Jihan Hussein1* , Mona El Bana1
, Mona El Bana1 ,Dalia Medhat1
,Dalia Medhat1 ,Yasmin Abdel Latif1,2
,Yasmin Abdel Latif1,2 , Samah M. El-sayed3
, Samah M. El-sayed3 , Ahmed M. Youssef4
, Ahmed M. Youssef4 and Mehrez E. El-Naggar5
and Mehrez E. El-Naggar5
1Medical Biochemistry Department, Medical Research Division, National Research Centre, Dokki, Giza, P.O. 12622 Egypt.
2Faculty of Biotechnology, October University for Modern Sciences and Arts, 6th. October, Giza, Egypt.
3Dairy Science Department, National Research Centre, Dokki, Giza, P.O. 12622 Egypt.
4Packing and Packaging Materials Department, National Research Centre, 12622, Dokki, Egypt.
5Textile Research Division, National Research Centre, Dokki, Giza, P.O. 12622 Egypt.
Corresponding Author E-mail: Jihan_husein@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2341
Abstract
Hibiscus sabdariffa L. has been widely cultivated in tropical areas, usually used in treatment of many disorders. Thus, in our study we aimed to evaluate the effect of dairy desserts supplemented with nanoform of Hibiscus sabdariffa L. extract (NHSE) against Ehrlich solid carcinoma (ESC) in mice. The NHSE was prepared by soaking the fine powder of plant in 90% ethanol by cold extraction. NHSE was evaluated using dynamic light scattering (DLS) and transmission electron microscope (TEM), then the prepared NHSE was added to dairy desserts using different concentrations. Sixty female albino mice were used and divided into six groups. After the end of the experimental period, blood was withdrawn; Serum was separated for determination of malondialdehyde (MDA), super oxidedismutase (SOD), catalase (CAT), tumor necrosis factor- α (TNF-α), matrix metalloproteinases-9 (MMP-9) and B-cell lymphoma-2 (Bcl-2). Serum homocystein (Hcy) level was estimated by high performance liquid chromatography (HPLC). Mice inoculated intramuscularly with Ehrlich cell line showed statistically marked increase in serum levels of MDA, TNF-α, MMP-9 and Hcy accompanied by marked decrease in SOD and CAT activities and Bcl-2 levels compared to the control group. Treatments with NHSE markedly triggered activity of anti-oxidant, attenuated the inflammatory response, reduced levels of Hcy and stimulated the apoptosis of tumor cells. Based on that, dairy desserts containing NHSE showed effective role in prohibiting the releasing of reactive oxygen species, ameliorating the immune response, and preventing tumor progression.
Keywords
Dairy Desserts; Ehrlich solid carcinoma; Hibiscus sabdariffa L nano-extract; Homocysteine; Matrix Metalloproteinases-9
Download this article as:| Copy the following to cite this article: Hussein J, El Bana M, Medhat D, Latif Y. A, El- sayed S. M, Youssef A. M, El-Naggar M. E. Hibiscus Sabdariffa L. Nanoparticles Offer a Preventive Potential Against Experimental Ehrlich Solid Carcinoma. Biomed Pharmacol J 2022;15(1). |
| Copy the following to cite this URL: Hussein J, El Bana M, Medhat D, Latif Y. A, El- sayed S. M, Youssef A. M, El-Naggar M. E. Hibiscus Sabdariffa L. Nanoparticles Offer a Preventive Potential Against Experimental Ehrlich Solid Carcinoma. Biomed Pharmacol J 2022;15(1). Available from: https://bit.ly/3AOEOUz |
Introduction
Cancer is characterized by dysfunction in the mechanisms underlying cell cycle regulation, including either over-proliferation of cells and/or reduction in cell removal 1. Solid tumors are the largest second tumor burden in some populations 2. Solid Ehrlich carcinoma is undifferentiated tumor which is used frequently to establish a tumor model and in chemotherapy investigations 3. Solid Ehrlich carcinoma characterized by large-scale virulence, quick development and infiltrative nature of the tumor reflect its high-grade malignancy 3. Tumor growth can cause antioxidant disturbances in certain tissues of the tumor host 4.
One of the characteristics of tumor growth and invasion is the increased flux of oxy-radicals and loss of cellular redox homeostasis. Cancer cells can generate large amounts of hydrogen peroxide, which may contribute to their ability to mutate, damage of normal tissues and invade other tissues. This suggests that there is a direct correlation between changes in the rate of cancer cell proliferation and changes in the antioxidant machinery. Furthermore, some anticancer agents can act as antioxidant 5. Recently, Varela Almanza et al., 6 hypothesized that Hyperhomocysteinemia (HHcy) is implicated in different pathologies, such as cardiovascular diseases, diabetes, hypertension, and breast cancer.
Hibiscus sabdariffa L. has belonged to the family Malvaceae. It is also called karkade in Arab-speaking countries. The calyces of Hibiscus sabdariffa L. have been extensively used in food applications. Furthermore, several medicinal applications of Hibiscus sabdariffa L. and its extract have been established around the world such as to treat hypertension, enhance the digestive system, prevent cancer, protect liver damage, treat kidney stone in addition to its hypolipidemic effects, antioxidant activity, and antimicrobial effects 7-11.
Hibiscus sabdariffa L (HS) is affluent source of phenolic compounds, including protocatechuic acid which suggested possessing in vitro protective impact against cytotoxicity and genotoxicity via its radical quenching capacity and enhancing DNA repair 10. It has been reported that HS inhibits skin tumor formation induced by 12-O-tetradecan-olyphorbol-13-acetate in CD1-mice 12 and prohibited human promyelocytic leukemia HL-60 cells growth 7. Medhat et al. 13 utilized the benefits of natural products through nanotechnology seems to be a safe and efficient process that may inhibit tumor growth and attenuate oxidative stress and inflammation. Moreover, there is a rising request for bioactive ingredients and natural antioxidants in dairy industries which have led to widely applied and have the potential benefits as nutraceuticals in the food industry..
From this point of view, this study was designed to evaluate the curative effect of dairy desserts loaded with different ratios of Hibiscus sabdariffa L. extract nanoparticles (NHSE) against ESC via evaluating oxidative stress and inflammatory markers in addition to the expected effect in enhancement of tumor cells apoptosis.
