
References
- Mukherjee P.K, Venkatesh, Kumar M.V. An Overview on the Development in Regulation and Control of Medicinal and Aromatic Plants in the Indian System of Medicine. Plant. Med. Aromatics., 2007;6(4): 129-136.
- Asadulla S. Pharmacognosy of Embelia ribes Burm F. Int. J. Curr. Res. Chem. Pharm. Sci., 2011; 1(4): 1236-1251.
- Patwardhan A, Ray S and Roy A. Molecular marker in phylogenetic studies-A review. J. Phylogen. Evolution. Biol., 2014; 2(2): 1-9.
- Sathe V. P and Dixit P. Quantitation of embelin from plant Embelia drupacea (Dennst) M.R. & S.M. Almeida by development and validation of HPTLC method. J. Biol. Sci., 2015; 5(1): 130-137.
- Chandrappa C.P., Anitha R., Jyothi P., Rajalakshmi K., Seema Mahammadi H., Govindappa M. and Sharanappa P. (2013) “Phytochemical analysis and antibacterial activity of endophytes of Embelia tsjeriam-cottam Linn” Research Article Biological Sciences 3(1): 467-473.
- Kuruvilla G. R, Neeraja M, Srikrishna A, et al. A new quinone from Maesa indica (Roxb) A DC, (Myrsinaceae). J. Chem., 2010; 49: 1637-1641.
- Mhaskar M, Joshi S, Chavan B, et al. Status of Embelia ribes Burm f. ‘Vidanga’, an important medicinal species of commerce from northern Western Ghats of India. Curr. , 2011; 100(4): 547-552.
- Ved D. K and Singh A. Identity of ‘Vidanga’ – a plant drug in trade. Newsletter- Medicinal plants of conservation concern; 2006.
- Venkatasubramanian P, Godbole A, Vidyashankar R, et al. Evaluation of traditional anthelmintic herbs as substitutes for the endangered Embelia ribes using Caenorhabditis elegans Curr. Sci., 2013; 105(11): 1593-1598.
- Sinha A, Das R, Deka B, et al. Authentication, micropropagation and conservation of Embelia ribes – a vulnerable medicinal plant. Indian. For., 2014; 140(7): 707-714.
- Kulkarni S. V and Chandu A. N. Physicochemical investigation of ‘Vidanga’ berries. IJSR. NET., 2015; 5(12): 1006-1009.
- Sharma G. K. Ethnomedicinal flora: Ayurvedic system of medicine in a remote part of the Indo-Tibetan Himalayas. J. Tenn. Acad. Sci., 1997; 72(3-4): 53-55.
- Rajakumar N and Shivanna M. B. Traditional herbal medicinal knowledge in Sagar taluk of Shimoga District, Karnataka, India. J. Nat. Prod. Resour.,2010; 1(1): 102-108.
- Souravi K and Rajasekharan P. E. Ethnopharmacological uses of Embelia ribes F.- A review. IOSR. J. Pharm. Biol. Sci., 2014; 9(3): 23-30.
CrossRef - Basak, U. C., & Mohapatra, M. (2015). Quantitative reckoning of embelin from fruits of Embelia tsjeriam- cottam using water bath process as an alternate method of extraction. Indian Journal of Pharmaceutical and Biological Research, 3(03), 15–23. http://doi:10.30750/ijpbr.3.3.4
CrossRef - Shanmugam, S., Baby, J. P., Chandran, R., Thankarajan, S., & Thangaraj, P. (2016). Maesa indica: a nutritional wild berry rich in polyphenols with special attention to radical scavenging and inhibition of key enzymes, α-amylase and α-glucosidase. Journal of Food Science and Technology, 53(7), 2957–2965. http://doi:10.1007/s13197-016-2263-3
CrossRef - Kasote D. M, Katyare S. S, Hegde M. V, et al. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci., 2015; 11(8): 982-991.
CrossRef - Liguori I, Russo, Curcio F, et al. Oxidative stress, aging, and diseases. Interv. Aging, 2018; 13: 757-772.
