Preeja Chandran , Khaviyaa Chandramohan
, Khaviyaa Chandramohan , Krithika Iyer
, Krithika Iyer , Felicia Mary Michael
, Felicia Mary Michael , Prakash Seppan
, Prakash Seppan and Sankar Venkatachalam*
and Sankar Venkatachalam*
Department of Anatomy, Dr.Arcot Lakshmanasamy Mudaliar Postgraduate Institute of Basic Medical Sciences, Taramani Campus, University of Madras, Chennai 600113, India.
Corresponding Author Email: venkatsankar@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2359
Abstract
Experimental studies found extracts of Mucuna pruriens (L.) DC, a plant used in the traditional medical systems to possess strong anti-inflammatory and anti-oxidant properties among a wide range of other beneficial effects. Hypothesizing the possibility for a multifaceted beneficial effect, the therapeutic potential of the ethanolic extract of Mucuna pruriens (MP) in treating spinal cord injury (SCI) was tested using the highly standardized Multicenter Animal Spinal Cord Injury Study (MASCIS) animal model of contusive SCI. Male Wister rats with SCI were treated with the ethanolic extract of MP at a dose of 200 mg/kg/day for 10 weeks. The outcome was assessed through molecular, biochemical, histological, and behavioral scoring parameters. Results indicated anti-apoptotic, anti-necrotic, anti-inflammatory, and neuroprotective effects of MP. Nevertheless, many of these beneficial effects were not statistically significant and there was no functional improvement due to MP treatment. MP at comparable doses was reported to be significantly effective in mitigating oxidative stress and/or inflammation under various other pathological conditions. Lack of significance in the present study could be due to the presence of blood-spinal cord-barrier; that might have prevented the components of MP from reaching spinal cord tissue in adequate quantities. Additionally, heterogeneity in the function of the cells typical to the CNS environment could be the reason for the failure of recovery. To conclude, the limitations posed by the structural and functional distinctions in the spinal cord environment in comparison to other non-CNS tissue environments deserve attention while adjudicating the efficacies of herbal remedies for SCI.
Keywords
Alternative Medicine; Herbal Product; Mucuna pruriens; Neuroprotection; Spinal cord injury; Spinal cord injury
Download this article as:| Copy the following to cite this article: Chandran P, Chandramohan K, Iyer K, Michael F. M, Seppan P, Venkatachalam S. Beneficial Effects of Ethanolic Extract of the Medicinal Herb Mucuna Pruriens Against Oxidative Stress and Inflammation Might be Limited in Contusive Spinal Cord Injury. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Chandran P, Chandramohan K, Iyer K, Michael F. M, Seppan P, Venkatachalam S. Beneficial Effects of Ethanolic Extract of the Medicinal Herb Mucuna Pruriens Against Oxidative Stress and Inflammation Might be Limited in Contusive Spinal Cord Injury. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3IGBSwn |
Introduction
Traumatic spinal cord injury (SCI) in humans is mostly of contusion type and is known for its poor prognosis 1,2. The initial trauma is referred to as ‘primary injury’ and is followed by ‘secondary injury’ which involves several pathological changes such as ischemia, thrombosis, edema, free radical release, electrolyte imbalances, excitotoxicity, etc. (1) The secondary injury is held responsible for delayed cell death, axonal degeneration, and demyelination 3,4 all of which hamper the functional recovery 5. Despite promising experimental approaches reported to counter secondary injury,6 contusion SCI remains a challenging neurological disorder with no definitive therapy 1 prompting the exploration of novel approaches.
Mucuna pruriens (L.) DC. a leguminous plant, found in India and other places (International Legume Database and Information Service (ILDIS) 2014] is used in Indian traditional medical systems viz. Ayurveda and Siddha to treat reproductive and neurological disorders 7,8. It has been referred to by different names in different languages and in English, it is referred to as Velvet bean or Cowhage 9. The book “Indian Materia Medica” reported the seed and leaf extracts as nervine tonic and were recommended for the treatment of nervous disorders such as facial paralyzes, hemiplegia, etc. 9.
Several experimental studies reported the efficacy of Mucuna pruriens (MP) seeds’ extract in treating conditions like rheumatoid arthritis, diabetes, atherosclerosis, nervous disorders, and male infertility 10. Its potential in treating Parkinsonism is a widely published finding owing to its high L-Dopa content 11. It possesses antioxidant properties by enhancing the levels of enzymic and non-enzymic antioxidants 10. Its anti-lipid peroxidation property 12 and anti-inflammatory activities were also previously reported 13. In addition to these properties, ethanolic extract of Mucuna pruriens seeds (MP) was reported to exhibit anti-apoptotic, anti-hyperglycemic, neuroprotective, androgenic, and aphrodisiac effects when used to treat male reproductive system disorders caused by a variety of factors such as diabetes, hyperlipidemia, testicular trauma and aging 14–17. Toxicity analysis did not indicate any potential harmful effects of Mucuna extracts. Systemic administration of mucuna extract was reported to be safe and it was concluded that mucuna extract can be used safely in rodent models 18.
Under these circumstances, it was intended to test the efficacy of the MP in treating contusion SCI. Inflammation, oxidative stress, and delayed cell death are among the primary reasons for the pathological changes after SCI, and the reported effects of MP in mitigating all these changes in other organ systems were the rationale for undertaking the present study. This reasons for the pathological changes after SCI, and the reported effects of MP in mitigating all these changes in other organ systems were the rationale for undertaking the present study. This
Materials and Methods
The study protocol was approved by the Institute’s Animal Ethics Committee (IAEC), which is functioning under the supervision of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Government of India. Animal maintenance standards were as per the specifications prescribed by the relevant international and national guidelines. Male rats of Wistar-Albino strain weighing 200 g to 250 g [70-90 days old) were procured from the central animal house facility of the institute. Animals were maintained under standard conditions prescribed for laboratory rats and all the maintenance standards were as per relevant national and international guidelines (CPCSEA Guidelines and Canadian Council for Experimental Animal Care Guidelines).
