Manuscript accepted on :18-11-2021
Published online on: 13-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Hanefi ÖZBEK
Second Review by: Dr. Nurul Diyana Sanuddin
Final Approval by: Dr. Gul Ozcan
Hanaa H. Ahmed , Soheir E. Kotob*
, Soheir E. Kotob* , Ahmed A. Abd-Rabou
, Ahmed A. Abd-Rabou , Hadeer A. Aglan
, Hadeer A. Aglan , Gamal A. Elmegeed
, Gamal A. Elmegeed and Ola A. Mohawed
and Ola A. Mohawed
Hormones Department, Medical Research and Clinical Studies Institute, National Research Centre, Dokki, Giza, Egypt.
Corresponding Author E-mail: soheir1010@hotmail.com
DOI : https://dx.doi.org/10.13005/bpj/2274
Abstract
This research aimed to formulate quercetin (Qu) and curcumin (CUR)-loaded PLGA NPs coated with chitosan (CS) & PEG and to explore their therapeutic effect against obesity in rats. Qu and CUR nanostructures were prepared and characterized by Zetasizer and TEM. Then, the formulated nanoparticles and their free couterparts were employed for mitigation of obesity in female rats. The size of NPs was in nanometer range with an average size distribution 307.9 nm for Qu NPs and 322.5 nm for CUR NPs. The Qu NPs and CUR NPs were appeared in the TEM image containing core in which the Qu or CUR was localized and surrounded by the coat of PLGA-CS-PEG. The Qu NPs exhibited negative zeta potential at -8.5 mV, while, CUR NPs exhibited positive zeta potential at +0.916 mV. Treatment with orlistat, free Qu, Qu NPs, free CURor CUR NPs elicited significant decline in body weight, BMI and Lee index. Orlistat and CUR NPs significantly diminished liver, heart and visceral adipose tissue weights. Furthermore, the suggested treatments significantly reduced the gonadal and subcutaneous adipose tissue weights. Orlistat significantly lessened kidney and adrenal weights. All treatments significantly minimized serum Chol., TG, LDL, glucose, INS, HOMA-IR, LH, MDA, TLR4 and NF-κB levels and elevated serum HDL, E2 and TAC levels. Orlistat significantly enhanced serum IL-10 level. Conclusively, Qu and CUR nanoformulations offer anti-obesity potency through their hypolipidemic, hypoglycemic,antioxidative and anti-inflammatory effects. Both Qu and CUR NPs manifested superior effect than their free counterparts, may be because of solubility elevation as well as bioavailability of the nanoencapsulation.
Keywords
Anthropometric Measurements; Biochemical Markers; Curcumin Nanoparticles; Lipid Profile; Obesity; Quercetin Nanoparticles
Download this article as:| Copy the following to cite this article: Ahmed H. H, Kotob S. E, Abd-Rabou A. A, Aglan H. A, Elmegeed G. A, Mohawed O. A. Pre-Clinical Evidence for the Anti-Obesity Potential of Quercetin and Curcumin Loaded Chitosan/PEG Blended PLGA Nanoparticles. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Ahmed H. H, Kotob S. E, Abd-Rabou A. A, Aglan H. A, Elmegeed G. A, Mohawed O. A. Pre-Clinical Evidence for the Anti-Obesity Potential of Quercetin and Curcumin Loaded Chitosan/PEG Blended PLGA Nanoparticles. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3oNgBcR |
Introduction
Obesity is a disease characterized by anomalous fatty acid biosynthesis and breakdown, which trigger supernumerary fat cumulation 1. The unusual widening of the adipose tissue mass generates from two discrete mechanisms: hyperplasia (augmented consistence of new adipocytes through adipogenesis process) and hypertrophy (enlarge in size of present adipocytes owing to lipogenesis and lipid cumulation). Hyperplasia comes from the differentiation of preadipocytes into mature adipocytes that have salient hypertrophic prospect, and the enlarged adipocyte size is neatly accompanied with elevated adipose tissue inflammation and systemic insulin resistance 2. Obesity is usually linked with several life-threatening ailments like hypertension, type 2 diabetes, cancer, respiratory complications, coronary heart disease, inflammation, and osteoarthritis 3.
