Manuscript accepted on :
Published online on: 22-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Vijay Rekulapally
Second Review by: Dr. Sharad Kamble
Final Approval by: Dr. Fai Poon
Fazeel Z A*
Department of Pharmacology, Malla Reddy Medical College for Women, Hyderabad, India.
Corresponding Author Email: fazeelzubair@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2333
Abstract
Background Potentiating activity of tablet apremilast 30mg BD against psoriasis in combination with 0.005% calcipotriol ointment was studied in comparison with calcipotriol monotherapy. Methods Single centre, prospective, parallel group, open label study compared efficacy and safety of calcipotriol+apremilast combination with calcipotriol monotherapy. Patients of mild to severe psoriasis in age group 18-60 years were randomized to two groups – calcipotriol+apremilast group and calcipotriol group. Calcipotriol+apremilast group received apremilast 30 mg BD p.o. and 0.005% calcipotriol ointment local application BD for 8 weeks. While calcipotriol group received 0.005% calcipotriol ointment local application BD for 8 weeks. Primary endpoint for efficacy was percentage of patients in whom mPASI decreased by 75% from baseline. Safety was also monitored throughout. Results 106 patients were randomized: calcipotriol+apremilast (n = 56) and calcipotriol group (n = 53). More patients of calcipotriol+apremilast achieved treatment success compared to calcipotriol was also higher (51.85% vs 34.61%; p < 0.001). Similar percentage of patients reported adverse events: Calcipotriol+apremilast 45.49% (n = 23) and calcipotriol 42.30% (n = 22) Conclusion Addition of apremilast to calcipotriol is significantly more efficacious than calcipotriol monotherapy. This combination is as safe as monotherapy.
Keywords
Apremilast; Calcipotriol; Corticosteroid; Psoriasis; PDE4 Inhibitors; Vitamin D
Download this article as:| Copy the following to cite this article: Fazeel Z. A. Comparison of Efficacy and Safety of Calcipotriol and Apremilast Combination Against Cacipotriol Monotherapy in Psoriasis. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Fazeel Z. A. Comparison of Efficacy and Safety of Calcipotriol and Apremilast Combination Against Cacipotriol Monotherapy in Psoriasis. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3FlRIuU |
Introduction
Psoriasis is an immunologically mediated inflammatory disease of the skin. Psoriasis prevalence throughput the globe ranges from 0.1% to 11.4% 1. Psoriasis hampers daily routine, productivity and life quality of a patient to a large extent. Often psoriasis is accompanied with comorbidities like psoriatic arthritis, cardiac disease, abnormal cholesterol levels, obesity, metabolic syndrome and depression 2,3.
Psoriasis is graded as mild, moderate and severe based on BSA, erythema, induration, and scaling of lesions. Topical therapy is commonly advocated for mild to moderate lesions. But certain oral drugs are also suitable for these categories. The choice of drugs has to be tailor made according to severity, and patient’s characteristics 2.
Past one and half decade has witnessed tremendous advances in psoriasis treatment but unfortunately, no drug offers complete cure and none of them is free from side effects 4,5. The latest guidelines for psoriasis treatment recommend topical vitamin D analogues like calcipotriol as first line therapy. Corticosteroids may be added to that depending on type and severity of psoriasis 2,6. Calcipotriol suppresses the proliferation of keratinocyte and also promotes differentiation of keratinocytes thereby restoring normal morphology and physiology of skin 7. Corticosteroid use is always associated with risks like suppression of adrenal gland, withdrawal symptoms, diabetes mellitus, glaucoma, cataract, osteoporosis 8,9. For steroid sparing, calcipotriol on weekdays and corticosteroids on weekdays is recommended 2,6.
Apremilast is one of the recently introduced oral drug for psoriasis 10. It is a phosphodiesterase-4 (PDE4) inhibitor. PDE4 is involved in the degradation of cAMP. When apremilast inhibits PDE4, cAMP concentration increases. cAMP inhibits the production of pro-inflammatory cytokines and promotes the production of anti-inflammatory cytokines 11. Currently, available data is not sufficient enough to establish guidelines regarding apremilast 2. However certain trials have proved that apremilast in a dose of 30 mg BD has decreased PASI by 75% in ~ 30 to 40% subjects 12,13.
Comparative assessment of effectiveness of therapy options deliver rational and evidence based treatment decisions to the practicing physicians. When certain comparisons are not available, it is prudent to perform comparative studies in order to explore possible future therapeutic options. In this study, we had compared cost efficacy and safety of calcipotriol apremilast combination against calcipotriol monotherapy. Since both drugs – calcipotriol and apremilast act by different mechanisms in psoriasis, combining both these drugs could enhance their efficacy and also reduce the duration of treatment hence possibly reduce side effects.