Animal groups
In the present study, data/observations from a total of 72 animals were included. These animals were from the following four groups of 18 each.
Control – Subjected to sham surgery
Control+MP – Sham-operated treated with MP
SCI – Spinal cord injured
SCI+MP – Spinal cord injured treated with MP
Under each group, animals were euthanized at 2 weeks and 10 weeks termed as short-term and long-term respectively. Results in the short term were analyzed using biochemical (n=6] and molecular biology methods (n=6]. Long-term animals were scored for motor deficit/recovery and spinal cords from euthanized animals were used for histological studies (n=6].
Contusion SCI and animal care
All surgeries were conducted under aseptic conditions. A contusion SCI at a lower thoracic level was created following the MASCIS protocol 19 as described previously. Briefly, under surgical anesthesia through a combination of ketamine 80mg/kg (Aneket, Neon Labs, Mumbai, India) and xylazine 10 mg/kg (Xyloxin, Indian Immunologicals Ltd, Hyderabad, India) administered intra-peritoneal, the spinal cord at the T10 spinal segment was exposed through standard laminectomy procedure19. After immobilizing the vertebral column using spinal clamps, with dura intact, a moderate contusion injury (12.5 mm drop) was created using MASCIS impactor version III after fixing the vertebral column using clamps (Spinal clamps and impactor obtained from W. M. Keck Center for Collaborative Neurosciences, Rutgers University, NJ, USA). Consistency in the lesion was verified through real-time monitoring of various parameters through the MASCIS III software, intra-operative observation of cord discoloration, and post-operative day one observations. Post-injury, the wound was closed in layers and skin incision approximated with surgical clips. The animals received prophylactic doses of 20 mg/kg/day of ampicillin (Campicilin, Cadila Pharma, Ahmedabad, India) and 2 mg/kg/day gentamycin (Garamycin, Fulford (India) Ltd, Mumbai, India) through intramuscular injections for 3 days following surgery to prevent any infection. Urine retention due to bladder paralysis was relieved through manual bladder expression thrice, daily. In general, the postoperative period of the animals was uneventful.
Mucuna pruriens extract preparation and characterization
An ethanolic extract of the seeds of the MP was used in the study. The authentication process of the acquired seeds, extract preparation and its characterization was as per the procedures described previously 15. Briefly, the seeds of MP were procured locally and after thorough washing in distilled water were dried in the shade for 7–12 days, and were powdered. The solvent ethanol was added to the powder and was incubated for 72 h with constant shaking. The filtrate was vacuum concentrated which gave a yield of around 20%. The extracts were used for studies when the L-dopa content assessed by HPLC is above 25%.
Dosage determination
The acute toxicity tests were conducted following the Organization for Economic Cooperation and Development (OECD) guidelines, 2001. Dosages from 50 mg to 2500 mg were tested. While no acute toxic effects were observed, long-term follow-up studies indicate mild toxic effects in doses excess of 200 mg/kg/day. In the literature, nephrotoxicity was reported by Gblotolorun et al. (Gbotolorun et al., 2018] at doses of 200 mg/kg. In a contemporary lab of our department, a dose of 200 mg/kg/day has been tested over a decade under various experimental conditions and was found to be very effective in mitigating the harmful effects of oxidative stress and inflammation. Therefore, for the present study, the dosage was fixed as 200 mg/kg/day. For the Control+MP and SCI+MP group animals, the determined dose was administrated orally, in saline as the vehicle from post-operative day one to the end of the experiment period.
Short-term outcome analyses
At two weeks post-injury, animals were euthanized by an overdose of anesthesia (Ketamine and Xylazine combo as described above), and spinal cord of 1 cm long segment with lesion epicenter in the middle was quickly removed and was processed suitably for qRT-PCR / Western Blot / Biochemical assays.
Estimation of mRNA levels using qRT-PCR
Total RNA was isolated using the Trizol method (Invitrogen, California, USA) following the kit manufacturer’s protocol. The purity of isolated RNAs was checked with Nanodrop and their integrities were assessed by running in agarose gel & visualization of bands under ethidium bromide staining. The mRNAs in the extracts were reverse transcribed to cDNA using Omniscript RT kit (Qiagen, Hilden, Germany). The cDNA was diluted at a 1:25 ratio in ultra-pure water and the mRNA levels of Casp3, Parp1, Gap43, and Gfap were analyzed using appropriate Taqman assays (Thermofisher Scientific (USA). Gapdh and Hprt were used as internal controls and the results were represented as relative fold changes using the 2^- ΔΔCT method 20. The Thermofisher Scientific catalogue numbers of the Taqman probes used in the study were – Casp3 (Rn 00563902_m1], Parp1 (Rn 00565018_m1], Gap43 (Rn 01474579_m1], Gfap (Rn 00566603_m1], Gapdh (Rn 01462662_g1] and Hprt (Rn 01527840_m1].
Western blotting
Protein from the spinal cord’s injury segment was extracted using RIPA buffer. Protein levels were estimated by using the Bradford assay kit (Biorad, California, USA). Equal amounts of total protein [30 µg) from all the samples were segregated in SDS-PAGE [7.5-12%) and were transferred to PVDF membranes following the standard western blotting protocol. Membranes with botted proteins were incubated with primary antibodies for ED1 (#Ab31630], NFkB (#SC114], VEGF (#SC152], NOS1 (#SC8309], GFAP (#Ab7260], and neuN (#Ab177487] from suppliers (Abcam, UK / Santa-Cruz, USA). In the next stage, membranes were incubated with appropriate HRP conjugated secondary antibodies (Abcam, UK) and bands were visualized by using an ultra-sensitive enhanced chemiluminescent (ECL) kit (SuperSignal West Femto Kit, Thermo Scientific, USA). Normalization of the data was carried out using total protein estimation. Values were represented as the percentage of protein levels when compared with control which was taken as 100%. 21.