Obesity, is identified as a global epidemic since it affects virtually all age and sociable classes in both developed and developing nations 4. It influences 1.5 billion humans globally, and is mostly a sequel of deleterious lifestyle preferences such as consumption of overeating food and paucity of exercise 5.
The available medications for obesity depends on lifestyle adjustments, decreasing energy consumption and eugmenting energy expenditure. Other than lifestyle changes like exercise and diet adjustment, the present clinical approaches to handle obesity give primacy to pharmacotherapy, comprising the drugs, lorcaserin and orlistat, and surgical interventions such as bariatric surgeries. Whilst, these classical treatments have many side effects which desire their enhancement 6, 7.
Utilization of a assortment of phytochemicals has protruded as an auspicious prospect for battling obesity and its comorbidities 8. A plenty of in vitro as well as in vivo researches have indicated that phytochemicals have anti-obesity response via restraining adipocyte proliferation and preadipocyte differentiation as well as potentiating apoptosis of adipocyte, dampening lipogenesis, lipolysis promotion as well as oxidation of fatty acid beta (β), and strengthening the regulatory networks controlling inflammation 9, 10.
3,3′,4′,5,7-pentahydroxyflavone (Quercetin; Qu) is a secondary metabolite existed plentifully in a wide diversity of foods, like vegetables, fruits and beverages. Accumulating evidence demonstrated that Qu can conserve against obesity and its accompanied metabolic disorders 11. The possible beneficial role of Qu was ascertained by its numerous bioactivities, comprising antioxidant, anti-inflammatory and anti-obesity impacts as well as other prospective biological characteristics 12, 13. Etxeberria et al. 14 recorded that Qu diminishes the serum INS level and prevents the gain of body weight in high-fat sucrose adminstered rats.
The prime curcuminoid polyphenolic compound of the spice turmeric (Curcuma longa) is called curcumin (CUR) 15; comprising 2–8% of the turmeric 16. Turmeric has well known biological activities like anti-inflammatory, anti-obesity, anti-oxidant anti-angiogenesis, and anti-carcinogenesis 17. The anti-obesity offect of CUR stems from hindering the differentiation of preadipocyte, defeating lipogenesis, enhancing oxidation of fatty acid, and minimizing adipose tissue inflammatory response 17-19.
Despite multiple health benefits, the commonly weak aqueous solubility, bioavailability, stability, and target specificity of phytochemicals, have restricted their clinical applications. Currently, nano-drug delivery tactic offers considerable promise for boosting the pharmacological properties of phytochemicals through augmenting their solubility and stability, elevating their bioavailability, protecting them from premature degradation in the body, prolonging their circulation time, and consequently intensifying their anti-obesity potential 8.
The current approach was constructed to examinate the advanced impact of Qu and CUR loaded chitosan/polyethylene glycol (PEG) blended poly D,L-lactide-co-glycolide (PLGA) nanoparticles on obesity induced via HFD and explored the mechanism of action of these nanoparticles and related signalling pathways, seeking to provide experimental basis for the development of clinical application of this type of treatment in patients.
Materials and Methods
Materials
Quercetin >95%, Curcumin >95%, poly D,L-lactide-co-glycolide (PLGA, ratio of copolymer 50:50, Inherent viscosity = 0.41), polyvinyl alcohol (PVA), chitosan (CS; MW 60–90 kDa, deacetylation degree = 85%), polyethylene glycol (2 kDa, PEG), dichloromethane, glacial acetic acid and Tween 80 were acquired from Sigma–Aldrich (St. Louis, MO). The remain chemicals were of analytical grade purchased from Sigma–Aldrich (St. Louis, MO).