Patients and methods
Patients
We had recruited cases of mild to severe psoriasis in the age group of 18 to 60 years. Grading of psoriasis as mild, moderate and severe was done according mPASI (modified Psoriasis Severity Index) 5,14,15. Cases having psoriasis since more than 6 months were taken. These cases were suitable candidates for topical therapy. Head was not considered in BSA and mPASI as it was excluded from treatment 14,16. All those patients who had received potent corticosteroids, biologic, systemic or phototherapy sixteen weeks preceding randomization were excluded. This period of sixteen weeks was decided to allow to avoid any interference of preceding therapy with trial treatment. Drugs which were stopped sixteen weeks ago would have been eliminated as five half lives are required for complete elimination of drug 17. Our OPD receives mostly pustular type of psoriasis hence we recruited only pustular type of psoriasis. This was also done to avoid bias as isolated cases of other types of psoriasis may not give us a sufficient sample size for those types of psoriasis. All those cases who were planned for phototherapy or change of therapy or having other skin diseases or other inflammatory disorders or hypercalcemia or systemic disease which required corticosteroid use or contraindication to calcipotriol or apremilast were also excluded.
Study design
This study was a single centre, prospective, parallel group, open label study. Patients were randomly allocated to any one of two groups – calcipotriol + apremilast group and calcipotriol group. Patients in calcipotriol + apremilast group (group 1) were administered tablet apremilast 30 mg twice daily orally along with topical 0.005% calcipotriol ointment twice daily for local application on all lesions for 8 weeks. Patients of calcipotriol group (group 2) were given only 0.005% calcipotriol ointment for local application twice daily for 8 weeks (2,10) (Figure 1)
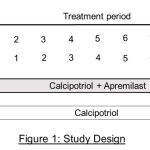 |
Figure 1: Study Design. |
Prior approval from the institute’s ethics committee was obtained. This study was done as per the ethical principles of the Declaration of Helsinki and good clinical practice guidelines. The procedure and purpose of the study was clearly explained individually to all study participants in their regional language. Written informed consent was obtained from all study subjects. This entire procedure was video-recorded.
Objectives and assessments
The primary objective of this study was to compare the efficacy of calcipotriol apremilast combination with calcipotriol monotherapy after 8 weeks. The primary endpoint for efficacy, also termed as “treatment success” was the percentage of patients in whom mPASI score decreased by 75% from baseline (mPASI75) 14,18.
Safety assessment was done by observing and evaluating adverse effects during the 8 week study period 19. All adverse effects were assessed as per the WHO – Uppsala Monitoring Centre Causality assessment and Naranjo ADR Probability Scale 20,21
Statistical analysis
Inter group efficacy endpoints – the percentage of patients achieving treatment success and the percentage of patients attaining mPASI75 were compared and analyzed using student’s unpaired t test. Significance tests were two-sided using 5% significance level and 95% confidence intervals (CIs). P value of < 0.001 was taken as significant. SPSS v20.0 by IBM was used for statistical analyses.
Results and Discussion
Patients
A total of 129 patients were recruited. The study was conducted at the department of dermatology, Viswabharathi general hospital and medical college, Kurnool, AP, India from January 2019 to December 2019. Patients were randomized to calcipotriol + apremilast group (n = 66) and calcipotriol group (n = 63) (Table 1). Twelve patients from calcipotriol + apremilast group and eleven patients from calcipotriol group did not follow up with the study. (Figure 2). The majority of patients had moderate psoriasis and overall mean mPASI was around 8. Psoriasis lesions covered < 15% of their body surface area (BSA). All cases were having modified Psoriasis Area and Severity Index (mPASI) of ≥2.
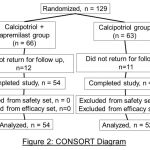 |
Figure 2: CONSORT Daigram |
Efficacy
Treatment success
Mean mPASI at baseline in calcipotriol + apremilast group was 7.8 and that in calcipotriol group was 8.1. Every week mean mPASI was lesser in calcipotriol + apremilast group compared to calcipotriol group till 8 weeks. Adjusted mPASI of calcipotriol + apremilast group was lesser than calcipotriol group and this difference was statistically significant. (4.45 vs. 5.25; adjusted difference -0.80; 95% CI: -1.05 to -0.35; P < 0.001) at week 1. This statistically significant difference was maintained until 8 weeks. (1.80 vs. 2.50; adjusted difference -0.70; 95% CI: -1.10, -0.20; P = 0.005).