Materials and Methods
Materials
Skimmed milk powder (medium heated, fat 1.25%, moisture 4%), white sugar, Hibiscus sabdariffa L. plant, whole milk (3% fat) and starch were obtained from local market. k-Carrageenan was obtained from Sigma Co., St Louis, MO, USA. Homocysteine standard (HPLC grade) was purchased from Sigma-Aldrich Company, St. Louis, MO, USA. All other chemicals were of HPLC grade.
Sixty female albino mice (20±5 g) were obtained from the animal house of National Research Centre (NRC), Giza, Egypt. Mice were fed a standard diet, water was available ad libitum for acclimatization before starting the experiment; mice were kept under constant environmental conditions at room temperature. The guidelines of the ethical care and treatment of the animals followed the regulations of the ethical committee of the NRC.
Methods
Preparation of Hibiscus sabdariffa L. extract nanoparticles (NHSE)
About 100 g of fresh Hibiscus sabdariffa L. was grinded to fine powder and soaked in 90% ethanol for extraction using cold extraction at room temperature for 24 h and then filtrated through a Whatman No. 1 filter paper. The filtrates were concentrated and evaporated using a rotary evaporator under pressure at 45°C. All dried extracts were stored at −20°C in a dark bottle free from for further experiments. Furthermore, the Hibiscus sabdariffa L. nanoform was prepared by dissolving desired amounts of dried extract in deionized distilled water to form a 100 mL solution. Then the prepared solution was left for sonicating (30 min) at 60 oC in sonication bath to form Hibiscus sabdariffa L. nanoparticles.
Manufacture of dairy desserts
Samples were prepared in batches of 1000 g, milk was weighed in a flask and preheated at 40°C then the skimmed milk powder (SMP) was dissolved in it and mixed under magnetic stirring for 10 min. Starch, sugar, and k-carrageen were then added to the cold milk and the mixture was stirred for 25 min at 90°C using a hot water bath. Then the sample was cooled in a water bath at 20°C with stirring for 10 min. Finally, when desserts cooled (0.1%, 0.3% and 0.6% of NHSE of total sample weight) were added and then samples were transferred to closed flasks and stored under refrigeration (5±1 °C). Desserts without additional NHSE were also prepared as control samples. The composition of the different formulas used in the experiment was presented in Table (1).
Table1: Composition (%) of different formula used in manufacture of NHSE dairy desserts.
| Ingredients | Control | Treat I (0.1% NHSE) | Treat II (0.3% NHSE) | Treat III (0.6% NHSE) |
| Milk | 85 | 84.9 | 84.7 | 84.4 |
| Sugar | 10 | 10 | 10 | 10 |
| SMP | 1.8 | 1.8 | 1.8 | 1.8 |
| Starch | 3 | 3 | 3 | 3 |
| k- carrageenan | 0.2 | 0.2 | 0.2 | 0.2 |
| NHSE | 0 | 0.1 | 0.3 | 0.6 |
Experimental design
Sixty female albino mice were classified into six groups (10 mice in each group) as follow: Group I (control group): healthy mice, received 250 µl of vehicle / mouse / day / orally for two weeks. Group II (solid tumor group): healthy mice inoculated once with 2.5×106 cells/mouse intramuscular in the hind limb. Group III (treated group I): healthy mice inoculated once with 2.5×106 cells/mouse intramuscular in the hind limb once and then received 250 µl dairy dessert (100 mg/kg body weight / day orally) for two weeks. Group IV (treated group II): healthy mice inoculated once with 2.5×106 cells/mouse intramuscular in the hind limb once and then received 250 µl of dairy dessert loaded with NHSE in a concentration of 0.1 (v/v)/daily by oral administration for 30 days. Group IV (treated group II): healthy mice inoculated with 2.5×106 cells/mouse intramuscular in the hind limb once and then received 250 µL of dairy dessert loaded with NHSE in a concentration of 0.3 (v/v)/daily by oral administration for two weeks. Group IV (treated group III): healthy mice inoculated with 2.5×106 cells/mouse intramuscular in the hind limb once and then received 250 µL of dairy dessert loaded with NHSE in a concentration of 0.6 (v/v)/daily by oral administration for two weeks.
After the experimental period, mice were kept fasting for 12 h before blood sampling, blood was withdrawn from the retro-orbital venous plexus of the eye using capillary tubes and collected in clean tubes for the biochemical analysis.
Characterization
Determination of total phenolic content of Hibiscus sabdariffa L. extract and dairy desserts samples
L. extract and dairy desserts samples were determined colorimetrically by a Folin-Ciocalteau reagent using calibration curve set with Gallic acid as a standard Results of TPC were stated as mg Gallic acid equivalents (GAE)/mL extract.
Determination of antioxidant activity of Hibiscus sabdariffa L. extract and dairy desserts products
samples were evaluated using DPPH radical scavenging method according to Matthus B.,15. The inhibition percentage of DPPH radical scavenging activity was calculated using the following formula, where: A, the absorbance at 515 nm of the control sample; A0, the final absorbance of the test sample at 515 nm 16.
Particle size of NHSE
The particle size of NHSE was evaluated using NICOMP 380 ZLS, Dynamic light scattering (DLS) instrument (PSS, Santa Barbara, CA, USA), using the 632 nm line of a HeNe laser as the incident light with angel 90o and Zeta potential with external angel 18.9o. Samples were diluted in 0.1M phosphate buffer pH 7.0 and filtered through 0.45 μm membrane (Mellipore, USA) to obtain a count rate in the appropriate range 100–450 nm, to avoid multiple scattering phenomena due to inter-particle interaction 17. Immediately, the diluted sample was transferred into a polystyrene cuvette for size determination.
Particle shape of NHSE
The particle shape was determined via transmission electron microscope (TEM) for the prepared NHSE through drying a drop of the NHSE solution on a carbon-coated copper grid.
Tumor transplantation:
Cell line of Ehrlich supplied through National Cancer Institute; Egypt was maintained in experimental female Swiss albino mice by inoculating 2.5×106 cells/mouse intramuscular with a fine needle in the hind limb of mice. Solid tumor observed after 10 to 13 days from the inoculation of EAC cells according to El-Gawish 18 and Medhat et al., 19.