Preparation of Qu and CUR PLGA-NPs surface modified with chitosan and PEG
Quercetin and CUR-loaded PLGA NPs were synthesized according to the procedure described by Abd-Rabou and Ahmed, 20 and Arya et al. 21 with tiny alterations. In 3 ml of dichloromethane, 100 mg of PLGA polymer & 10 mg of quercetin or curcumin were dissolved. In 1% glacial acetic acid, 5 ml of chitosan solution (12% w/v) was made, filtered & then titrated to 5 ml of PVA aqueous solution (2% w/v) before emulsification. Thereafter, PEG (2 kDa), 2% w/v, was provided to the prepared chitosan-PVA aqueous solution prior to emulsification to gain PEG-coated (PLGA-CS-PEG) NPs. In their organic phase PLGA and Qu or CUR were emulsified in the previous mentioned chitosan-PEG-PVA aqueous solution, then sonicated by a microtip probe sonicator (VC 505, Vibracell Sonics, Newton, USA) adjusted at energy output of 55 W in an ice bath for 2 min. The organic solvent was evaporated by stiring the emulsion at room temperature on a magnetic stirring plate overnight. In the next day, ultracentrifugation at 35,000 rpm at 4 °C for 20 min (Sorvall Ultra-speed Centrifuge, Kendro) was done to obtaine the formed NPs , then the unbound PVA, chitosan, PEG and un-encapsulated Qu or CUR were removed by double distilled water washing.
Methods
Physicochemical characterization of Qu and CUR NPs
Measurement of particle size analysis and zeta potential
Particle size and size distribution (polydispersity index) of Qu and CUR NPs were analyzed using dynamic light scattering (DLS) using a Zetasizer (Nano ZS, Malvern Instrument, UK). Zeta potential of the Qu and CUR NPs was also investigated using the same Zetasizer. ∼ 1 mg/ml of NPs suspension in MilliQ water was sonicated for 30 sec in an ice bath using a sonicator (VC 505, Vibracell Sonics, Newton) set at 55 W of energy output. 100 μl of these NPs suspensions was diluted to 1 ml in MilliQ water and used for particle size and zeta potential measurements.
Transmission electron microscopic (TEM)
Particle morphology of the synthesized nanoparticles was tested using transmission electron microscopy (TEM; JEOL JEM-2100, Japan). Briefly, into aFormvar-coated copper grids 100 μg/ml of the nano-suspensions were dropped, then after drying complete, the samples were stained by uranyl acetate, 2% w/v, (Electron Microscopy Services, Ft. Washington, PA). Digital Micrograph and Soft Imaging Viewer Software was applied for mage capture & analysis wer.
In vivo study
Animal handling
A 64 adult female Wistar strain albino rats of, with body weight of 130 ± 10 g and age of 90 d, were applied in the current experimental setting. The rats were comes from the Animal Facility Unit of the National Research Centre, Dokki, Giza, Egypt and placed in cages of polypropylene (8 rats/cage) in ideal environmental conditions, clean and controlled air room, a temperature of 24±1°C, a 12/12 hrs ligh/dark cycle, a 60±5% of relative humidity & free entrance to tap water as well as standard rodent chow. Rats were left to adapt to these conditions for 2 weeks before the experiment beginning.
Ethical Statement
Housing and management of animals followed the care and use of laboratory animals recommendations and guidelines. The research was done in compliance with the code of ethics of the World Medical Association (Helsinki Declaration) for animal experiments as well as the experimental protocol was agreed by the institution Ethical Committee for Medical Research, National Research Centre, Egypt (Code No:18175).