Percentage of patients reaching mPASI75 by 8 weeks was higher in calcipotriol + apremilast group compared to calcipotriol group and this difference was statistically significant. (51.85 % [n = 28/54] vs 34.61 % [n = 18/52]; OR: 2.81, 95% CI: 1.37, 3.47; p < 0.001) (Figure 3). In calcipotriol + apremilast group, mPASI was attained by 58.33 % (n = 7/12), 47.37% (n = 18/38) and 75% (n = 3/4) of cases with mild, moderate and severe psoriasis at baseline respectively. While in calcipotriol group, mPASI was attained by 40 % (n = 4/10), 35.13% (n = 13/37) and 20 % (n = 1/5) of cases with mild, moderate and severe psoriasis at baseline respectively.
Table 1: Patient demographics and baseline characteristics
| CALCIPOTRIOL + APREMILAST
(n = 54) |
CALCIPOTRIOL
(n = 52) |
|
| Age:
Mean ± SD (years) |
49.2 ± 13.84 | 50.2 ± 14.86 |
| Male:Female ratio | 32:22 | 29:23 |
| BMI:
Mean ± SD (kg/m2) |
31.7 ± 6.58 | 29.5 ± 5.68 |
| Affected BSA:
Mean ± SD (%) |
7.2 ± 4.50 | 7.4 ± 4.75 |
| Duration of psoriasis: Mean ± SD (years) | 17.6 ± 14.80 | 18.5 ± 12.28 |
| mPASI:
Mean±SD |
7.8 ± 4.15 | 8.1 ± 3.30 |
| Baseline PGA:
n(%) |
||
| Mild | 12 (22.22) | 10 (19.23) |
| Moderate | 38 (70.37) | 37 (71.15) |
| Severe | 4 (7.4) | 5 (9.62) |
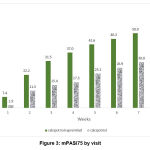 |
Figure 3: mPASI75 by visit. |
Safety
Almost a similar percentage of patients in both groups reported adverse events during the 8 week treatment period. 45.49 % (n = 23) patients in calcipotriol + apremilast group and 42.30% (n = 22) patients in calcipotriol group reported adverse events. Common adverse events in both groups were upper respiratory tract infection (URTI), nasopharyngitis, and vitamin D deficiency. (Table 2). In calcipotriol + apremilast group, nasopharyngitis was reported by 6 (26.09 %) patients, vitamin D deficiency was seen in 5 (21.74 %) patients and URTI was seen in 5 (21.74 %) patients. While in calcipotriol group, naspopharyngitis, vitamin D deficiency and URTI were observed in 5 (22.73 %), 6 (27.27 %) and 6 (27.27 %) patients respectively. No severe adverse events or fatalities were noted nor were there any withdrawals due to AE’s.
Table 2: Adverse events noted during 8 week treatment
| ADVERSE EVENT | CALCIPOTRIOL + APREMILAST
n (%) |
CALCIPOTRIOL
n (%) |
| No. of patients by whom AE’s were reported | 23 (45.59) | 22 (42.30) |
| Total no. of AE’s reported | 38 | 40 |
| Nasopharyngitis | 6 (26.09) | 5 (22.73) |
| Vitamin D deficiency | 5 (21.74) | 6 (27.27) |
| Upper respiratory tract infection | 5 (21.74) | 6 (27.27) |
| Infections and infestations | 4 (17.39) | 5 (22.73) |
| Headache | 4 (17.39) | 5 (22.73) |
| Back pain | 2 (8.70) | 3 (13.63) |
| Diarrhoea | 3 (13.04) | 2 (9.09) |
| Pruritus | 3 (13.04) | 2 (9.09) |
| Influenza-like illness | 2 (8.70) | 2 (9.09) |
| Psoriasis | 1 (4.34) | 2 (9.09) |
| Lower respiratory tract infection | 2 (8.70) | 0 (0) |
| Gastro-oesophageal reflux disease | 1 (4.34) | 1 (4.55) |
| Arthralgia | 0 (0) | 1 (4.55) |
Discussion
In this study, combination of calcipotriol + apremilast was significantly more effective than calcipotriol monotherapy at 8 weeks of treatment in psoriasis. This higher efficacy of combination was apparent since the first week and was maintained till the end of 8 weeks treatment period. Another point to be noted was that the efficacy of calcipotriol + apremilast combination was effective in all 3 severities of baseline psoriasis. Calcipotriol + apremilast combination was well tolerated, no extra adverse events were noted in combination compared to calcipotriol monotherapy. Patients were continuously getting more benefit in the calcipotriol + apremilast group compared to calcipotriol group in terms of treatment success as well as mPASI75. (Figure 3). This suggests that extending the treatment beyond 8 weeks can help in clearing all lesions in those cases whose lesions were not clear or almost clear by 8 weeks.