Biochemical assays for lipid peroxidation and antioxidants
Estimation of Lipid peroxidation (LPO) was carried out following the procedure of Hogberg et al., 22. Accordingly, Malondialdehyde (MDA), formed as an end product of peroxidation of lipids, served as an index of the intensity of oxidative stress and level of lipid peroxidation 23. The amount of protein in the tissue homogenate was estimated by Bradford assay using bovine serum albumin as the standard.
The enzyme superoxide dismutase (SOD) activity was measured by using the degree of inhibition of auto-oxidation of pyrogallol, in an alkaline pH by SOD 24. The enzyme activity is defined as the enzyme required for 50% inhibition of pyrogallol auto-oxidation/min (units/ mg protein). The assay described by Sinha was applied to evaluate the catalase (CAT) activity 25. Ascorbic acid 26 and α-Tocopherol 27 were measured and the levels were expressed as μg/g protein. To facilitate comparison, the assay results of enzymic and non-enzymic antioxidants were presented as a percentage compared to the respective controls.
Long-term outcome analyses
Behavior-based scoring for functional deficit
Animal behavior-based scoring was carried out to quantify the motor functional deficit/recovery. General gross locomotor ability was quantified using the open field locomotor test score viz. BBB scoring method 28. Any animal not showing zero scores on the 1st day of the postoperative period was not included in the study. In the long-term animals, at weekly intervals, BBB scoring was carried out. Additionally, animals were tested using the narrow beam walking test and horizontal ladder crossing test. These two tests were meant for assessing fine motor control in the animals29,30. An inclined plane balancing test was also conducted at weekly intervals to assess the extrapyramidal tract functions 31.
Histological analysis
Long-term animals at the end of 10 weeks were euthanized by an overdose of anesthesia and tissues were fixed immediately in 4% paraformaldehyde in PBS which was administered through transcardial perfusion. The vascular tree was washed by PBS before the administration of the fixative. A 1 cm length spinal cord with the lesion epicenter in the middle was used. The paraffin-embedded tissues were sectioned in a sagittal plane and prepared for histological analysis. The lesion area was estimated from the H & E stained serial sections using ImageJ software and was expressed in percentage in comparison to the control group.
Proximal and distal segments to the injury segment of the spinal cord mentioned above were also used to check demyelination status and cell death induced by the SCI. Myelination was measured from 20 µm thickness cross-sections stained by LFB using ImageJ software after supersaturating the signals as described previously 32. Results were illustrated as a percentage of myelinated area out of the total area. Cross-sections cut at 10 µm thickness were stained with a modified trichrome staining method to identify and quantify apoptotic cells 33. Results were expressed as the number of apoptotic cells identified in the grey matter under a 40X objective lens. All the tissue processing, sectioning, and staining procedures were carried out as described in standard histopathological textbooks 34
Data representation and statistical analysis
Numeric data were presented as histograms/line charts and values represent group mean + standard error of the mean. Statistical analysis was performed with GraphPad Prism Version 7.0. Data were compared using one-way ANOVA. Statistical significance was tested at p < 0.05 levels.
Results
Real-time PCR results showed increased Casp3, Parp1 expressions in the SCI group indicative of increased cell death due to apoptosis and necrosis. MP treatment reduced the corresponding mRNA levels, signaling a reduction in cell death. (Fig. 1 A & B). Gap43 mRNA levels were more and Gfap mRNA levels were less in MP treated group when compared with the SCI group (Fig. 1 C & D); which symbolizes increased axon regeneration attempts and reduction in glial scarring. Reductions in Parp1 and Gfap expressions seen in the MP group were statistically significant when compared with the values in the SCI group. Other differences were not significant.
 |
Figure 1: Real-time RT-PCR analyses of mRNAs associated with cell death and regeneration. |
MP treatment appears to reverse the changes induced by SCI in the mRNA levels of genes responsible for cell death (casp3, Parp1), axon sprouting (Gap43), and glial scarring (Gfap). At p < 0.05 level, * represents statistical significance when compared with the SCI group and # comparison with the Ctrl group. Values represent group mean + standard error of the mean.
Western blotting for relative quantification of protein levels largely corroborates PCR results. Increased levels of ED1, NOS1, and NFkB in the SCI group when compared with control, indicated the expected increased inflammatory and cellular stress levels. MP treatment was found to reverse the trend. MP treatment increased VEGF and NeuN levels thus could mean neovascularization and better survival of neurons (Fig. 2] which are supportive of the pro-regenerative signs seen in RT-PCR analysis. Out of these, changes induced by MP in NFkB levels were significant. –
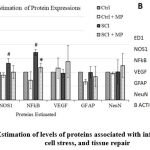 |
Figure 2: Estimation of levels of proteins associated with inflammation, cell stress, and tissue repair. |
An increase in ED1, NOS1, and NFkB levels in SCI shall indicate increased inflammation and cell stress. MP treatment reversed these changes with a significant reduction in NFkB levels. MP increased the levels of VEGF (vasculogenesis), NeuN (neuron survival), and reduce GFAP (glial scarring) all indicative of neuroprotection. Nevertheless, these changes were not significant statistically. At p < 0.05 level, * represents statistical significance when compared with the SCI group and # comparison with the Ctrl group. Values represent group mean + standard error of the mean.
The biochemical assay showed a significantly increased lipid peroxidation due to SCI and was found to be reduced by MP treatment. The levels of antioxidant enzymes sodium dismutase (SOD) were also reduced significantly due to SCI. Levels of catalase (CAT), as well as non-enzymic antioxidants viz. vitamin C & E, were not altered significantly by the SCI or by MP treatment (Fig. 3].
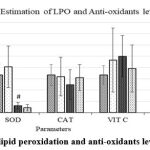 |
Figure 3: Estimation of lipid peroxidation and anti-oxidants levels at 2 weeks after SCI. |
SCI caused significantly increased lipid peroxidation (LPO) and reduced levels of Sodium Dismutase and Catalase. While MP reduced LPO, there was no effect on SOD and CAT. Vit C and E were unaffected by SCI. At p < 0.05 level, # represents statistical significance when compared with the Ctrl group. Values represent group mean + standard error of the mean.