Experimental groups
Next to the period of acclimatization, according to Buettner et al. 22 a standard rodent chow, with protein of 26.5%, fat of 3.8%, carbohydrate of 40%, and crude fiber of 4.5% in 100 g of chow, was administreted to rats (eight rats) for the experimental period (18 weeks) and deemed as the lean control group (control group). Obese model was generated in the other 56 rats by the 12thweeks of HFD feeding (protein of 19.93%, cholesterol of 15%, carbohydrate of 57.50%, and dietary fiber of 2.81% in 100 g of chow according to Soliman et al. 23 method (Obese group). The obese group was categorized into 7 groups (8 rats/ group). (1): untreated obese group; (2): obese rats treated with orlistat orally (200 mg/kg b.wt.) every day in accordance with Nishioka et al. 24 for 6 weeks; (3):obese rats orallyadministered 1 ml/rat of PLGA-CS-PEG nano-void daily for 6 weeks; (4)and (5): obese rats supplemented orally with freeQu or Qu NPs respectively in a dose of 10 mg\kg b.wt. dissolved in 1 ml distilled water/rat 25 every day for 6 weeks; (6) & (7): obese rats orally received free CURor CUR NPs respectively (20 mg\kg b.wt.) dissolved in 1 ml distilled water/rat [26] daily for 6 weeks.
Anthropometric measurements
After final administration, the existence of obesity was assessed using Lee index according the equation of Bernardis and Patterson 27:
Lee index = cube root of body weight (g) / nose-to-anus length (cm).
The immediately anterior to the forefoot (abdominal circumference (AC)), immediately behind the foreleg (thoracic circumference (TC)), and nose-to-anus or nose-anus length (body length) were also determined. Also, body mass index (BMI) was measured.
BMI (g/cm2) = (Body weight (g)/Length2 (cm2)) according to Novelli et al. [28] was determined.
Blood and tissue sampling
After taking the anthropometric parameters, the rats were fasted for 12-14 hrs (overnight), & the samples of blood were collected, under anesthesia of diethyl ether, from the tail vein. The blood specimens were collected in a centrifuge tube in the absence of any agent of anticoagulant, then the samples left for 45 minutes to coagulate at room temperature to collect the serum. The samples were separated using centrifugation for 15 minutes at 1800 × g and 4°C by the cooling centrifuge apparatus. Serum samples were frozen and stored at −20°C for further biochemical analyses. After blood samples collection, the rats were euthanized using the cervical dislocation technique.
Liver, kidneys (right and left), heart, spleen, adrenal, thymus, and visceral adipose tissue (VAT),gonadaladipose tissue (GAT) as well as subcutaneous adipose tissue (GAT) were removed according to the defined anatomical landmarks. The dissected organs and tissues were washed with isotonic saline, blotted dry and weighed immediately.
Biochemical determinations
Serum total cholesterol (Chol), triglycerides (TG), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) levels were assessed using colorimetric method through the kits acquired from Reactivos GPL Co. (Barcelona, Spain) according to Meiattini 29 methods, Bucolo and David 30 and Naito 31 respectively. Serum glucose and malondialdhyde (MDA) concentrations were assigned colorimetrically applying kits purchased from Biodiagnostic (Egypt) following Trinder 32 and Ohkawa et al. 33 methods respectively.
Quantitativeanalysis of serum insulin (INS) level was performed using ELISA kit secured from Immunoscopec Co. CA (Netherlands) following the National Committee for Clinical Laboratory Standards Procedures for the collection of diagnostic blood specimens by venipuncture 34 method. Estimation of insulin resistance was done through applying the method of homeostasis model assessment, HOMA-IR, and was determined using the following formula:
Serum glucose (mg/dl) × fasting serumINS (MIU/mL) divided by 405 35.
Serum leutinizing hormone (LH) level wasevaluated using ELISA kit obtained from Immunoscopec Co. CA (Netherlands) following Uotilla et al. 36 method. Moreover, assessment of the serum estradiol (E2) level was carried out using ELISA kit provided fromBiocheck, Inc, (USA) according to Tietz 37 method. Serum total antioxidant capacity (TAC), nuclear factor kappa B (NF-κB) as well as interleukin 10 (IL-10) levels werequantified by ELISA using kitssupplied from Sino Gene Clon Biotech. Co., Ltd (China) following the manufacturer’s manual. Finally, serum toll–like receptor 4 (TLR4) level was assessed using ELISA kit procured from Sunlong Biotech. Co., Ltd. (China) following the operating instructions.
