Sanjay Kumar Mahapatra1 , Rinu Rani Dash2*
, Rinu Rani Dash2* , Bhabagrahi Rath2
, Bhabagrahi Rath2 and Prajit Saswat Hota2
and Prajit Saswat Hota2
1Department of Urology, VSS Institute of Medical Science, Burla, India, 768017
2Department of Pharmacology, VSS Institute of Medical Science, Burla, India, 768017
Corresponding Author E-mail: rinu.rani2009@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2389
Abstract
Overactive bladder (OAB) is a common medical condition, especially in the elderly population, that affects the quality of life. The primary treatment of OAB is a combination of behavioural measures and widely used anti-muscarinic drug therapy like solifenacin, which brings about significant side effects, thereby affecting the quality of life adversely. Mirabegron, a recently approved drug for treatment of OAB is a β3 - adrenergic agonist, which relaxes the detrusor muscle. With this background, the current study was done to compare the efficacy and safety of solifenacin, mirabegron and combination of low doses of solifenacin and mirabegron on various parameters of OAB. A total no. of 70 patients with OAB symptoms coming to urology OPD were included in this study and they were divided into 3 groups - Group 1: 24 patients receiving solifenacin 10mg/day, Group 2: 23 patients receiving solifenacin 5mg/day plus mirabegron 25mg/day, Group 3: 23 patients receiving mirabegron 50 mg/day. Patients were assessed for change in mean numbers of micturition, urgency, incontinence per 24 hour and quality of life using Patient Perception of Bladder Control (PPBC) Questionnaire. They were followed up after 4 weeks and 12 weeks and change in the above parameters from baseline were noted. Patients receiving combination therapy achieved a significant reduction in urgency, frequency, nocturia and urge incontinence and decrease in side effects when compared with solifenacin and mirabegron alone. Combination therapy with solifenacin and mirabegron significantly improved quality of life and reduced side effects like dry mouth, blurred vision, constipation in OAB patients in comparison to solifenacin and mirabegron monotherapy.
Keywords
Overactive Bladder; Mirabegron; Solifenacin; Quality of life
Download this article as:| Copy the following to cite this article: Mahapatra S. K, Dash R. R, Rath B, Hota P. S. Solifenacin and Mirabegron Monotherapies Versus Combination Therapy in Overactive Bladder: A Prospective Observational Study. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Mahapatra S. K, Dash R. R, Rath B, Hota P. S. Solifenacin and Mirabegron Monotherapies Versus Combination Therapy in Overactive Bladder: A Prospective Observational Study. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3HM90lm |
Introduction
Overactive bladder (OAB) is defined as a symptom complex characterised by urinary frequency (≥8 micturitions/24 hours) and urgency, with or without urge incontinence and nocturia in the absence of local pathologic or metabolic factors that would account for these symptoms.1 OAB can significantly interfere with daily routines and health related quality of life (HRQoL), including social, physical and psychological well-being, productivity and sexual health and can cause stress and depression.2
Antimuscarinic (AM) drugs are well-established oral pharmacological treatment for OAB, which relaxes the detrusor muscle and by inhibiting muscarinic receptor subtypes, M2 and M3 reduces sensory symptoms during the storage phase of the micturition cycle. Both subtypes are expressed in multiple tissues, increasing the risk of bothersome, anticholinergic adverse events (AEs) such as dry mouth, which along with lack of efficacy, is the most frequently cited reason for discontinuation of antimuscarinic treatment.3
Recent advances in the understanding of OAB have identified three β- adrenoceptor subtypes (β1, β2, β3) in the detrusor muscle and urothelium, β3 being the predominant β- receptor subtype in human urinary bladder.4-8
Mirabegron is the first β3 adrenoceptor agonist that has been shown to relax the detrusor muscle during the bladder storage phase and increase bladder capacity.9
As these agents have different mechanisms of action, combining a β3-adrenoceptor agonist with an AM agent may improve efficacy in OAB treatment; combinations with reduced doses may deliver an improved tolerability profile compared with monotherapy, without compromising efficacy.10
The PPBC (Patient Perception of Bladder Control) Questionnaire, recommended by the EMEA (European Medicine Evaluation Association) is a valid and responsive global measure. 11-12
However, there is paucity of data that demonstrate the effectiveness and superiority of combination therapy over monotherapy.
Aims and Objectives
To evaluate the efficacy and safety of solifenacin and mirabegron monotherapies compared with low dose combination therapy.
Methods
This prospective, observational & questionnaire-based study was conducted in the Department of Urology and Department of Pharmacology of V.S.S. Institute of Medical Science & Research, Burla, for a period of 6 months (April-September 2019), after obtaining approval from IEC (Institutional Ethics Committee).
A total number of 70 patients aged ≥18 years with OAB symptoms, coming to urology OPD were included in the study after taking written informed consent. They were divided into 3 groups:
Group 1: 24 patients receiving solifenacin 10mg/day.