CrossRef - Vargas R. A, Guerrero R. V and Petricevich V. L. Evaluation of anti-arthritic potential of partitioned extracts of bougainvillea x buttiana (var. Rose) Holttum and Standl. Int. J. Pharm. , 2018; 10(3): 117-123.
CrossRef - Brand-Williams W, Cuvelier M. E and Berset C. Use of a free radical method to evaluate antioxidant activity. Sci. Technol., 1995; 28: 25–30.
CrossRef - Benzie I. F and Strain J. J. The Ferric reducing ability of plasma (FRAP) as a measure of ‘antioxidant power’: the FRAP assay. Anal. Biochem., 1996; 239: 70-76.
CrossRef - Wade C. R, Jackson P. G and Rij A. M. Quantitation of malondialdehyde (MDA) in plasma, by ion-pairing reverse phase HPLC. Med., 1985; 33: 291–296.
CrossRef - Sahira, B. K and Cathrine L. General techniques involved in phytochemical analysis. J. Adv. Res. Chem. Sci., 2015; 2(4): 25-32.
- Njoya E. M, Munvera A. M and Mkounga P. Phytochemical analysis with free radical scavenging, nitric oxide inhibition and antiproliferative activity of Sarcocephalus pobeguinii Complement.Altern. Med., 2017; 17(199): 1-9.
CrossRef - Zhang J, Shen X, Wang K, et al. Antioxidant activities and molecular mechanisms of the ethanol extracts of Baccharis propolis and Eucalyptus propolis in RAW64.7 cells. Biol., 2016; 54: 2220-2235.
CrossRef - Amari N. O, Bouzouina M, Berkani A, et al. Phytochemical screening and antioxidant capacity of the aerial parts of Thymelaea hirsuta Asian Pac. J. Trop. Dis., 2014; 4(2): 104–109.
CrossRef - Senhaji S, Lamchouri F and Toufik H. Phytochemical content, antibacterial and antioxidant potential of endemic plant, Anabasis aretioïdes coss. & Moq. (Chenopodiaceae). Res. Int., 2020; doi: 10.1155/2020/6152932.
CrossRef - Khan W, Subhan S, Shams D. F, et al. Antioxidant potential, phytochemicals composition, and metal contents of Datura alba.Res. Int., 2019; 2019: 1-8.
CrossRef - Batool R, Khan R, Sajid M, et al. Estimation of phytochemical constituents and in vitro antioxidant potencies of Brachychiton populneus (Schott & Endl.) R.Br. Chem., 2019; 13(32): 1-15.
CrossRef - Kytidou K, Artola M, Herman S, et al. Plant glycosides and glycosidases: A treasure-trove for therapeutics. Plant Sci., 2020; 11(357): 1-21.
CrossRef - Jantan I, Ahmad W and Bukhari S. N. A. Plant-derived immunomodulators: an insight on their preclinical evaluation and clinical trials. Plant Sci., 2015; 6(655): 1-18.
CrossRef - Iqbal J, Abbasi A, Mahmood T, et al. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed., 2017; 7(12): 1129-1150.
CrossRef - Jiang L, Zhang G, Li Y, Shi G, Li M. Potential application of plant-based functional foods in the development of immune boosters. Front. Pharmacol.,2021;12:398. DOI=10.3389/fphar.2021.637782
CrossRef - He Y, Hu Z, Li A, Zhu Z, Yang N, Ying Z, et al. Recent advances in biotransformation of saponins. ,2019;24:23-65. doi:10.3390/molecules24132365
CrossRef - Ning Y. C, Qiao H. X, Pan C. M, and Zhang X. J. Effects of fermented gypenosides on immune function in mice. Hubei Agr. Sci., 2016; 55: 2304–2307. doi: 10.14088/j.cnki.issn0439-8114.2016.09.038
- Rajput Z. I, Hu S. H, Xiao C. W, and Arijo, A. G. Adjuvant effects of saponins on animal immune responses. Zhejiang Univ. Sci., 2007; B.8 (3): 153–161. DOI:10.1631/jzus. 2007.B0153
CrossRef - Rainer E. Häusler, F. L, Stephan K. Amino acids – A life between metabolism and signaling. Plant. Sci., 2014; 229: 225-237, DOI: https://doi.org/10.1016/j.plantsci.2014.09.011.