Statistical analysis
The results of the experiment were illustrated as arithmetic means and their standard errors (SE) (Mean±SE). The analysis of data were done by one-way analysis of variance using the Statistical Package for the Social Sciences program (SPSS), version 17 followed by Bonferroni post hoc test to compare significance among the studied groups [38]. In all analyses, p<0.05 was taken to improve statistical significance.
Results
Physicochemical characterization of Qu and CUR NPs
The particle size distribution as well as zeta potential of the formulated NPs were specified using dynamic light scattering (DLS) by a Zetasizer, whereas the morphology of NPs was detected by TEM. In this setting, our findings indicated that the designed NPs were of nanometer size range with an average size distribution 307.9 nm for Qu NPs (Fig. 1A) and 322.5 nm for CUR NPs (Fig. 2A).
The Qu NPs configuration was appeared in the TEM image containing core in which the Qu was localized and surrounded by the coat of PLGA-CS-PEG (Fig. 1B). The zeta potential of Qu NPs was illustrated in Fig. (1C), which demonstrated the negative zeta potential at -8.5 mV. On the other hand, the CUR NPs pattern was seen in the TEM image containing core in which the CUR was centralized and enclosed by the coat of PLGA-CS-PEG (Fig. 2B). The zeta potential of CUR NPs was represented in Fig. (2C), which revealed the positive zeta potential at +0.916 mV.
Effect of orlistat, free Qu, Qu NPs, free CUR or CUR NPs on anthropometrical parameters
The results in Table (1) evaluated the influence of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on anthropometric parameters of obese rats. Prolonged feeding of rats with HFD provoked significant (p<0.05) elevation in the body weight, TC, AC, BMI and Lee index versus the control group. Obese rats treated with orlistat elicited significant (p<0.05) reduction in body weight, TC, AC, BMI and Lee index in comparison with the untreated obese rats. Meanwhile, treatment of obese rats with free Qu, Qu NPs, free CUR and CUR NPs evoked significant (p<0.05) detraction in body weight, BMI and Lee index relative to the untreated obese rats. Additionally, all treated groups experienced insignificant (p˃0.05) change in body length and TC comparing with the untreated obese rats. Obese rats treated with free Qu, Qu NPs and free CUR exhibited insignificant (p˃0.05) change in AC while those treated with CUR NPs recorded significant (p<0.05) lowering in AC versus the untreated obese rats.
The obese groups treated with PLGA-CS-PEG nano-void or free Qu displayed significant (p<0.05) enhancement in weight gain versus the obese group treated with orlistat. Moreover, obese rats treated with PLGA-CS-PEG nano-void exhibited significant (p<0.05) increase in BMI and Lee index when compared with the obese group treated with orlistat.
Table 1: Effect of treatment with orlistat, free Qu, Qu NPs, free CURor CUR NPs on anthropometric parameters of obese rats.