Group 2: 23 patients receiving solifenacin 5mg/day plus mirabegron 25mg/day
Group 3: 23 patients receiving mirabegron 50 mg/day.
They were followed up after 4 weeks and 12 weeks and change in the parameters from baseline were noted. During the follow up period 6 patients from group 1 and 4 patients from group 3 were lost to follow up.
Patients were assessed for change in mean numbers of micturition, urgency, incontinence, nocturia per 24 hour and quality of life using PPBC questionnaire.
Inclusion Criteria
Male or female patient ⩾18 years of age with symptoms of OAB
Exclusion Criteria
Pregnant / lactating Female
Neurogenic bladder.
Patient with significant stress/urgency incontinence
An indwelling catheter.
Patient with diabetic neuropathy.
Uncontrolled hypertension.
Evidence of a symptomatic UTI, interstitial cystitis, bladder stones, malignant disease
of the pelvic organs.
Uncontrolled narrow angle glaucoma, urinary or gastric retention.
Severe renal impairment or End Stage Renal disease.
Statistical Analysis
Statistics have been done using Graph pad prism version 6.0 using ANOVA and non-parametric scales. p-value < 0.05 was taken as statistically significant.
Results
Table 1: Age Distribution of Patients with OAB
| Age | No./ Percentage of cases |
| < 25 year | 02 (3.33%) |
| 25 – 50 year | 15 (25%) |
| > 50 year | 43 (71.67%) |
| Mean age (Mean ± SD) | 51.3 ± 12.7 |
Overall, 70 patients complaining of bladder difficulties had been recruited at the beginning, of which 10 patients were lost to follow up. Out of 60 patients, majority of patients belong to age group> 50 years as shown in Table 1.
Table 2: Sex Distribution of Patients with OAB
| Gender | No./ Percentage of cases |
| Males | 18 (30%) |
| Females | 42 (70%) |
Out of 60 cases, 18 (30%) were males and 42(70%) were females as presented in Table 2.
Table 3: Duration of Symptom and Baseline Data of OAB Patients
| Baseline Data | Mean ± SD |
| Duration of symptoms (in months) | 38.01± 9.91 |
| Number of micturitions/24hr | 9.58± 1.68 |
| Urgency episodes/24hr | 3.25± 2.24 |
| Incontinence episodes/24hr | 2.11± 1.78 |
| Nocturia/24hr | 2.25± 1.12 |
The mean duration of symptoms was 38.01± 9.91 months. The baseline micturition data in terms of mean number of micturitions, urgency, incontinence episodes and nocturia has been presented in Table 3.
Table 4: Mean Change in Frequency of Micturition/24hr among Different Drug Groups
| SOLI 10
n = 18 |
SOLI5+MIRA25
n = 23 |
MIRA 50
n =19 |
|
| Baseline | 9.778±0.249 | 9.793±0.289 | 9.211±0.554 |
| 4thweek (1st Visit) | 8.056±0.235 | 7.478±0.250 | 7.316±0.367 |
| 12th week (2ndVisit) | 6.167±0.020 | 4.696±0.146*** | 5.474±0.159 |
Data expressed as mean ± SEM and analysed by ANOVA *P<0.05, (**) P<0.01, (***) P<0.001
Compared with monotherapies, the combination therapy resulted in a significant improvement in reducing the mean number of micturitions/ 24hr from baseline to End of Treatment (EoT).
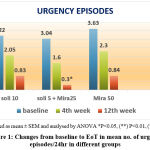 |
Figure 1: Changes from baseline to EoT in mean no. of urgency episodes/24hr in different groups |
Out of all the three groups the frequency of urgency episodes was significantly reduced (3.04-0.3) in patients taking combination therapy as compared to the solifenacin and mirabegron monotherapy.
Table 5: Change from baseline to EoT in mean no of incontinence episodes/24 hr in all three groups
| SOLI 10
n = 18 |
SOLI5+MIRA25
n = 23 |
MIRA 50
n =19 |
|
| Baseline | 1.889±0.386 | 2.087±0.343 | 2.368±0.496 |
| 4th week (1st Visit) | 1.222±0.274 | 0.652±0.161 | 1.316±0.359 |
| 12th week (2ndVisit) | 0.500±0.166 | 0.173±0.080 | 0.368±0.156 |
In all the treatment groups a trend towards a decrease in mean number of incontinence episodes/24hr was observed from baseline to EoT but the reduction was not statistically significant.
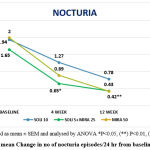 |
Figure 2: mean Change in no of nocturia episodes/24 hr from baseline to EoT. |
The above graph showed statistically significant improvement in mean no of nocturia episodes/24hr in the patients taking combination therapy.