Observations from histological studies were in line with the molecular analyses. SCI had significantly increased the lesion area in comparison to the control status as one would expect. MP treatment had reduced this lesion area (Fig. 4A) thus suggesting reduced cell death which can be further supported by the apoptotic cell counting estimates (Fig. 5B). Apoptotic cell quantification using modified Trichrome staining showed increased cell death in SCI which was mitigated by MP treatment (Fig. 5B). Surprisingly, the loss of myelination caused by SCI appears to be unaffected by the MP treatment (Fig. 5A).
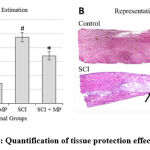 |
Figure 4: Quantification of tissue protection effects of MP |
A. Estimation of tissue loss by quantifying space in serial sagittal sections of the spinal cord indicated a tissue loss of up to 50% due to SCI. MP treatment reduced this loss significantly. At p < 0.05 level, * represents statistical significance when compared with the SCI group and
# comparison with the Ctrl group. Values represent group mean + standard error of the mean.
B. Representative reconstructed histology images illustrating the effect of tissue loss due to SCI. Arrows bound the lesion epicenter with the typical narrowing of the spinal cord and formation of the necrotic cavity (c) in the lesion site.
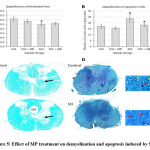 |
Figure 5: Effect of MP treatment on demyelination and apoptosis induced by SCI |
The myelinated area as a percentage of the total cross-sectional area of the spinal cord showed reduction due to SCI as expected due to demyelination. MP treatment had no effect on it.
Apoptotic cells were found to be significantly increased due to the SCI. The numbers of such cells were significantly reduced in the MP treatment group. At p < 0.05 level, * represents statistical significance when compared with the SCI group and # comparison with the Ctrl group. Values represent group mean + standard error of the mean.
Representative LFB stained spinal cord sections used for ImageJ-based quantification of myelination area. SCI can be seen to have caused demyelination and loss of cortico-spinal tract (CST). Demyelination may be evident not only in the white matter but also in the grey matter as arrows here show the differences between normal and injured spinal cord.
Representative images of spinal cord sections stained by the modified trichrome method used to calculate apoptotic cells. Insets represent enlarged portions marked in while rectangles illustrate normal (yellow arrow) and apoptotic cells (red arrows).
Behavior-based scoring to assess motor deficit/recovery showed barely small differences induced by MP treatment in the open field locomotion test and other fine motor skill tests like narrow beam walking test and inclined plane test while no effect in the horizontal ladder walking test (Fig. 6).
 |
Figure 6: Role of MP treatment in producing functional recovery after SCI. |
In an open field locomotion test, a normal animal would score 21 points on the BBB scale. As seen here, SCI severely affects the motor ability with a score of around 8 only.
Normal animals cross a horizontally kept ladder with 40 rungs with only 1 or 2 foot placing errors. SCI causes paralysis with a theoretical maximum error of 40. Over the period, performance improved but never reaches near-normal levels.
Normal animals easily balance an inclination angle of 80 to 85 degrees. SCI animals could never balance beyond 65 degrees.
In the narrow beam tests, 6 is the maximum score for normal performance. The score never goes beyond 1.5 in SCI animals for 10 weeks post-injury.
PLEASE NOTE: To avoid cluttering, only SCI and SCI + MP group values were plotted here and Ctrl and Ctrl + MP group values are to the full extent as mentioned vide supra. At the p < 0.05 level, the differences between the group means plotted here are not statistically significant. Values represent group mean + standard error of the mean.
It is most important to note that as illustrated (please refer to the illustrations and legends), barring a few parameters, the differences between SCI and MP treated groups were not statistically significant.
Discussion
Prospects of using MP to treat SCI
Inflammation and oxidative stress are the key players of secondary injury which prevents recovery in SCI 35,36. Anti-inflammatory and anti-oxidant properties are the much acclaimed beneficial aspects of MP treatment 12,13,37. Therefore, MP can be a prospective candidate for SCI treatment. In addition to the anti-inflammatory and anti-oxidant properties, MP was reported to exhibit anti-tumorigenic, anti-diabetic, anti-microbial, anti-venom, and androgenic properties through various bio-active mechanisms 7,10,38,39. The high L-dopa content of MP 11 had led to its application against Parkinsonism for achieving neuroprotection 40 in dopaminergic neurons. It is noteworthy here that dopamine is also a potential candidate for circuit reorganization after SCI 41. Administration of MP increased body weight 42 as it can be a rich source of protein and minerals 43–45. Reports about the anti-apoptotic effects of MP and its ability to maintain mitochondrial membrane potential were impressive 17.
Thus MP appears to possess a multitude of beneficial aspects through different bio-active mechanisms. These aspects are in favor of MP usage in SCI. It is indeed a surprise that there were no previous studies about the role of MP in SCI. Therefore, the present study was undertaken to test whether this seemingly “wonder cocktail” could be of some help in treating SCI.
Role of MP in treating SCI
Plant extracts or traditional medicine compounds were reported to be useful in SCI. Effectiveness of whole plant extracts of Nigella sativa 46, grape seed 47, Scutellaria baricalensis 48, garlic 49, spirulina 50, ginseng 51, Ginkgo biloba 52, and polyherbal formulations like JSK was reported to offer benefits 53. Isolated compounds from plants such as flavonoids, quercetin, resveratrol, curcumin, Epigallocatechin Gallate, etc. were also reported as potential candidates for consideration 54. Based on an ethnobotanical survey Mikawlrawng et al. 55, have listed about 40 different plants including MP used for the treatment of various paralyzes in Indian traditional medical practices. Due to varied experimental conditions, type of lesions, and outcome measures, a simple comparison of these herbal sources for their efficacy is difficult. However, invariably, all the works reported about the anti-inflammatory, anti-oxidant, and anti-apoptotic/neuroprotective effects of the plants/agents tested.