| Parameters
Groups |
Body weight
(g) |
Body length
(cm) |
TC
(cm) |
AC
(cm) |
BMI
(g/cm2) |
Lee Index
|
| Control group | 129.3±3.05 | 20.31±0.31 | 11.12±0.23 | 12.50±0.23 | 0.31±0.01 | 0.249±0.004 |
| Obese group | 275.2±4.09a | 21.17±0.24 | 12.24±0.16a | 14.43±0.24a | 0.61±0.02a | 0.312±0.002a |
| Ob+Orlistat | 135.0±4.22b
|
20.52±0.29 | 11.56±0.14b | 13.20±0.28b | 0.33±0.01b | 0.256±0.005b |
| Ob+ PLGA-CS-PEG nano-void | 263.1±7.61c
|
20.92±0.35 | 12.02±0.10 | 14.06±0.35 | 0.58±0.02c | 0.298±0.009c |
| Ob+free Qu | 161.4±3.62bc | 20.81±0.13 | 11.80±0.07 | 13.65±0.14 | 0.37±0.01b | 0.273±0.004b |
| Ob+ Qu NPs | 147.5±5.90b | 20.67±0.17 | 11.75±0.09 | 13.50±0.16 | 0.346±0.02b | 0.267±0.009b |
| Ob+free CUR | 153.7±5.96b | 20.70±0.21 | 11.76±0.08 | 13.61±0.16 | 0.358±0.01b | 0.270±0.004b |
| Ob+ CUR NPs | 141.0±3.40b | 20.57±0.22 | 11.66±0.11 | 13.34±0.15b | 0.33±0.01b | 0.260± 0.005b |
Data were depicted as Mean ± S.E for each group of rats n=8.
a: Change of significant at P>0.05 comparing with the control rats.
b: Change of significant at P>0.05 comparing with the obese rats.
c: Change of significant at P>0.05 comparing with the rats treated with orlistat.
Effect of orlistat, free Qu, Qu NPs, free CUR or CUR NPs on different organs and tissues weight
The obtained data in Table (2) illustrated the effect of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on the weight of different organs and tissues of obese rats. Prolonged feeding of rats with HFD brought about significant (p<0.05) enhancement in the weight of the liver, left kidney, heart, adrenal, thymus and visceral, gonadal as well as subcutaneous adipose tissues versus the control rats. However, supplementation of HFD-fed rats with orlistat or CUR NPs inspired significant (p<0.05) drop in liver, heart and visceral, gonadal as well as subcutaneous adipose tissues weight in comparison with those left without treatment. Also, treatment of obese rats with orlistat yielded significant (p<0.05) decline in the weight of the left kidney and adrenal relative to the untreated obese rats. Furthermore, the group of obese rats treated with Qu NPs exhibited significant (p<0.05) depletion in visceral, gonadal and subcutaneous adipose tissues weight relative to the untreated obese rats. Treatment of obese rats with free Qu or free CUR caused significant (p<0.05) drop in the weight of gonadal and subcutaneous adipose tissues contrary to those left without treatment.
The group of obese rats treated with PLGA-CS-PEG nano-void revealed significant (p<0.05) elevation in the weight of the visceral, gonadal and subcutaneous adipose tissues in comparison with that treated with orlistat. Moreover, the obese rats in the groups treated with free Qu or free CUR manifested significant (p<0.05) rise in the weight of gonadal adipose tissues versus the obese rats in the group treated with orlistat.
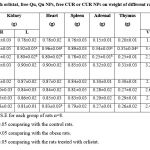 |
Table 2: Effect of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on weight of different rat’s organs of obese rats. |
Data were depicted as Mean ± S.E for each group of rats n=8.
a: Change of significant at P>0.05 comparing with the control rats.
b: Change of significant at P>0.05 comparing with the obese rats.
c: Change of significant at P>0.05 comparing with the rats treated with orlistat.
Effect of orlistat, free Qu, Qu NPs, free CUR or CUR NPs on serum lipid profile
The represented data in Table (3) showed the influence of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on serum lipid profile of obese rats. Feeding rats with HFD for a long period of time induced significant (p<0.05) promotion in Chol, TG and LDL serum levels in concomitant with significant (p<0.05) drop in HDL serum level by contrast with the control rats. While, treatment of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs produced significant (p<0.05) decline in Chol, TG and LDL serum levels along with significant (p<0.05) rise in HDL serum level versus those left without treatment.
The group of obese rats treated with PLGA-CS-PEG nano-void exhibited significant (p<0.05) enhancement in Chol, TG and LDL serum levels along with significant (p<0.05) diminution in HDL serum level in contrary to the group of obese rats treated with orlistat.