Table 6: mean change in PPBC SCORE from baseline to EoT
| SOLI 10
n = 18 |
SOLI5+MIRA25
n = 23 |
MIRA 50
n =19 |
|
| baseline | 4.444±0.145 | 4.391±0.163 | 4.737±0.200 |
| 4th week (1st Visit) | 3.500±0.121 | 3.217±0.156 | 2.947±0.178 |
| 12th week (2ndVisit) | 2.222±0.152 | 1.609±0.104** | 1.789±0.144 |
Patients with major PPBC improvement from baseline to EoT belonged to the combination therapy when compared to the other two monotherapies.
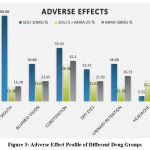 |
Figure 3: Adverse Effect Profile of Different Drug Groups |
The frequency of antimuscarinic side effects (dry mouth, constipation, urinary retention) had lower incidence in combination therapy compared with solifenacin group. Mirabegron group had more incidence of headache compared to other two groups.
Discussion
OAB is a common and bothersome symptom complex, which significantly affects patient’s quality of life.13
The PPBC (Patient Perception of Bladder Control) is a valid and responsive global measure of bladder condition that performs well among patients with OAB. The PPBC was also highly responsive to improvements in micturition frequency, urgency episodes, incontinent episodes, nocturia and patient-reported HRQL.11
In this study the mean age was found to be 51.3 years, which corroborates with the study conducted by Abrams et al which showed mean age to be 54.6 years.14 This signifies that OAB is more common in older age group. In the present study, majority of study cases were females, mean percentage being 71.42 which is similar to a study done by Khuller et al where female prevalence was 72%.15 This reveals that females are more prone for developing OAB related symptoms.
The mean duration of symptoms in this study was 38.01 months which was comparable to a study done by Paul Abrams et al, which showed mean value to be 48.5 months.14
In the current study combination therapy has significantly reduced the mean no. of micturitions /24hr from 9.79 at baseline to 4.69 at 12 weeks (Table 1). A study done by Krauwinkle et al, showed that the mean number of micturition was decreased from 10.73 to 8.24.16
In the present study the mean reduction in urgency episodes/day was from 3.09 to 0.30 in the combination group, which was statistically significant and better than the findings in a study by Yamaguchi et al (2.86 to 1.82).17
In this study the mean reductions in incontinence episodes shown by the combination group was comparable to the other two monotherapies. No statistically significant difference was seen among different drug groups in reduction of incontinence episodes. This finding corroborates with the study done by Christian Gratzke et al.18
In the current study, the mean reduction in number of nocturia episodes was comparable in two groups that is in combination group (1.6 to 0.4) and mirabegron monotherapy (2 to 0.42) Figure 2. There was statistically significant reduction in mean number of nocturia episodes per 24hr in combination group. This finding was in agreement to study done by Yamaguchi et al.17
The mean reduction in PPBC score in the current study improved significantly with combination therapy (4.3 to 1.3) compared to solifenacin group (4.4 to 2.2) and mirabegron group (4.7 to 1.7).
As shown in Figure 3, higher incidence of adverse effects like dry mouth, blurred vision, constipation and urinary retention was seen in solifenacin group as compared to mirabegron and combination group. Incidence of headache was found to be higher in mirabegron group as compared to the other two groups. Occurrence of the above-mentioned adverse effects were comparable to a study done by Jose E. Batista et al.19
Limitation of the study
Small sample size, short follow up duration and the fact that a single item questionnaire cannot provide the depth or breadth of information that can be obtained from multi-item measures.
Conclusion
Analysis of combination therapy of solifenacin and mirabegron demonstrated significant improvements over monotherapies (solifenacin 10 mg & mirabegron 50 mg) in frequency of micturition, urgency and nocturia episodes without increasing bothersome adverse effects associated with antimuscarinic therapy. The combination of mirabegron and an antimuscarinic agent may provide an attractive therapeutic approach to maximise efficacy and minimise the side effect burden.
Acknowledgement
We would like to acknowledge the help extended by Department of Urology and Department of Pharmacology. We would also like to thank all the patients who took part in our study.
Conflict of Interest
There is no conflict of interest.
Funding source
There is no funding Sources.
References
- Abrams P, Artibani W, Cardozo L, Dmochowski R, Van Kerrebroeck, Sand P. Reviewing the ICS 2002 terminology report: the ongoing debate. Neurourol Urodyn., 2009; 28(4): 287.
CrossRef - Coyne KS, Sexton CC, Irwin DE, et al. The impact of overactive bladder, incontinence and other lower urinary tract symptoms on quality of life, work productivity, sexuality and emotional well-being in men and women: Results from the EPIC study. BJU Int., 2008; 10: 1388–1395.