Determination of serum oxidant/ anti-oxidant markers
Serum malondialdehyde (MDA), superoxide dismutase (SOD) and catalase activity (CAT) were estimated according to the methods of Nishikimi et al., 20 using standard spectrophotometric assays.
Determination of serum inflammatory and apoptotic markers
Quantitative determination of inflammatory markers including tumor necrosis factor- α (TNF-α) and matrix metallopeptidase 9 (MMP-9) in addition to the anti- apoptotic marker B-cell lymphoma 2 (Bcl-2 ) were determined using enzyme-linked immunosrbent assay (ELISA) according to the manufacturer’s instructions (Glory Science Co, Ltd, Del Rio, TX, USA).
Determination of serum homocysteine (Hcy)
Serum Hcy was estimated by high performance liquid chromatography (HPLC) system, Agilent technologies 1100 series, equipped with a quaternary pump (Quat. pump, G131A model) according to Hussein et al.,21. Briefly, 400 µl serum were mixed with 30 µl of 1.2 mol/l trichloroacetic acid (TCA), incubated in ice for 30 min to precipitate protein, centrifuged for 20 min at 4000 rpm at 4°C, and supernatants were filtered through 0.2 µm filter then 30 µl from the solution were injected into HPLC. Separation was achieved on reversed phase column (C18, 25, 0.46 cm i.d. 5 µm), the mobile phase consisted of 40 mmol/L sodium phosphate monobasic monohydrate; 8 mmol/L heptanesul fonic acid and 18% (v/v) methanol adjusted to pH 3.1 by addition of phosphoric acid and filtered through a 0.45-µm membrane filter and was delivered at a flow rate of 1 ml/min at 40°C. UV detection was performed at 260 nm. Serial dilutions of standards were injected, and their peak areas were determined. A linear standard curve was constructed by plotting peak areas versus the corresponding concentrations. The concentrations in samples were obtained from the standard curve.
Statistical analysis
Statistical package for social science (SPSS software version 12, Chicago, Illinois) was used. All numeric variables were expressed as mean ± standard error (SE) and analyzed by one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test for post-hoc comparison of group means. For all tests a probability (P < 0.05) was considered significant.
Results and discussions
Hibiscus sabdariffa L was extracted using ethanol as extraction solvent. It has been expected that the efficiency was increased by utilizing Hibiscus sabdariffa L in nanoemulsion and then blended with different concentration of dairy dessert. Scheme 1 represents the steps for the extraction of Hibiscus sabdariffa L (HSE), nanoemulsion preparation (NHSE), blending with dairy dessert and the final products were injected for mice to be used as natural drug against ESC in mice.
 |
Scheme 1: photo images that created from every steps of extraction, nanoemulsion preparation and blending with dairy dessert then treatment of animals. |
Table 2 shows the chemical composition of dairy desserts; total solids (T.S) ranged from 24.51 to 25.61 %. Total solids significantly reduced and moisture increased as added of NHSE dairy desserts. This result is an agreement with El-Shibiny et al., 22 and El-Sayed 17. The fat and protein content of dairy desserts prepared with or without a combination of NHSE was similar. The ash content of dairy desserts significantly increased in NHSE dairy dessert treatments contrary to that the carbohydrates content decreased as raised of added NHSE to the treatments. The carbohydrate content was significantly highest with control is also related to its total solids content. Ash content was ranged from 0.69 to 0.79%, the highest ash content observed with treatment that prepared by 0.6% NHSE (0.79 %) and, this may be owing to the chemical composition of NHSE 23.
Table 2: Chemical composition of dairy desserts contains different ratios of NHSE.
| Components | Control | Treat. 0.1% NHSE | Treat. 0.3% NHSE | Treat. 0.6% NHSE |
| T.S% | 25.61±0.06
|
25.53b±0.05
|
24. 69c±0.06
|
24.51d±0.06
|
| Moisture % | 74.39d±0.05
|
74.47c±0.05
|
75.31b±0.06
|
75.49a±0.06
|
| Fat% | 3.22a±0.06 | 3.20a±0.05 | 3.18a±0.06 | 3.16a±0.07 |
| Protein% | 3.02a±0.05
|
3.02a±0.06
|
3.00a±0.06
|
2.99a±0.06
|
| Ash % | 0.69d±0.05 | 0.72c±0.06
|
0.74c±0.06 | 0.79a±0.05
|
| Carbohydrate % | 20.06a±0.06 | 18.59b±0.07 | 17.77c±0.05 |
17.57d±0.06 |
Data expressed as mean of 3 replicates ± standard error. Means in the same row showing the same capital letters are not significantly different (p≤0.05).
Total phenolic content and antioxidant activity of Hibiscus sabdariffa L. nano-extract and different treatments of dairy desserts fortified with NHSE are exposed in Table 3. The total phenolic compound of Hibiscus sabdariffa L. extract was 47.51 mg/g extract, and this could be payable to natural Phyto compounds, for instance,
citric acid, hibiscus acid, hydroxy citric acid, and protocatechuic acid as main phenolic composites in Hibiscus sabdariffa L. extract nanoparticles. Furthermore, Table 3 displayed that the antioxidant activity of Hibiscus sabdariffa L. extract nanoparticles was 81.21%, which can be related to the anthocyanins, which principally produce the abundant color of the Hibiscus sabdariffa L. plant 23-25. Additionally, the total phenolic content and antioxidant activity of the different treatments of dairy desserts contain NHSE at the level of 0.1, 0.3 and 0.6 % were significantly (p<0.05) raised with increasing ratios of NHSE into dairy desserts. These results proved the several useful applications of Hibiscus sabdariffa L. extract nanoparticles, specifically as a good source of antioxidant activity and bioactive components
Table 3: Total phenolic content and antioxidant activity of Hibiscus sabdariffa L. extract nanoparticles and different treatments of dairy desserts supplemented by NHSE.
| Samples | Total phenol content (mg/g) | Antioxidant activity (%) |
| NHSE | 52.43±0.97a | 81.21±1.43a |
| Treat. 0.1% NHSE | 4.65±0.27d | 11.38±0.61d |
| Treat. 0.3% NHSE | 12.24±0.51c | 18.57±0.85c |
| Treat. 0.6% NHSE | 28.73±0.73b | 37.62±1. 32b |
Data expressed as mean of 3 replicates ± standard error. Means in the same column with different capital letters are significantly different at p≤0.05.