Table 3: Effect of treatment with orlistat, free Qu, Qu NPs as well as free CUR and CUR NPs on lipid profile of obese rats.
| Parameters
Groups |
Chol
(mg/dL) |
TG
(mg/dL) |
HDL
(mg/dL) |
LDL
(mg/dL) |
| Control group | 69.2±1.2 | 83.1±2.8 | 35.5±2.7 | 23.2±1.4 |
| Obese group | 156.2±5.9a | 128.3±5.9a | 22.9±0.88a | 93.8±5.7a |
| Ob+Orlistat | 75.9±1.9b | 87.2±2.2b | 33.9±0.9b | 32.7±3.0b |
| Ob+ PLGA-CS-PEG nano-void | 150.1±3.6c | 121.6±1.4c | 25.1±0.9c | 89.5±1.9c |
| Ob+free Qu | 88.0±3.3b | 97.0±2.2b | 30.1±1.5b | 41.5±2.4b |
| Ob+ Qu NPs | 79.2±2.6b | 94.30±6.4b | 31.6±1.4b | 38.5±1.1b |
| Ob+free CUR | 83.3±2.5b | 96.2±3.5b | 30.8±1.1b | 39.0±0.9b |
| Ob+CUR NPs | 77.3±1.9b | 90.7±2.4b | 32.9±1.2b | 34.9±2.1b |
Data were depicted as Mean ± S.E for each group of rats n=8.
a: Change of significant at P>0.05 comparing with the control rats.
b: Change of significant at P>0.05 comparing with the obese rats.
c: Change of significant at P>0.05 comparing with the rats treated with orlistat.
Effect of orlistat, free Qu, Qu NPs, free CUR and CUR NPs on serum glucose, INS and HOMA-IR values
Table (4) demonstrated the influence of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on serum glucose, INS and insulin resistance values of obese rats. Extended feeding of rats with HFD provoked significant (p<0.05) advancement in glucose, INS serum levels and HOMA-IR value relative to the control rats. On the opposite side, treatment of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs brought about significant (p<0.05) drop in glucose, INS serum levels and HOMA-IR values versus the untreated obese rats.
In comparison with the group of obese rats receiving orlistat, the HFD-fed rats treated with PLGA-CS-PEG nano-void, free Qu or free CUR displayed significant (p<0.05) rise in glucose serum level. Moreover, the groups of obese rats treated with PLGA-CS-PEG nano-void or free Qu exhibited significant (p<0.05) elevation in INS serum level and HOMA-IR values versus that treated with orlistat. Lastly, the group of obese rats treated with free CUR recorded significant (p<0.05) rise in HOMA-IR value relative to those treated with orlistat.
Table 4: Effect of treatment with orlistat, free Qu, Qu NPs as well as free CUR and CUR NPs on serum glucose, INS and insulin resistance values of obese rats.
| Parameters
Groups |
Glucose
(mg/dL) |
INS
(MlU/ml) |
HOMA-IR |
| Control group | 69.8±1.7 | 13.5±0.14 | 2.3±0.07 |
| Obese group | 153.7±1.4a | 27.8±0.12a | 10.5±0.13a |
| Ob+Orlistat | 73.0±2.0b | 14.6±0.83b | 2.6±0.19b |
| Ob+ PLGA-CS-PEG nano-void | 150.1±4.3c | 25.4±1.12c | 10.2±0.35c |
| Ob+free Qu | 88.3±1.7bc | 19.3±0.91bc | 4.2±0.17bc |
| Ob+ Qu NPs | 83.8±2.1b | 17.5±1.33b | 3.6±0.28b |
| Ob+free CUR | 86.9±2.1bc | 18.6±1.25b | 3.9±0.24bc |
| Ob+ CUR NPs | 79.5±3.6b | 15.0±0.54b | 2.9±0.13b |
Data were depicted as Mean ± S.E for each group of rats n=8.
a: Change of significant at P>0.05 comparing with the control rats.
b: Change of significant at P>0.05 comparing with the obese rats.
c: Change of significant at P>0.05 comparing with the rats treated with orlistat.