We could not find any study where the potentiating effect of apremilast to calcipotriol was studied. Although studies were available which have compared apremilast with placebo or calcipotriol + betamethasone combination 10,18,19.
The rate of development of adverse events and adverse drug reactions was low and no serious ADR was noted. Headache, diarrhoea, URTI observed in our study were also reported by Krishnamoorthy et. al. and Mallick et. al. 22,23
This observation was similar to previous studies of calcipotriol and apremilast monotherapies 19,24,25. Our study confirms that the addition of apremilast to calcipotriol is well tolerated and may have a higher benefit:risk ratio for psoriasis.
Lack of adherence to psoriasis treatment is attributed to poor perception by a patient about effectiveness and prolonged duration of therapy 26,27. Previous studies have proved that patient’s thought about a treatment being effective, easy to use and shorter duration of treatment makes a patient more adherent to treatment which leads to better treatment outcomes 28,29.
Conclusion
Our study proves that the addition of apremilast to calcipotriol is significantly more efficacious than calcipotriol monotherapy and safety wise, this combination is as safe as monotherapy. This superior efficacy, shorter duration and similar safety profile of calcipotriol + apremilast combination should contribute to better compliance, improved quality of life and real world treatment outcomes.
Acknowledgement
We are thankful to the department of dermatology, Viswabharathi medical college for their full cooperation in carrying out this study. We are also thankful to the management of Viswabharathi medical college for sponsoring test drugs.
Conflict of Interest
All authors declare no conflicts of interest.
Funding Sources
There is no funding source.
References
- Michalek IM, Loring B, John SM, World Health Organization. Global report on psoriasis. 2016.
- Menter A, Cordoro KM, Davis DMR, Kroshinsky D, Paller AS, Armstrong AW, et al. Joint American Academy of Dermatology–National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2019 Nov;S0190962219326556.
- Strober B, Greenberg JD, Karki C, Mason M, Guo N, Hur P, et al. Impact of psoriasis severity on patient-reported clinical symptoms, health-related quality of life and work productivity among US patients: real-world data from the Corrona Psoriasis Registry. BMJ Open. 2019 20;9(4):e027535.
CrossRef - Strober BE, van der Walt JM, Armstrong AW, Bourcier M, Carvalho AVE, Chouela E, et al. Clinical Goals and Barriers to Effective Psoriasis Care. Dermatol Ther. 2019 Mar;9(1):5–18.
CrossRef - Nazeer M, Ravindran S, Gangadharan G, Criton S. A survey of treatment practices in management of psoriasis patients among dermatologists of Kerala. Indian Dermatol Online J. 2019;10(4):437.
CrossRef - Kleyn EC, Morsman E, Griffin L, Wu JJ, Cm van de Kerkhof P, Gulliver W, et al. Review of international psoriasis guidelines for the treatment of psoriasis: recommendations for topical corticosteroid treatments. J Dermatol Treat. 2019 May 19;30(4):311–9.
CrossRef - John Berth‐Jones. Principles of Topical Therapy. In: Rook’s Textbook of Dermatology. 9th ed. West Sussex, UK: WILEY Blackwell; 2016. p. 18.24-25.
- Francis IE, Seedahmed K, Khumalo N, Ross IL. Topical Steroids Inducing Cushing’s Syndrome and Subsequent Adrenal Axis Suppression. Ann Med Health Sci Res [Internet]. 2019 [cited 2019 Dec 6]; Available from: https://www.amhsr.org/abstract/topical-steroids-inducing-cushings-syndrome-and-subsequent-adrenal-axis-suppression-5218.html
- Andersen YMF, Egeberg A, Ban L, Gran S, Williams HC, Francis NA, et al. Association Between Topical Corticosteroid Use and Type 2 Diabetes in Two European Population-Based Adult Cohorts. Diabetes Care. 2019 Jun;42(6):1095–103.
CrossRef - Papp K, Cather JC, Rosoph L, Sofen H, Langley RG, Matheson RT, et al. Efficacy of apremilast in the treatment of moderate to severe psoriasis: a randomised controlled trial. Lancet Lond Engl. 2012 Aug 25;380(9843):738–46.
CrossRef - Li H, Zuo J, Tang W. Phosphodiesterase-4 Inhibitors for the Treatment of Inflammatory Diseases. Front Pharmacol. 2018 Oct 17;9:1048.