Structure evaluations of the prepared Hibiscus sabdariffa L. extract (NHSE)
TEM images revealed that the Hibiscus sabdariffa L. extract nanoparticles was in spherical shape as well as very smooth surface as obtainable in (Figure 1 a, b). Furthermore, the dynamic light scattering effect of particle size investigation of the prepared NHSE was presented in (Figure 1c) which displays the average diameter of is approximately 105 nm, which recognizes that the particle size of NHSE in nanoform during the solubilization of NHSE in water. Moreover, the polydispersity of NHSE remarks narrowing particle size distribution approving to relative literature measurements for several nanoparticles with homogenous particle mean diameters and good particle distribution 26.
 |
Figure 1: (a,b) TEM, and (c) mean diameter and relative particle size of the prepared NHSE |
Biochemistry evaluation of the prepared Hibiscus sabdariffa L. extract (NHSE)
Oxidative stress is a key player in the progression of many diseases and it is suggested to have a considerable role in carcinoma 27. Excess of highly reactive free radicals production in cancer initiate a chain reaction that cause inadequate levels of antioxidants and destruction of biological macromolecules including lipids, proteins, carbohydrates, and nucleic acids, and imbalance body homeostasis 28.
This study reinforces the hypothesis that positively correlates between the imbalance of oxidant/anti-oxidant and the progression of ESC 29. Our results revealed a statistically marked increase in serum levels of MDA followed by a marked decrease in serum levels of SOD and CAT in Ehlich solid carcinoma group compared to the control group.
Thus, in this study inoculating mice by cell line of Ehrlich intramuscular in the hind limb caused the appearance of visible solid tumor. The results showed a marked increase in serum levels of MDA by 89.1%compared to the control group, while the percent of changes in pre-treatment with dairy dessert supplemented in different concentrations of HSE (0.1 v/v,0.3 v/v, 0.6 v/v) were -13.3%, -24.2%, -28.43%, and -40.9% in serum level of MDA respectively compared to solid tumor group (Figure 2a).
On the contrary, induction of solid tumor showed a marked decrease in serum levels of SOD (Figure 2 b) and CAT (Figure 2 c) by -59.4%and -62% respectively compared to the control group, while levels of SOD were markedly increased in groups pre-treated with dairy dessert (free of HSE) and dairy desserts supplemented with different concentration of HSE (0.1 v/v, 0.3 v/v, 0.6 v/v) by 26.9%, 44.8%, 85.2%, 110.7% respectively compared to solid tumor group. In addition, serum levels of CAT were markedly increased in groups treated with dairy dessert (free of HSE) and dairy desserts supplemented with different concentration of HSE (0.1 v/v, 0.3 v/v, 0.6 v/v) by 19.6%, 79%, 119.6, 137.9% respectively compared to solid tumor group. Pre-treatment with dairy dessert supplemented with HSE (0.6 v/v) concentration accounted for the best results compared to the Ehrlich solid carcinoma group.
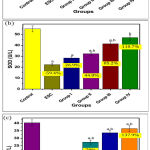 |
Figure 2: Serum levels (a) MDA, (b) SOD and (c) CAT of oxidant /anti-oxidant parameters in different studied groups. |
P: a significant difference compared to Pa) the control group, Pb) solid tumor group.
% of change a = percent of change compared to control group.
% of change b = percent of change compared to ESC group.
In the same line, Abd El-Aziz et al., 31 reported that Ehrlich carcinoma manifested elevation in lipid peroxidation product and appreciable diminish in SOD and CAT. This alteration of antioxidant levels may be launched by tumorigenesis. The reduced levels of GSH found in tumor-bearing mice might be due to the transformation rate of GSH to oxidized one 31.
Figure 3 summarized serum levels of TNF-α (Figure 3a), MMP-9 (Figure 3b) and Bcl-2 (Figure 3c). It has been shown that there is marked increase in Ehrlich solid carcinoma group compared to the control group by 184% and 253.3% ,and 114.89 % respectively. Pre-treatment with dairy dessert (free of HSE) and dairy desserts supplemented with different concentration of HSE (0.1 v/v, 0.3 v/v, 0.6 v/v) appeared a significant reduction of TNF-α concentration by -16.19%, -38.7% – -47.18% -59.15% respectively compared to solid tumor group. Pre-treatment with the dairy dessert supplemented with different concentrations of HSE (0.1 v/v, 0.3 v/v, 0.6 v/v) cause significant decrease in serum levels of MMP-9 by -15.8%, – 23.39%, -32.83% , and -45.28% respectively
Inoculation of Ehrlich cells results in a statistically marked increase in Bcl-2 level in Ehrlich solid carcinoma group compared to the control group by 114.89%, this level was decreased with the pre-treatment with dairy desserts supplemented in different concentrations of HSE (0.1 v/v, 0.3 v/v, 0.6 v/v) by -17.2%, -22.77%, -46, and -50.49% respectively. Pre-treatment with dairy dessert fortified with HSE (0.6 v/v) concentration showed high compatibility against ESC group.
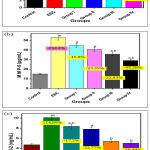 |
Figure 3: Serum levels of (a) TNF-α, (b) MMP-9 and (c) Bcl-2 |
P: a significant difference compared to Pa) the control group, Pb) solid tumor group.
% of change a = percent of change compared to control group.