The effects of these herbal products in mitigating inflammation, enhancing antioxidant effects, reducing cellular death, and preventing tissue necrosis reported in those reports were seen in the present study also. Interestingly, in the studies cited above, either the functional recovery observed was small or not addressed.
Reduced ED1, NOS1, NFkB in the MP treatment group indicate reduced inflammation and macrophage infiltration to the injury site 56,57. Reduced Casp3 & Parp1, increased Gap43 & NeuN mRNA levels due to MP treatment suggest a reduced cell death and regenerative attempts. This can be supported by increased VEGF and reduced GFAP protein levels. As stated, histological parameters could indicate cytoprotection by MP and its pro-regenerative properties 58–60.
Given there is no standard therapy available for SCI and there is no previous study of MP usage for SCI, it is difficult to compare the effects observed with either a “standard drug” or with other studies. While the results of the present study, in general, corroborate the effects of other herbal products, unfortunately, there was no statistical significance for many biochemical and molecular parameters. Extremely small differences in the behavior scores without any statistical significance observed in the present study, seal the conclusion about the inadequacy of MP treatment effects in traumatic SCI.
Possible reasons for the lack of significant beneficial effects
It is indeed disappointing to note the lack of significant recovery after MP treatment in SCI. Nevertheless, a search for the possible reason(s) revealed interesting facts that may have some implications for testing herbal products for spinal cord injury repair and to design future studies using this extract.
The anti-inflammatory and anti-oxidant properties (reduction in NOS1 leading to a reduction in Ca++ influx and free radicals) of MP were suggested as the probable reason behind the observed anti-apoptotic effects of MP on the Schwann cells of the dorsal nerve of the penis 17. Hence, other effects such as anti-apoptotic, anti-necrotic, vasculogenic, tissue-protective, etc claimed under MP treatments could be epiphenomenon to its two fundamental effects viz. anti-inflammatory and anti-oxidant properties.
The lack of significant reduction of oxidative stress/inflammation by MP in the present study as revealed by parameters shall indicate the inadequacy of MP in achieving a potent antioxidant/anti-inflammatory role in SCI. The intriguing question is, while MP at comparable doses was found to be most effective in other conditions, why did it fail in the SCI?
The possibility of a botched extract preparation and species variation could be ruled out as the species authentication, extract preparation and its characterization were all performed as per established protocols referred above. Also, MP extract from the same lot of preparation when tested for other conditions was found to exhibit potent anti-oxidant and anti-inflammatory properties (data not shown). One curious observation was the high variations noted within the groups used for molecular parameters. The reason for such high intra-group variations is not known at present and in this regard, the intervention time of 2 weeks to study these changes may be inappropriate. Nevertheless, even in the long term, MP treatment failed to produce any significant functional recovery.
We speculate that the difference in the tissue environment between the central nervous system (CNS) and other organ systems could also be a reason for the observed inadequacy of MP effects in the spinal cord. The components in the MP extract might have failed to reach the spinal cord in adequate quantity as the spinal cord is a CNS organ protected by the blood-spinal cord – barrier. Complexly, its components might be metabolized in the liver before they can act on their targets. With no information available regarding the complete list of components/ metabolites and their permeability through the blood-spinal cord – barrier, this speculation is hard to either prove or disprove.
Need for further studies and future directions
Previous publications about MP had reported some interesting observations. Not only by its high L-Dopa content but MP was also reported to be a highly probable drug for Parkinsonism owing to its action involving NADH and Q10 61. Interestingly, in standard Parkinsonism therapy, L-Dopa is combined with carbidopa to inhibit the premature conversion of L-dopa in the periphery before reaching CNS. MP’s effectiveness for Parkinsonism without the co-administration of carbidopa could indicate that MP’s action might involve some other component and not just its L-Dopa content. Likewise, the reported differential action of MP in mediating cell death is also interesting. It is held that anti-oxidants jeopardize anti-cancer treatments 62,63. In contrast, despite its high anti-oxidant property and anti-apoptotic property elsewhere, Rajeshwar et al.10 and others 64 reported about the cell death induced in malignant cells treated with MP. This type of contrasting effect could be due to galic acid, a malignant cells treated with MP. This type of contrasting effect could be due to galic acid, a component of MP, which is a mutagenic and DNA damaging compound 65.
Failure of MP or its adverse effects were also reported by a few studies earlier. For e.g. the anti-inflammatory effect of MP in carrageenin-induced paw inflammation 66 was found to be no better than the standard drug aspirin 13. In one case, MP treatment was reported to be the cause of the precipitation of mania 67 possibly due to the surge in brain dopamine. Nephrotoxicity was reported by Gblotolorun et al. 68 at doses of 50 to 200 mg/kg. Importantly, in two clinical trials involving MP usage for Parkinsonism, the advantage of MP treatment could not be established 40,69.
From the aforesaid discussions, it would be obvious that MP can be a novel drug source yet unpredictable outcomes and failures are not uncommon. All these could be due to the variation in the composition of its contents. Thus it is possible that MP apart from having the already established components might contain yet to identify components whose variations can potentially impact the outcome. Identifying all the components in a plant extract might be a challenging task as the medicinal values (active components) of a given plant are influenced by external facts like soil type, rainfall, sunshine, etc 70,71.
In these contexts, further studies are required to know the components of MP and factors that can influence their levels. Such studies can eventually lead to the preparation of the appropriate components under synthetic/semisynthetic drug manufacturing processes as done in the case of some of the widely used drugs like metformin and paclitaxel 72.
Conclusion
The damage to the spinal neurons and severance of brain connection caused by the primary SCI are exacerbated by the secondary injury. Pharmacological interventions for SCI focus on mitigating the secondary injury thereby creating a conducive condition for the regenerative efforts by the host. In this regard, MP treatment to counter the secondary injury offered limited benefits at the cellular/tissue level but not at the functional level.
Through further studies, it may be necessary to identify the active components/metabolites of MP and the ways to improve their pass through the blood-spinal cord – barrier. Once that is accomplished, it would be interesting to see whether a statistically significant recovery can be achieved. Until such establishment of credibility, MP as a novel therapeutic agent for treating SCI could not be supported from the available data.