CrossRef - Rachel A, Gad G, Hagai C. The metabolic roles of free amino acids during seed development, Plant. Sci. 2018; 275: 11-18, ISSN 0168-9452, https://doi.org/10.1016/j.plantsci.2018.06.011.
CrossRef - Bhandari U, Kanojia R and Pillai Effect of ethanolic extract of Embelia ribeson dyslipidemia in diabetic rats. Int. J. Exp. Diab. Res., 2002; 3: 159–162.
CrossRef - Vite M. H, nangude S. L, gorte S. M. Anti-inflammatory effect of ethanolic extract of Embelia tsjeriam cottam. Int. J. Pharm. Pharm. Sci., 2011; 3(4): 101-102.
CrossRef - Bhandari U, Jain N, Ansari M.N, Pillai K.K. Beneficial effect of Embelia ribes ethanolic extract on blood pressure and glycosylated hemoglobin in streptozotocin-induced diabetes in rats. Fitoterapia., 2008;79(5):351-355. DOI: https://doi.org/10.1016/j.fitote.2008.04.001.
CrossRef - Ibrahim K.M, Afzal A, Akram M, Mohiuddin E, Khan U, Sultan A, Shah S. M. A, Asif M, Ghazala S, Khalil A and Riaz U. R. Monograph of Embelia ribes f. Afr. J. Plant. Sci., 2010; 4(12):503-505.
- Lal B and Mishra N. Importance of Embelia ribes: an update. J. Pharm. Sci., 2013; 4(10): 3823-3838.
- Xu X, Xie B, Pan S, Yang E, Wang K, Cenkowski S, Hydamake A. W, and Rao S.A new technology for extraction and purification of proanthocyanidins derived from sea buckthorn bark. J. Sci. Food Agric., 2006; 86: 486– 492.
CrossRef - Nedić M, Wassermann T.N, Larsen R. W, Suhm M.A.A combined Raman‐ and infrared jet study of mixed methanol–water and ethanol–water clusters. Phys. Chem. Chem. Phys., 2011;31:14050– 14063.
CrossRef - Chinedum E, Kate E, Sonia C, Ironkwe A, Andrew I. Polyphenolic Composition and Antioxidant Activities of 6 New Turmeric (Curcuma Longa) Accessions. Recent. Pat. Food. Nutr. Agric. 2015;7(1):22-27. DOI: 10.2174/2212798407666150401104716. PMID: 25827393.
CrossRef - Sinha S, Raghuwanshi R. Evaluation of Phytochemical, Antioxidant and Reducing Activity in Whole Plant Extract of Andrographis paniculata (Burm.f.) Wall. ex Nees. Biosc. Biotech. Res. Comm. 2020;13(4):1734-1742.
CrossRef - Dimitrios S, Nikolaos P, Chryssa S, Dimitrios M, Nektarios A, Eliza C, Christos P, Eleni R, Leandros S, Aristidis M. T, Dimitrios K. Correlation of total polyphenolic content with antioxidant and antibacterial activity of 24 extracts from Greek domestic Lamiaceae species, Food. Chem. Toxicol., 2012; 50(11):4115-4124.https://doi.org/10.1016/j.fct.2012.08.033.
CrossRef - Huh M.K and Han M.D. Inhibitory effects of hyaluronidase and DPPH radical scavenging activity using extraction of Equisetum arvens. European Journal of Advanced Research in Biological and Life Sciences (EJARBLS).,2015; 3: 47-51.
CrossRef - Sen S, De B, Devanna N, Chakraborty R. Total phenolic, total flavonoid content, and antioxidant capacity of the leaves of Meyna spinosa, an Indian medicinal plant. Chin. J. Nat. Med., 2013;11(2):149-57. DOI: 10.1016/S1875-5364(13)60042-4. PMID: 23787182.
CrossRef - Wang Z, Lee Y, Eun J. S. et al. Inhibition of adipocyte inflammation and macrophage chemotaxis by butein. J. Pharmacol., 2014; 738: 40–48.