% of change b = percent of change compared to solid tumor group
Many studies reported a positive correlation between inflammation and the development of the tumor where the inflammatory response is the inception mechanisms of carcinogenesis 19,33. The usual response to the injured tissue which is characterized by involvement of chemical signals initiating recruitment and infiltration of leukocytes to the damaged sites in order to recover the affected tissue. This kind of physiological inflammatory response is self-limiting and is terminated after the assaulting agent is removed or the repair is completed 34. Some cytokines such as TNF and interleukins haves an important impact in immune homeostasis, and inflammation 34.. In this study, we observed excessive expression of serum TNF-α in ESC group compared to the control group; which may be explained by the fact that the uncontrolled inflammatory respond that is accompanied by continuous release of cytokines enhance not only the rate of malignant growth but also the induction of tumor in the surrounding tissue in addition the liberation of reactive oxygen species (ROS), reactive nitrogen species (RNS) and boosted levels of macrophages releasing ROS caused alteration of tissue DNA and resulting in genomic modifications 35. In agreement with our results Aldubayan et al., 29 reported that mice treated by Ehrlich cell line showed increased tumor size; high expression level of inflammatory markers including TNF-α.
Another significant marker in many types of cancers is matrix metalloproteinases (MMPs) which are implicated in the differentiation, morphogenesis and tissue remodeling during angiogenesis, tumor invasion and metastasis 36. The MMP-9, a 92-kDa gelatinase B type IV collagenase is associated with the pathogenesis of different diseases because of its considerable regulation action 37, thus ,investigating MMP-9 activity with ESC could be valuable in tracking the consequence of this model. In this study, serum levels of MMP-9 were markedly increased in ESC group compared to the control group. It has been evaluated levels of mRNA and protein levels of MMP-9; they found that protein levels of MMP-9 were increased in papillary thyroid cancer patients. Zarkesh et al., 38 suggested that MMP-9 secreted by macrophages, neutrophils, and transformed cells enhances tissue damages particularly in the absence of their inhibitors. In carcinomas where the major function of MMP-9 is the degradation of type IV collagen which is known as the main component of the basement membrane, hence stimulating tumor invasion.
Apoptosis is a complicated process and alterations occur at any stage along these pathways. Once the imbalance between anti-apoptotic proteins (Bcl-2) and apoptotic promoter proteins (Bax) occurs; the affected cells become malignant, spreads from its site of origin to another part of the body and become resistant to the anti-cancer drugs.
Thus, During carcinogenesis, the repression of apoptosis acts as the major impulse in cancer progression 1.. The present results revealed a significant decrease in serum concentration of Bcl-2 was determined in ESC group compared to the control group. According to Hochman et al., the expression of Bcl-2 is regulated by oxidative stress. In this study, ESC motivated the augmentation of oxidant and the imbalance in oxidant and antioxidant enzymes resulting in impaired functions of Bcl-2 which is in agreement with the previous study 39.
Another promising marker which is associated with increasing oncogenic risk is Hcy. In this study, serum level of Hcy was markedly increased in Ehrlich solid carcinoma group compared to the control group by 215 %. There was a marked decrease in Hcy level by -24.35%, -32.85%, -40% and -59.2% in all prophylactic groups respectively compared to ESC group (Figure 4). It is worthy to mention that pre-treatment with dairy dessert supplemented with HSE (0.6 v/v) concentration gave the better results when compared to ESC group than the lower concentrations (0.1 and 0.3 v/v).
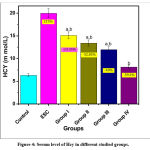 |
Figure 4: Serum level of Hcy in different studied groups. |
P: a significant difference compared to Pa) the control group, Pb) solid tumor group.
% of change a = percent of change compared to control group.
% of change b= percent of change compared to ESC group.
Akilzhanova et al., 40 suggested that Hcy metabolism control the expression of specific pathways that affects tumor behavior. Zhu et al., 41 supposed that HHcy increased the intracellular accumulation of S-adenosylhomocysteine (Hcy), which induces the production of oxidative metabolites of estrogens (catechol estrogens) that contribute to estrogen-induced tumors in animal models and in some human cancers such as breast cancer.
In this study, mice inoculated intramuscularly with Ehrlich cells showed significant increase in serum levels of Hcy compared to the control group. Concomitant with our results, Hasan et al., 42 reported that increased plasma Hcy is closely related to cancer development. Since Hcy is a pro-oxidant, and the formation of Hcy-Hcy dimers and Hcy-protein adducts which stimulate the formation of highly reactive free radicals such as homocysteine thiolactone that in turn produces covalent adducts with lysine or arginine residues in proteins, resulting in the formation of insoluble toxic protein aggregates or amyloids leading to biotoxicity to the endothelial cells 43.
Calyces of NHSE are a rich source of polyphenols, flavonoids (anthocyanins, deichlphinidin, hibiscetin, queNHSE tin and gossypetin, protocatechuic acid (PCA)), alkaloids (L-ascorbic acid, carotenoids, anisaldehyde, galactose, mucopolysaccharides, pectin), polysaccharides, and stearic acid 44. Anthocyanin, flavonoids, PCA, and L-ascorbic acid have been demonstrated to have antioxidant effect in vitro and in vivo 45. Thus, in this study we evaluated the effect of dairy dessert supplemented with different concentrations of nano-NHSE against Ehrlich solid carcinoma. According to Hussein et al., 46, the development of therapeutic forms of nanoparticles could be useful in enhancement of the bioactivity and effectiveness of natural extracts in treatment of different diseases.
In this study, treatment of ESC with dairy dessert (free of NHSE) and dairy dessert supplemented with different concentrations of NHSE regulated levels of oxidant via its activity in scavenging ROS through protecting cells against lipid peroxidation product, blocking the activity of xanthine oxidase, enhancing activity of glutathione, SOD, and CAT 10. Besides, dairy dessert supplemented with different concentrations of NHSE attenuated the inflammatory response initiated by Ehrlich cells. This anti-inflammatory and immune stimulatory effect is exerted by enhancement the releasing of IL-10, inhibiting the liberation of TNF-α 47, and reducing cyclooxygenase-2 activity by down-regulating JNK and p38 MAPK 48.
HSE is a rich source of anthocyanins and polyphenolic compounds; these compounds induced apoptosis against human leukaemia cells 49 via the p38-FasL and Bid pathway and ROS-mediated mitochondrial dysfunction pathway and against smooth muscle cells via p38 and p53 pathway 50. The anti-cancer activity of NHSE were assessed against human prostate cancer cells in vitro and in vivo through Bax/cytochromec-mediated caspase 9 and Fas-mediated caspase 8/t-Bid pathways 51-53. These results may explain the remarkable increase of Bcl-2 in ESC treated groups.