CrossRef - Benner J., Nichol M., Rovner E., Jumadilova Z., Alvir J., Hussein M., et al. (2010) Patient-reported reasons for discontinuing overactive bladder medication. BJU Int., 2010 May; 105(9): 1276–1282.
CrossRef - Takeda M, Obara K, Mizusawa T et al. Evidence for b3-adrenoceptor subtypes in relaxation of the human urinary bladder detrusor: analysis by molecular biological and pharmacological methods. J Pharmacol Exp Ther., 01 Mar 1999; 288(3): 1367-1373.
- Igawa Y, Yamazaki Y, Takeda H et al. Functional and molecular biological evidence for a possible b3-adrenoceptor in the human detrusor muscle. Br J Pharmacol., 1999; 126: 819–825.
CrossRef - Fujimura T, Tamura K, Tsutsumi T et al. Expression and possible functional role of the b3-adrenoceptor in human and rat detrusor muscle. J Urol., 1999; 161(2): 680–685.
CrossRef - Kullmann FA, Downs TR, Artim DE et al. Urothelial b3-adrenergic receptors in the rat bladder. Neurourol Urodyn., Jan 2011; 30(1): 144-150.
CrossRef - Otsuka A, Shinbo H, Matsumoto R, Kurita Y, Ozono S. Expression and functional role of b-adrenoceptors in the human urinary bladder urothelium. NaunynSchmiedebergs Arch Pharmacol., June 2008; 377(4-6): 473–81.
CrossRef - Nitti VW, Rosenberg S, Mitcheson DH, He W, Fakhoury A, Martin NE. Urodynamics and safety of the b3-adrenoceptor agonist mirabegron in males with lower urinary tract symptoms and bladder outlet obstruction. J Urol., Oct 2013; 190(4): 1320–1327.
CrossRef - Korstanje C, Someya A, Yanai H, et al. Additive effects for increased bladder storage function with the antimuscarinic drug solifenacin and the selective b3-adrenoceptor agonist mirabegron in two rat models for in vivo bladder function. Poster 81 presented at: 43rd Meeting of the International Continence Society; August 26–30,2013; Barcelona, Spain.
- Matza LS, Thompson CL, Krasnow J, Brewster-Jordan J, Zyczynski T, CoyneKS. Test-retest reliability of four questionnaires for patients with overactive bladder: the overactive bladder questionnaire (OAB-q), patient perception of bladder condition (PPBC), urgency questionnaire (UQ), and the primary OAB symptom questionnaire (POSQ). Neurourol Urodyn., 2005; 24(3): 215–225.
CrossRef - Karin S. Coyne, Louis S. Matza, Zoe Kopp, et al. The Validation of the Patient Perception of Bladder Condition (PPBC): A Single-Item Global Measure for Patients with Overactive Bladder. Neurourol., June 2006; 49(6): 1079-1086.
CrossRef - Irwin D., Milsom I., Hunskaar S., Reilly K., Kopp Z., Herschorn S., et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: results of the EPIC Study. Eur Urol., Dec 2006; 50(6): 1306-1314.
CrossRef - Abrams P, Kelleher C, Staskin D et al. Combination treatment with mirabegron and solifenacin in patients with overactive bladder: efficacy and safety results from a randomised, double-blind, dose-ranging, phase 2 study (Symphony). Eur Urol., Mar 2015; 67(3): 577–88.
CrossRef - Khullar V, Amarenco G, Angulo JC, et al. Efficacy and tolerability of mirabegron, ab (3)-adrenoceptor agonist, in patients with overactive bladder: Results from a randomised European-Australian phase 3 trial. Eur Urol., Feb 2013; 63(2): 283–95.
CrossRef - Walter J J Krauwinkel, Virginie M M Kerbusch, John Meijer,Reiner Tretter, Gregory Strabach, et al. Evaluation of the pharmacokinetic interaction between the b3-adrenoceptor agonist mirabegron and the muscarinic receptor antagonist solifenacin in healthy subjects. Clin Pharmacol Drug Dev., July 2013; 2(3):255–263.
CrossRef - Yamaguchi O, Kakizaki H, Homma Y, et al. Safety and efficacy of mirabegron as ‘add-on’ therapy inpatients with overactivebladder treated with solifenacin: a post-marketing, open-label study in Japan (MILAI study). BJU Int., 2015; 116(4): 612–622.
CrossRef - White WB, Chapple C, Gratzke C, et al. Cardiovascular safety of the b3-adrenoceptor agonist mirabegron and the antimuscarinic agent solifenacin in the SYNERGY trial. J Clin Pharmacol., Aug 2018; 58(8): 1084-1091.
CrossRef - Batista J., Kolbl H., Herschorn S., Rechberger T., Cambronero J., Halaska M., et al. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a noninferiority, randomized, phase IIIb trial. Ther Adv Urol., Aug 2015; 7(4): 167–179.
CrossRef