Acknowledgment
This study was partly supported by a research grant [No.58/55/2013-BMS Dated 19-03-2015] from the Indian Council of Medical Research (ICMR), Government of India to Dr. SP (PI), and Dr. VS (Co-PI). Ms. PC was supported by a fellowship from the Department of Biotechnology (DBT), Government of India. Ms. KC received a university research fellowship from the University of Madras. Dr.FMM was a recipient of the INSPIRE fellowship from the Department of Science and Technology (DST), Government of India. Ms.KI was supported by a fellowship from the Science and Engineering Research Board (SERB), Government of India. Funding agencies did not interfere in any aspect of the study.
Conflict of Interest
The Author declare that there is no conflict of interest.
Funding Sources
This study was partly supported by a research grant [No.58/55/2013-BMS Dated 19-03-2015] from the Indian Council of Medical Research (ICMR), Government of India to Dr. SP (PI), and Dr. VS (Co-PI).
References
- Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front Neurol. 2019;10(March):1–25.
CrossRef - Cheriyan T, Ryan DJ, Weinreb JH, Cheriyan J, Paul JC, Lafage V, et al. Spinal cord injury models: A review. Spinal Cord [Internet]. 2014;52(8):588–95. Available from: http://dx.doi.org/10.1038/sc.2014.91
CrossRef - DeRisi Joseph, Penland Lolita BPO, Tyagi S, Kramer FR, Group NP, DeRisi Joseph, Penland Lolita BPO. ©199 7 Nature Publishing Group http://www.nature.com/naturemedicine. Group [Internet]. 1996;4:303–8. Available from: http://www.ncbi.nlm.nih.gov/ pubmed/9585240
- Lu J, Ashwell KWS, Waite P. Advances in secondary spinal cord injury: Role of apoptosis. Spine (Phila Pa 1976). 2000;25(14):1859–66.
CrossRef - Hausmann ON. Post-traumatic inflammation following spinal cord injury. Spinal Cord. 2003;41(7):369–78.
CrossRef - Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7(8):628–43.
CrossRef - Lampariello L, Cortelazzo A, Guerranti R, Sticozzi C, Valacchi G. The magic velvet bean of mucuna pruriens. J Tradit Complement Med [Internet]. 2011;2(4):331–9. Available from: http://dx.doi.org/10.1016/S2225-4110(16)30119-5
CrossRef - Pathak- Gandhi N, Vaidya ADB. Management of Parkinson’s disease in Ayurveda: Medicinal plants and adjuvant measures. J Ethnopharmacol [Internet]. 2017 Feb;197:46–51. Available from: https://linkinghub.elsevier.com/retrieve/pii/ S0378874116305463
CrossRef - Nadkarni, K.M.; Nadkarni AK. The Indian Materia Medica. 3rd Editio. New Delhi: Popular Prakashan Pvt. Ltd.; 1976. 818–820 p.
- Rajeshwar Y, G M, Mazumber UK. Antitumor Activity and in vivo Antioxidant Status of Mucuna pruriens (Fabaceae) Seeds against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Iran J Phamacology Ther. 2005;4(1):46–53.
- Singh AP, Sarkar S, Tripathi M, Rajender S. Mucuna pruriens and Its Major Constituent L-DOPA Recover Spermatogenic Loss by Combating ROS, Loss of Mitochondrial Membrane Potential and Apoptosis. PLoS One. 2013;8(1):1–10.
CrossRef - Tripathi YB, Upadhyay AK. Antioxidant property of Mucuna pruriens Linn. Curr Sci. 2001;80(11):1377–8.
- Javed N, Alam SS, Subhani H, Akhtar MS, Khan AH. Evaluation of Anti-inflammatory Activity of Mucuna. Proceeding SZPGMI. 2010;24(2):97–102.
- Suresh S, Prithiviraj E, Prakash S. Effect of Mucuna pruriens on oxidative stress mediated damage in aged rat sperm. Int J Androl. 2010;33(1):22–32.
CrossRef - Suresh S, Prithiviraj E, Prakash S. Dose- and time-dependent effects of ethanolic extract of Mucuna pruriens Linn. seed on sexual behaviour of normal male rats. J Ethnopharmacol. 2009;122(3):497–501.
CrossRef - Suresh S, Prithiviraj E, Venkata Lakshmi N, Karthik Ganesh M, Ganesh L, Prakash S. Effect of Mucuna pruriens (Linn.) on mitochondrial dysfunction and DNA damage in epididymal sperm of streptozotocin induced diabetic rat. J Ethnopharmacol [Internet]. 2013;145(1):32–41. Available from: http://dx.doi.org/10.1016/j.jep.2012.10.030
CrossRef - Seppan P, Muhammed I, Mohanraj KG, Lakshmanan G, Premavathy D, Muthu SJ, et al. Therapeutic potential of Mucuna pruriens (Linn.) on ageing induced damage in dorsal nerve of the penis and its implication on erectile function: an experimental study using albino rats. Aging Male [Internet]. 2018 Feb 15;1–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29447059
- Ahmed S, Qureshi B, Mohtasheem M, Hasan U, Ahmed SW, Azhar I. Toxicity Assessment of Mucuna Pruriens Linn. Seeds. Int Res J Pharm. 2011;(May 2014):1–4.
- Young W. Spinal cord contusion models. Prog Brain Res. 2002;137(1975):231–55.
CrossRef - Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–8.