The percent of changes in the above-mentioned results appeared the potency of supplemented agents in attenuating oxidative stress as well as inflammation according to the increasing in its concentration (0.6 is more potent than 0.3 and the latter is more potent than 0.1) and all of them more potent than the extract alone. This to confirm our hypothesis about the role of using nanoparticles in enhancing the efficacy of this extract; in addition to appear the idea in using different doses to give the best result. So, we can confirm that the effectiveness of Hibiscus sabdariffa L. extract nanoparticles is dose dependent.
Conclusion
In the current study, Hibiscus sabdariffa L. was extracted and used for the preparation of Hibiscus sabdariffa L extract nanoparticles (NHSE) that were prepared in small size and well distribution. Different concentrations of the as prepared nanoparticles (NHSE) were blended with dairy dessert to be used as nutrient for mice in order to evaluating its effect against Ehrlich solid carcinoma. Also, it is used for modulating the balance between oxidant and antioxidant enzyme activity, attenuating levels of inflammatory markers and prohibiting tumor growth. The data obtained revealed that dairy desserts combined with NHSE play a valuable role in banning the release of reactive oxygen species, enhancing the immune response and preventing the progression of the tumour.
Conflict of Interest
There is no conflict of interest.
Funding Sources
There is no funding source.
References
- Hassan, M.; Watari, H.; AbuAlmaaty, A.; Ohba, Y.; Sakuragi, N. Apoptosis and Molecular Targeting Therapy in Cancer. Biomed Res. Int. 2014, 2014.
CrossRef - Travis, L. B. Therapy-Associated Solid Tumors. Acta Oncol. (Madr). 2002, 41 (4), 323–333.
CrossRef - Silva, L. A.; Nascimento, K. A. F.; Maciel, M. C. G.; Pinheiro, M. T.; Sousa, P. R. A.; Ferreira, S. C. P.; Azevedo, A. P. S.; Guerra, R. N. M.; Nascimento, F. R. F. Sunflower Seed Oil-Enriched Product Can Inhibit Ehrlich Solid Tumor Growth in Mice. Chemotherapy 2006, 52 (2), 91–94.
CrossRef - Wenger, F. A.; Kilian, M.; Mautsch, I.; Jacobi, C. A.; Steiert, A.; Peter, F. J.; Guski, H.; Schimke, I.; Müller, J. M. Influence of Octreotide on Liver Metastasis and Hepatic Lipid Peroxidation in BOP-Induced Pancreatic Cancer in Syrian Hamsters. Pancreas 2001, 23 (3), 266–272.
CrossRef - Gupta, M.; Mazumder, U. K.; Kumar, R. S.; Kumar, T. S. Antitumor 5. Activity and Antioxident Role of Bauhinia Racemosa against Ehrlich Ascites Carcinoma in Swiss Albino Mice. Acta Pharmacol Sin 2004, 25 (8), 1070–1076.
- Varela Almanza, K. M.; Puebla-Pérez, A. M.; Delgado-Saucedo, J. I.; Rodríguez-Arévalo, F.; Zúñiga-González, G. M.; Figuera, L. E.; Morán-Mendoza, A.; Gallegos-Arreola, M. P. Increased Homocysteine Plasma Levels in Breast Cancer Patients of a Mexican Population. Exp. Oncol. 2018.
CrossRef - Tseng, T.-H.; Kao, T.-W.; Chu, C.-Y.; Chou, F.-P.; Lin, W.-L.; Wang, C.-J. Induction of Apoptosis by Hibiscus Protocatechuic Acid in Human Leukemia Cells via Reduction of Retinoblastoma (RB) Phosphorylation and Bcl-2 Expression. Biochem. Pharmacol. 2000, 60 (3), 307–315.
CrossRef - Hirunpanich, V.; Utaipat, A.; Morales, N. P.; Bunyapraphatsara, N.; Sato, H.; Herunsale, A.; Suthisisang, C. Hypocholesterolemic and Antioxidant Effects of Aqueous Extracts from the Dried Calyx of Hibiscus Sabdariffa L. in Hypercholesterolemic Rats. J. Ethnopharmacol. 2006, 103 (2), 252–260.
CrossRef - Prasongwatana, V.; Woottisin, S.; Sriboonlue, P.; Kukongviriyapan, V. Uricosuric Effect of Roselle (Hibiscus Sabdariffa) in Normal and Renal-Stone Former Subjects. J. Ethnopharmacol. 2008, 117 (3), 491–495.
CrossRef - Da-Costa-Rocha, I.; Bonnlaender, B.; Sievers, H.; Pischel, I.; Heinrich, M. Hibiscus Sabdariffa L.–A Phytochemical and Pharmacological Review. Food Chem. 2014, 165, 424–443.
CrossRef - El-Sayed, S. M.; El-Sayed, H. S.; Ibrahim, O. A.; Youssef, A. M. Rational Design of Chitosan/Guar Gum/Zinc Oxide Bionanocomposites Based on Roselle Calyx Extract for Ras Cheese Coating. Carbohydr. Polym. 2020, 116234.
CrossRef - Tseng, T.-H.; Hsu, J.-D.; Lo, M.-H.; Chu, C.-Y.; Chou, F.-P.; Huang, C.-L.; Wang, C.-J. Inhibitory Effect of Hibiscus Protocatechuic Acid on Tumor Promotion in Mouse Skin. Cancer Lett. 1998, 126 (2), 199–207.
CrossRef - Medhat, D.; El-mezayen, H. A.; El-Naggar, M. E.; Farrag, A. R.; Abdelgawad, M. E.; Hussein, J.; Kamal, M. H. Evaluation of Urinary 8-Hydroxy-2-Deoxyguanosine Level in Experimental Alzheimer’s Disease: Impact of Carvacrol Nanoparticles. Mol. Biol. Rep. 2019, 46 (4), 4517–4527. https://doi.org/10.1007/s11033-019-04907-3.
CrossRef - Lafka, T.-I.; Sinanoglou, V.; Lazos, E. S. On the Extraction and Antioxidant Activity of Phenolic Compounds from Winery Wastes. Food Chem. 2007, 104 (3), 1206–1214.