CrossRef - WCG Center Watch. Otezla (apremilast) [Internet]. 2019. Available from: https://www.centerwatch.com/directories/1067-fda-approved-drugs/listing/3974-otezla-apremilast
- Celgene Corporation. HIGHLIGHTS OF PRESCRIBING INFORMATION [Internet]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205437s006lbl.pdf
- Mahil SK, Wilson N, Dand N, Reynolds NJ, Griffiths CEM, Emsley R, et al. Psoriasis treat to target: defining outcomes in psoriasis using data from a real-world, population-based cohort study (the British Association of Dermatologists Biologics and Immunomodulators Register, BADBIR). Br J Dermatol. 2019 Jul 8;
CrossRef - Callis Duffin K, Bushmakin AG, Cappelleri JC, Mallbris L, Mamolo C. A multi-item Physician Global Assessment scale to assess psoriasis disease severity: validation based on four phase III tofacitinib studies. BMC Dermatol. 2019 Dec;19(1):8.
CrossRef - Koo J, Tyring S, Werschler WP, Bruce S, Olesen M, Villumsen J, et al. Superior efficacy of calcipotriene and betamethasone dipropionate aerosol foam versus ointment in patients with psoriasis vulgaris – A randomized phase II study. J Dermatol Treat. 2016 Mar 3;27(2):120–7.
CrossRef - Iain L. O. Buxton. Pharmacokinetics: The Dynamics of Drug Absorption, Distribution, Metabolism, and Elimination. In: Goodman’s and Gilman’s The Pharmacological Basis of Therapeutics. 13th ed. New York: McGraw Hill Education; 2018. p. 13–29.
- Paul C, Stein Gold L, Cambazard F, Kalb RE, Lowson D, Bang B, et al. Calcipotriol plus betamethasone dipropionate aerosol foam provides superior efficacy vs. gel in patients with psoriasis vulgaris: randomized, controlled PSO-ABLE study. J Eur Acad Dermatol Venereol. 2017 Jan;31(1):119–26.
CrossRef - Reich K, Gooderham M, Green L, Bewley A, Zhang Z, Khanskaya I, et al. The efficacy and safety of apremilast, etanercept and placebo in patients with moderate-to-severe plaque psoriasis: 52-week results from a phase IIIb, randomized, placebo-controlled trial (LIBERATE). J Eur Acad Dermatol Venereol. 2017 Mar;31(3):507–17.
CrossRef - Munshi R, Belhekar M, Taur S. A study of agreement between the Naranjo algorithm and WHO-UMC criteria for causality assessment of adverse drug reactions. Indian J Pharmacol. 2014;46(1):117.
CrossRef - Naranjo algorithm: A method for estimating the probability of adverse drug reactions | pmidCALC online calculators [Internet]. [cited 2019 Dec 11]. Available from: http://www.pmidcalc.org/?sid=7249508&newtest=Y
- Krishnamoorthy G, Kotecha A, Pimentel J. Complete resolution of erythrodermic psoriasis with first-line apremilast monotherapy. BMJ Case Rep. 2019 Jan 31;12(1).
CrossRef - Mallick B, Praharaj DL, Nath P, Panigrahi SC. Apremilast induced chronic diarrhea and malnutrition. Drug Discov Ther. 2018;12(6):379–80.
CrossRef - Vujic I, Herman R, Sanlorenzo M, Posch C, Monshi B, Rappersberger K, et al. Apremilast in psoriasis – a prospective real-world study. J Eur Acad Dermatol Venereol. 2018 Feb;32(2):254–9.
CrossRef - Hugar L, H. R. A comparative study of efficacy and safety of topical calcitriol and topical calcipotriol in stable chronic plaque type psoriasis. Int J Basic Clin Pharmacol. 2019 Feb 23;8(3):402.
CrossRef - Zschocke I, Mrowietz U, Karakasili E, Reich K. Non-adherence and measures to improve adherence in the topical treatment of psoriasis. J Eur Acad Dermatol Venereol. 2014;28(Suppl 2):4–9.
CrossRef - Bewley A, Page B. Maximizing patient adherence for optimal outcomes in psoriasis. J Eur Acad Dermatol Venereol. 2011;25(Suppl 4):9–14.
CrossRef - Puig L, Carrascosa JM, Belinchon I. Adherence and patient satisfaction with topical treatment in psoriasis, and the use, and organoleptic properties of such treatments: a Delphi study with an expert panel and members of the Psoriasis Group of the Spanish Academy of Dermatology and Venereology. Actas Dermosifiliogr. 2013;104:488–496.
CrossRef - Tan X, Feldman SR, Chang J, Balkrishnan R. Topical drug delivery systems in dermatology: a review of patient adherence issues. Expert Opin Drug Deliv. 2012;9:1263–1271.
CrossRef