CrossRef - Michael FM, Chandran P, Chandramohan K, Iyer K, Jayaraj K, Sundaramoorthy R, et al. Prospects of siRNA cocktails as tools for modifying multiple gene targets in the injured spinal cord. Exp Biol Med [Internet]. 2019 Oct;244(13):1096–110. Available from: http://journals.sagepub.com/doi/10.1177/1535370219871868
CrossRef - Högberg J, Larson RE, Kristoferson A, Orrenius S. NADPH-dependent reductase solubilized from microsomes by peroxidation and its activity. Biochem Biophys Res Commun [Internet]. 1974 Feb;56(3):836–42. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4151195
CrossRef - Devasagayam TP. Lipid peroxidation in rat uterus. Biochim Biophys Acta [Internet]. 1986 May;876(3):507–14. Available from: http://www.ncbi.nlm.nih.gov/pubmed/ 3085721
CrossRef - Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem [Internet]. 1974 Sep;47(3):469–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/4215654
CrossRef - Sinha AK. Colorimetric assay of catalase. Anal Biochem [Internet]. 1972 Jun;47(2):389–94. Available from: https://linkinghub.elsevier.com/retrieve/pii/ 0003269772901327
CrossRef - Omaye ST, Turnbull JD, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues, and fluids. Methods Enzymol [Internet]. 1979;62:3–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/440112
CrossRef - QUAIFE ML, SCRIMSHAW NS, LOWRY OH. A micromethod for assay of total tocopherols in blood serum. J Biol Chem [Internet]. 1949 Oct;180(3):1229–35. Available from: http://www.ncbi.nlm.nih.gov/pubmed/18139216
CrossRef - Basso DM, Beattie MS, Bresnahan JC. A Sensitive and Reliable Locomotor Rating Scale for Open Field Testing in Rats. J Neurotrauma. 1995;12(1):1–21.
CrossRef - Blanco Lezcano L, Del L, Lorigados Pedre C, Fernández Verdecia CI, Serrano Sánchez T, Fuentes NP, et al. Convenience of the traversal beam test modified to evaluate the model of Parkinson’s disease in Rat lesioned in SNPC. J Cell Anim Biol [Internet]. 2009;3(9):145–51. Available from: http://www.academicjournals.org/JCAB
- Metz GA, Whishaw IQ. The ladder rung walking task: A scoring system and its practical application. J Vis Exp. 2009;(28):2–5.
CrossRef - Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp Neurol. 1995;132(2):220–8.
CrossRef - Ek CJ, Habgood MD, Dennis R, Dziegielewska KM, Mallard C, Wheaton B, et al. Pathological Changes in the White Matter after Spinal Contusion Injury in the Rat. PLoS One. 2012;7(8).
CrossRef - Anton E. Detection of apoptosis by a modified trichrome technique. J Histotechnol. 1999;22(4):301–4.
CrossRef - Culling C. Handbook of Histopathological and Histochemical Techniques. 3rd Editio. London: Butterworths, London.; 1972. 1–250 p.
- Fehlings MG, Nguyen DH. Immunoglobulin G: A potential treatment to attenuate neuroinflammation following spinal cord injury. J Clin Immunol. 2010;30(SUPPL. 1):109–12.
CrossRef - Xiong Y, Rabchevsky AG, Hall ED. Role of peroxynitrite in secondary oxidative damage after spinal cord injury. J Neurochem. 2007;100(3):639–49.
CrossRef - Bala V, Debnath A, Shill AK, Bose U. Anti-inflammatory, diuretic and antibacterial activities of aerial parts of mucuna pruriens linn. Vol. 7, International Journal of Pharmacology. 2011. p. 498–503.
CrossRef - Misra L, Wagner H. Extraction of bioactive principles from Mucuna pruriens seeds. Indian J Biochem Biophys. 2007;44(1):56–60.
- Sathiyanarayanan L, Arulmozhi S. Mucuna pruriens Linn. – A comprehensive review. Pharmacogn Rev. 2007;1(1):157–62.
- Katzenshlager R, Evans A, Manson A, Palsalos PN, Ratnaraj N, Watt H, et al. Mucuna pruriens in Parkinson’s disease: A double blind clinical and pharmacological study. J Neurol Neurosurg Psychiatry. 2004;75(12):1672–7.
CrossRef - Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: A parallel pathway for the modulation of spinal locomotor networks. Front Neural Circuits. 2014;8(JUNE):1–16.
CrossRef - Bal Krishna A, Manikyam HK, Sharma VK, Sharma N. Acute oral toxicity study in rats with Mucuna pruriens seed extract. Asian J Plant Sci Res [Internet]. 2016;6(2):1–5. Available from: http://www.imedpub.com/articles/acute-oral-toxicity-study-in-rats-with-mucuna-pruriens-seed-extract.pdf
- Khan SA, Khan L, Hussain I, Marwat KB, Akhtar N. Profile of Heavy Metals in Selected Medicinal Plants. J Weed Sci Res. 2008;14(12):101–10.
- Bhaskar A, Vidhya VG, Ramya M. Hypoglycemic effect of Mucuna pruriens seed extract on normal and streptozotocin-diabetic rats. Fitoterapia [Internet]. 2008;79(7–8):539–43. Available from: http://dx.doi.org/10.1016/j.fitote.2008.05.008
CrossRef - Asif K. Unique Journal of Engineering and Advanced Sciences. 2013;01(01):31–7.