CrossRef - Matthäus, B. Antioxidant Activity of Extracts Obtained from Residues of Different Oilseeds. J. Agric. Food Chem. 2002, 50 (12), 3444–3452.
CrossRef - El-Naggar, M. E.; Hussein, J.; El-Sayed, S. M.; Youssef, A. M.; El Bana, M.; Latif, Y. A.; Medhat, D. Protective Effect of the Functional Yogurt Based on Malva Parviflora Leaves Extract Nanoemulsion on Acetic Acid-Induced Ulcerative Colitis in Rats. J. Mater. Res. Technol. 2020, 9 (6). https://doi.org/10.1016/j.jmrt.2020.10.047.
CrossRef - El-Sayed, S. M. Use of Spinach Powder as Functional Ingredient in the Manufacture of UF-Soft Cheese. Heliyon 2020, 6 (1), e03278.
CrossRef - El-Gawish, M. Antitumor Activity of Inositol Hexaphosphate (Phytic Acid) in Mice Loaded with Solid Tumor. Egypt. J. Biomed. Sci 2003, 11, 106–121.
- Medhat, D.; Hussein, J.; El-Naggar, M. E.; Attia, M. F.; Anwar, M.; Latif, Y. A.; Booles, H. F.; Morsy, S.; Farrag, A. R.; Khalil, W. K. B.; Khalil, W. K. B.; El-Khayat, Z. Effect of Au-Dextran NPs as Anti-Tumor Agent against EAC and Solid Tumor in Mice by Biochemical Evaluations and Histopathological Investigations. Biomed. Pharmacother. 2017, 91, 1006–1016. https://doi.org/10.1016/ j.biopha.2017.05.043.
CrossRef - Nishikimi, M.; Roa, N. A.; Yogi, K. Measurement of Superoxide Dismutase. Biochem. Biophys. Res. Commun 1972, 46, 849–854.
CrossRef - Hussein, J.; El-Khayat, Z.; Farrag, A. R.; Medhat, D.; Abdel, Y. Inhibition of Hyperhomocysteinemia in Indomethacin Induced Peptic Ulcer: Impact of Pomegranate Juice Supplementation. Inhib. hyperhomocysteinemia Indomethacin Induc. peptic ulcer Impact pomegranate juice Suppl. 2014, 6, 131–138.
- El-Shibiny, S.; El-Gawad, M. A. E.-K. M. A.; Assem, F. M.; El-Sayed, S. M. The Use of Nano-Sized Eggshell Powder for Calcium Fortification of Cow? S and Buffalo? S Milk Yogurts. Acta Sci. Pol. Technol. Aliment. 2018, 17 (1), 37–49.
CrossRef - El-Sayed, S. M.; Youssef, A. M. Potential Application of Herbs and Spices and Their Effects in Functional Dairy Products. Heliyon 2019, 5 (6), e01989.
CrossRef - Borrás-Linares, I.; Fernández-Arroyo, S.; Arráez-Roman, D.; Palmeros-Suárez, P. A.; Del Val-Díaz, R.; Andrade-Gonzáles, I.; Fernández-Gutiérrez, A.; Gómez-Leyva, J. F.; Segura-Carretero, A. Characterization of Phenolic Compounds, Anthocyanidin, Antioxidant and Antimicrobial Activity of 25 Varieties of Mexican Roselle (Hibiscus Sabdariffa). Ind. Crops Prod. 2015, 69, 385–394.
CrossRef - Wang, H.; Cao, G.; Prior, R. L. Oxygen Radical Absorbing Capacity of Anthocyanins. J. Agric. Food Chem. 1997, 45 (2), 304–309.
CrossRef - Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10 (2), 57.
CrossRef - Boz, H. Phenolic Amides (Avenanthramides) in Oats-a Review. Czech J. Food Sci. 2015, 33 (5), 399–404.
CrossRef - Elejalde, J. I. G. Oxidative Stress, Diseases and Antioxidant Treatment. In Anales de medicina interna (Madrid, Spain: 1984); 2001; Vol. 18, pp 326–335.
- Aldubayan, M. A.; Elgharabawy, R. M.; Ahmed, A. S.; Tousson, E. Antineoplastic Activity and Curative Role of Avenanthramides against the Growth of Ehrlich Solid Tumors in Mice. Oxid. Med. Cell. Longev. 2019, 2019.
CrossRef - Coello, F. P.; Orosco-Vargas, C.; Peraza-Marrero, M.; Pinto-Catari, I.; Ramirez-Azuaje, D. Effect of an Extract of Hibiscus Sabdariffa L., on Oxidative Stress Induced in Saccharomyces Cerevisiae. Ciencia, Ambient. y Clima 2020, 3 (1), 41–46.
CrossRef - Abd El-Aziz, A. F.; Hefni, M. E.; Shalaby, A. M. Inhibitory Effects of Rosemary (Rosmarinus Officinalis L.) on Ehrlich Ascites Carcinoma in Mice. Int. J. Curr. Res. Aca. Rev 2014, 2 (9), 330–357.
- Villalpando-Arteaga, E. V.; Mendieta-Condado, E.; Esquivel-Solís, H.; Canales-Aguirre, A. A.; Gálvez-Gastélum, F. J.; Mateos-Díaz, J. C.; Rodríguez-González, J. A.; Márquez-Aguirre, A. L. Hibiscus Sabdariffa L. Aqueous Extract Attenuates Hepatic Steatosis through down-Regulation of PPAR-γ and SREBP-1c in Diet-Induced Obese Mice. Food Funct. 2013, 4 (4), 618–626.
CrossRef - Moselhy, S. S. Chemopreventive Effect of Lycopene Alone or with Melatonin against the Genesis of Oxidative Stress and Mammary Tumors Induced by 7, 12 Dimethyl (a) Benzanthracene in Sprague Dawely Female Rats. Mol. Cell. Biochem. 2008, 319 (1–2), 175–180.
CrossRef - Todoric, J.; Antonucci, L.; Karin, M. Targeting Inflammation in Cancer Prevention and Therapy. Cancer Prev. Res. 2016, 9 (12), 895–905.
CrossRef - Karin, M.; Clevers, H. Reparative Inflammation Takes Charge of Tissue Regeneration. Nature 2016, 529 (7586), 307–315.