CrossRef - Kamble R. C. Torane K. S, Mundhe N, Deshpande R and Salvekar J.P. Evolution of Free Radical Scavenging Potential of Embelia Basal. Chem. Pharm. Res., 2011, 3(2):465-471.
- Singh J. P, Kaur A, Singh N, et al. In-vitro antioxidant and antimicrobial properties of Jambolan (Syzygium cumini) fruit polyphenols. LWT-Food. Sci. Technol., 2016; 65: 1025-1030.
CrossRef - Pandey M. M, Khatoon S and Rastogi S. Determination of flavonoids, polyphenols and antioxidant activity of Tephrosia purpurea: a seasonal study. Integr. Med., 2016, 14(6): 447–455.
- D’Amelia V, Aversano R, Chiaiese P, et al. The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem. Rev., 2018; 17: 611–625.
CrossRef - Zebeaman M and Gebeyehu R. Phytochemical Screening and Antioxidant Activity of the fruit of Embelia Schimperi (family Myrsinaceae) Int. J. Phytochem., 2018; 1(2): 2018;27-32.
- Singh J. P, Kaur A, Singh N, et al. In vitro antioxidant and antimicrobial properties of Jambolan (Syzygium cumini) fruit polyphenols. LWT-Food Sci. Technol., 2016; 65: 1025-1030.
CrossRef - Pandey M. M, Khatoon S and Rastogi S. Determination of flavonoids, polyphenols and antioxidant activity of Tephrosia purpurea: a seasonal study. Integr. Med., 2016, 14(6): 447–455.
CrossRef - D’Amelia V, Aversano R, Chiaiese P, et al. The antioxidant properties of plant flavonoids: their exploitation by molecular plant breeding. Phytochem. Rev., 2018; 17: 611–625.
CrossRef - Kim H. P, Son K. H, Chang H. W, et al. Anti-inflammatory plant flavonoids and cellular action mechanism. Pharmacol. Sci., 2004; 96(3): 229-245.
CrossRef - Kumar S and Pandey A. K. Chemistry and biological activities of flavonoids: an overview. Sci. World. J., 2013: 1-16.
CrossRef - Balu M, Sangeetha P, Haripriya D, et al. Rejuvenation of antioxidant system in central nervous system of aged rats by grape seed extract. Neurosci. Lett., 2005; 383: 295–300.
CrossRef - Medpilwar M, Maru D, Upadhyay M, et al. In vitro antioxidant and anti-lipid peroxidation activity of ethanolic extracts of Bougainvillea shubhra, Bougainvillea peruviana and Bougainvillea bhuttiana Golden Glow: A comparative study. Nat. Med., 2015; 15(1): 1-6.
CrossRef - Sreelekshmi, Arafat M. M, Shyamal S, Shine V. J, Anuja G. I, Suja S. R. Anti-inflammatory, analgesic and anti-lipid peroxidation studies on stem bark of Ficusreligiosa Indian J. Nat Prod. Resour., 2007: 377-381.
- Osmund C. E, Christian E. O, Clement P. W. Evaluation of the in vitro anti-oxidant activity of Alternanthera brasiliana leaves. J. Pharm. Res., 2013; 6(9):919-924, DOI:https://doi.org/10.1016/j.jopr.2013.09.006.
CrossRef
- Singh D, Singh R, Singh P, et al. Effects of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Clin. Pharmacol., 2009; 105(4): 243-248.
CrossRef - Peng W and Kuo S. M. Flavonoid structure affects the inhibition of lipid peroxidation in caco-2 intestinal cells at physiological concentrations. J. Nutr., 2003; 133(7): 2184–2187.
CrossRef - Tavares W. R, Seca A. M. L Inula L. Secondary metabolites against oxidative stress-related human diseases. Antioxidants (Basel, Switzerland)., 2019; 8(122): 1-20.
CrossRef - Apak R, Ozyürek M, Guçlu K, et al. Antioxidant activity/capacity measurement. 1. Classification, physicochemical principles, mechanisms and electron transfer (ET)-based assays. Agr. Food. Chem., 2016; 64(5): 997-1027.
CrossRef