- Kanter M, Coskun O, Kalayci M, Buyukbas S, Cagavi F. Neuroprotective effects of Nigella sativa on experimental spinal cord injury in rats. Hum {\&} Exp Toxicol [Internet]. 2006 Mar;25(3):127–33. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16634331
CrossRef - Sakr HF, Abbas AM, Bin-Jaliah I. Modulation of the neurological and vascular complications by grape seed extract in a rat model of spinal cord ischemia–reperfusion injury by downregulation of both osteopontin and cyclooxygenase-2. Can J Physiol Pharmacol [Internet]. 2016 Jul;94(7):719–27. Available from: http://www.nrcresearchpress.com/doi/10.1139/cjpp-2015-0498
CrossRef - Yune TY, Lee JY, Cui CM, Kim HC, Oh TH. Neuroprotective effect of Scutellaria baicalensis on spinal cord injury in rats. J Neurochem [Internet]. 2009 Aug;110(4):1276–87. Available from: http://doi.wiley.com/10.1111/j.1471-4159. 2009.06214.x
CrossRef - Cemil B, Gokce EC, Erdamar H, Karabork A, Onur O, Okcu AH, et al. Effects of the aged garlic extract in spinal cord injury in the rat. Turkish J Trauma Emerg Surg [Internet]. 2012;18(6):463–8. Available from: https://www.journalagent.com/travma/ pdfs/UTD-84829- RESEARCH%7B%5C_%7DARTICLE-CEMIL.pdf
CrossRef - Aziz I, Che Ramli MD, Mohd Zain NS, Sanusi J. Behavioral and Histopathological Study of Changes in Spinal Cord Injured Rats Supplemented with Spirulina platensis. Evidence-Based Complement Altern Med [Internet]. 2014;2014:1–8. Available from: http://www.hindawi.com/journals/ecam/2014/871657/
CrossRef - Wang W, Shen H, Xie J-J, Ling J, Lu H. Neuroprotective effect of ginseng against spinal cord injury induced oxidative stress and inflammatory responses. Int J Clin Exp Med [Internet]. 2015;8(3):3514–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/ 26064243
- Ao Q, Sun X-H, Wang A-J, Fu P-F, Gong K, Zuo H-Z, et al. Protective effects of extract of Ginkgo biloba (EGb 761) on nerve cells after spinal cord injury in rats. Spinal Cord [Internet]. 2006 Nov;44(11):662–7. Available from: http://www.nature.com/articles/ 3101900
CrossRef - Su C, Zhang D, Truong J, Jiang C, Lee S, Jarouche M, et al. Effects of a novel herbal formulation JSK on acute spinal cord injury in rats. Restor Neurol Neurosci. 2013;31(5):597–617.
CrossRef - Zhang Q, Zhang L-X, An J, Yan L, Liu C-C, Zhao J-J, et al. Huangqin flavonoid extraction for spinal cord injury in a rat model. Neural Regen Res [Internet]. 2018;13(12):2200. Available from: http://www.nrronline.org/text.asp?2018/13/12/ 2200/241472
CrossRef - Mikawlrawng K, Rani R, Kumar S, Bhardwaj AR, Prakash G. Anti-paralytic medicinal plants – Review. J Tradit Complement Med [Internet]. 2018 Jan;8(1):4–10. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2225411017300159
CrossRef - Karova K, Wainwright J V., MacHova-Urdzikova L, Pisal R V., Schmidt M, Jendelova P, et al. Transplantation of neural precursors generated from spinal progenitor cells reduces inflammation in spinal cord injury via NF-κB pathway inhibition 11 Medical and Health Sciences 1107 Immunology. J Neuroinflammation. 2019;16(1):1–11.
CrossRef - Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88(6):1335–44.
CrossRef - Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, et al. Interaction of reactive astrocytes with type i collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat Med. 2017;23(7):818–28.
CrossRef - Curtis R, Green D, Lindsay RM, Wilkin GP. Up-regulation of GAP-43 and growth of axons in rat spinal cord after compression injury. J Neurocytol. 1993;22(1):51–64.
CrossRef - Ahn YH, Lee G, Kang SK. Molecular insights of the injured lesions of rat spinal cords: Inflammation, apoptosis, and cell survival. Biochem Biophys Res Commun. 2006;348(2):560–70.
CrossRef - Manyam B V, Dhanasekaran M, Hare TA. Neuroprotective effects of the antiparkinson drug Mucuna pruriens. Phyther Res. 2004;18(9):706–12.
CrossRef - Sayin VI, Ibrahim MX, Larsson E, Nilsson JA, Lindahl P, Bergo MO. Antioxidants Accelerate Lung Cancer Progression in Mice. Sci Transl Med [Internet]. 2014 Jan;6(221):221ra15–221ra15. Available from: https://stm.sciencemag.org/lookup/doi/ 10.1126/scitranslmed.3007653
CrossRef - Piskounova E, Agathocleous M, Murphy MM, Hu Z, Huddlestun SE, Zhao Z, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature [Internet]. 2015 Nov;527(7577):186–91. Available from: http://www.nature.com/articles/nature 15726
CrossRef - Minari JB, Ogar GO, Bello AJ. Antiproliferative potential of aqueous leaf extract of Mucuna pruriens on DMBA-induced breast cancer in female albino rats. Egypt J Med Hum Genet [Internet]. 2016 Oct;17(4):331–43. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1110863015001317
CrossRef - Kumar P, Saha S. An updated review on Taxonomy, Phytochemistry, Pharmacology and Toxicology of Macuna pruriens. IC J J Pharmacogn Phytochem [Internet]. 2013;8192(1):2668735. Available from: www.phytojournal.com
- Iauk L, Galati EM, Kirjavainen S, Forestieri AM, Trovato A. Analgesic and antipyretic effects of mucuna pruriens. Pharm Biol. 1993;31(3):213–6.
CrossRef - Vaish S, Choudhary S, Khosla N, Sharma S, Singh J, Sudarsanan S. Mucuna pruriens (Konch Beej) precipitates manic symptoms. J Ment Heal Hum Behav. 2014;19(2):85.
CrossRef - Gbotolorun SC, Isah PK, Adebajo OA. Toxicity of Mucuna pruriens seed extract on the kidney of adult Sprague-Dawley rats. 2018;7(1):27–33.
- Cilia R, Laguna J, Cassani E, Cereda E, Pozzi NG, Isaias IU, et al. Mucuna pruriens in Parkinson disease . Neurology. 2017;89(5):432–8.
CrossRef - Ncube B, Finnie JF, Van Staden J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. South African J Bot [Internet]. 2012 Sep;82:11–20. Available from: https://linkinghub.elsevier.com/retrieve/pii/ S0254629912000968
CrossRef - Ghosh D. Quality issues of herbal medicines: internal and external factors. Int J Complement Altern Med. 2018;11(2):67–9.
CrossRef - Cao H, Liao S, Zhong W, Xiao X, Zhu J, Li W, et al. Synthesis, characterization, and biological evaluations of 1,3,5-triazine derivatives of metformin cyclization with berberine and magnolol in the presence of sodium methylate. Molecules. 2017;22(10).
CrossRef