CrossRef - Warren, M. A.; Shoemaker, S. F.; Shealy, D. J.; Bshara, W.; Ip, M. M. Tumor Necrosis Factor Deficiency Inhibits Mammary Tumorigenesis and a Tumor Necrosis Factor Neutralizing Antibody Decreases Mammary Tumor Growth in Neu/ErbB2 Transgenic Mice. Mol. Cancer Ther. 2009, 8 (9), 2655–2663.
CrossRef - Shaked, H.; Hofseth, L. J.; Chumanevich, A.; Chumanevich, A. A.; Wang, J.; Wang, Y.; Taniguchi, K.; Guma, M.; Shenouda, S.; Clevers, H. Chronic Epithelial NF-ΚB Activation Accelerates APC Loss and Intestinal Tumor Initiation through INOS up-Regulation. Proc. Natl. Acad. Sci. 2012, 109 (35), 14007–14012.
CrossRef - Egeblad, M.; Werb, Z. New Functions for the Matrix Metalloproteinases in Cancer Progression. Nat. Rev. cancer 2002, 2 (3), 161–174.
CrossRef - López-Otín, C.; Overall, C. M. Protease Degradomics: A New Challenge for Proteomics. Nat. Rev. Mol. cell Biol. 2002, 3 (7), 509–519.
CrossRef - Zarkesh, M.; Zadeh-Vakili, A.; Akbarzadeh, M.; Fanaei, S. A.; Hedayati, M.; Azizi, F. The Role of Matrix Metalloproteinase-9 as a Prognostic Biomarker in Papillary Thyroid Cancer. BMC Cancer 2018, 18 (1), 1199.
CrossRef - Hochman, A.; Sternin, H.; Gorodin, S.; Korsmeyer, S.; Ziv, I.; Melamed, E.; Offen, D. Enhanced Oxidative Stress and Altered Antioxidants in Brains of Bcl‐2‐deficient Mice. J. Neurochem. 1998, 71 (2), 741–748.
CrossRef - Akilzhanova, A.; Nurkina, Z.; Momynaliev, K.; Ramanculov, E.; Zhumadilov, Z.; Rakhypbekov, T.; Hayashida, N.; Nakashima, M.; Takamura, N. Genetic Profile and Determinants of Homocysteine Levels in Kazakhstan Patients with Breast Cancer. Anticancer Res. 2013, 33 (9), 4049–4059.
- Zhu, B. T. Medical Hypothesis: Hyperhomocysteinemia Is a Risk Factor for Estrogen-Induced Hormonal Cancer. Int. J. Oncol. 2003, 22 (3), 499–508.
- Hasan, T.; Arora, R.; Bansal, A. K.; Bhattacharya, R.; Sharma, G. S.; Singh, L. R. Disturbed Homocysteine Metabolism Is Associated with Cancer. Exp. Mol. Med. 2019, 51 (2), 1–13.
CrossRef - Sharma, G. S.; Kumar, T.; Dar, T. A.; Singh, L. R. Protein N-Homocysteinylation: From Cellular Toxicity to Neurodegeneration. Biochim. Biophys. Acta (BBA)-General Subj. 2015, 1850 (11), 2239–2245.
CrossRef - Pérez-Torres, I.; Ruiz-Ramírez, A.; Baños, G.; El-Hafidi, M. Hibiscus Sabdariffa Linnaeus (Malvaceae), Curcumin and Resveratrol as Alternative Medicinal Agents against Metabolic Syndrome. Cardiovasc. Hematol. Agents Med. Chem. (Formerly Curr. Med. Chem. Hematol. Agents) 2013, 11 (1), 25–37.
CrossRef - Lin, H.-H.; Chen, J.-H.; Wang, C.-J. Chemopreventive Properties and Molecular Mechanisms of the Bioactive Compounds in Hibiscus Sabdariffa Linne. Curr. Med. Chem. 2011, 18 (8), 1245–1254.
CrossRef - Hussein, J.; El-Banna, M.; Mahmoud, K. F.; Morsy, S.; Latif, Y. A.; Medhat, D.; Refaat, E.; Farrag, A. R.; El-Daly, S. M. The Therapeutic Effect of Nano-Encapsulated and Nano-Emulsion Forms of Carvacrol on Experimental Liver Fibrosis. Biomed. Pharmacother. 2017, 90, 880–887.
CrossRef - Fakeye, T. O.; Pal, A.; Bawankule, D. U.; Khanuja, S. P. S. Immunomodulatory Effect of Extracts of Hibiscus Sabdariffa L.(Family Malvaceae) in a Mouse Model. Phyther. Res. An Int. J. Devoted to Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2008, 22 (5), 664–668.
CrossRef - Kao, E.-S.; Hsu, J.-D.; Wang, C.-J.; Yang, S.-H.; Cheng, S.-Y.; Lee, H.-J. Polyphenols Extracted from Hibiscus Sabdariffa L. Inhibited Lipopolysaccharide-Induced Inflammation by Improving Antioxidative Conditions and Regulating Cyclooxygenase-2 Expression. Biosci. Biotechnol. Biochem. 2009, 73 (2), 385–390.
CrossRef - Chang, Y.-C.; Huang, H.-P.; Hsu, J.-D.; Yang, S.-F.; Wang, C.-J. Hibiscus Anthocyanins Rich Extract-Induced Apoptotic Cell Death in Human Promyelocytic Leukemia Cells. Toxicol. Appl. Pharmacol. 2005, 205 (3), 201–212.
CrossRef - Lo, C.; Huang, H.; Lin, H.; Chien, C.; Wang, C. Effect of Hibiscus Anthocyanins‐rich Extract Induces Apoptosis of Proliferating Smooth Muscle Cell via Activation of P38 MAPK and P53 Pathway. Mol. Nutr. Food Res. 2007, 51 (12), 1452–1460.
CrossRef - Lin, H.-H.; Chan, K.-C.; Sheu, J.-Y.; Hsuan, S.-W.; Wang, C.-J.; Chen, J.-H. Hibiscus Sabdariffa Leaf Induces Apoptosis of Human Prostate Cancer Cells in Vitro and in Vivo. Food Chem. 2012, 132 (2), 880–891.
CrossRef