Effect of orlistat, free Qu, Qu NPs, free CUR and CUR NPs on serum LH and E2 levels
Fig. (3a & b) explained the influence of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on serum LH and E2 levels of obese rats. Feeding rats with HFD for a long period of time prompted significant (p<0.05) enhancement in serum LH level in concomitant with significant (p<0.05) decline in E2 serum level in contrary to the control rats. However, supplementation of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs caused significant (p<0.05) drop in LH serum level along with significant (p<0.05) elevation in E2 serum level in contrast with the untreated obese rats. Furthermore, the groups of obese rats treated with PLGA-CS-PEG nano-void, free Qu or free CUR displayed significant (p<0.05) drop in E2 serum level versus the group treated with orlistat.
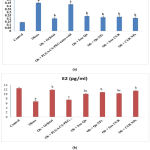 |
Figure 3: Effect of orlistat, free Qu, Qu NPs, free CUR and CUR NPs on serum (a) LH and (b) E2 levels of obese rats. |
Data were depicted as Mean ± S.E for each group of rats n=8.
a: Change of significant at P>0.05 comparing with the control rats.
b: Change of significant at P>0.05 comparing with the obese rats.
c: Change of significant at P>0.05 comparing with the rats treated with orlistat.
Effect of orlistat, free Qu, Qu NPs, free CUR and CUR NPs on oxidant/anti-oxidant status
The results depicted in Table (5) represented the impact of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on serum MDA and TAC levels of obese rats. Prolonged feeding of rats with HFD yielded significant (p<0.05) elevation in MDA serum level along with significant (p<0.05) inhibition in TAC serum level versus the control rats. Whereas, treatment of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs triggered significant (p<0.05) decline in serum MDA level in concomitant with significant (p<0.05) elevation in serum TAC relative to the untreated obese rats. At the same time, the group of obese rats treated with PLGA-CS-PEG nano-void exhibited significant (p<0.05) advancement in MDA serum level and significant depletion (p<0.05) in TAC serum level versus that treated with orlistat.
Table 5: Effect of treatment with orlistat, free Qu, Qu NPs as well as free CUR and CUR NPs on oxidant/antioxidant status of obese rats.
| Parameters
Groups |
MDA
(nmol/mL) |
TAC
(U/ml)
|
| Control group | 42.7±1.3 | 7.1±0.41 |
| Obese group | 86.4±3.9a | 2.4±0.14a |
| Ob+Orlistat | 48.2±1.2b | 5.9±0.12b |
| Ob+ PLGA-CS-PEG nano-void | 80.7±3.9c | 2.9±0.21c |
| Ob+free Qu | 52.3±2.1b | 4.9±0.18b |
| Ob+ Qu NPs | 50.8±2.2b | 5.37±0.27b |
| Ob+free CUR | 51.4±2.4b | 5.1±0.29b |
| Ob+CUR NPs | 49.9±2.6b | 5.42±0.31b |
Data were depicted as Mean ± S.E for each group of rats n=8.
a: Change of significant at P>0.05 comparing with the control rats.
b: Change of significant at P>0.05 comparing with the obese rats.
c: Change of significant at P>0.05 comparing with the rats treated with orlistat.
Effect of orlistat, free Qu, Qu NPs, free CUR and CUR NPs on inflammatory mediators
The data in Table (6) demonstrated the impact of treatment with orlistat, free Qu, Qu NPs, free CUR or CUR NPs on serum TLR4, NF-κB and IL-10 levels of obese rats. Feeding rats with HFD for a long period of time elicited significant (p<0.05) rise in TLR4 and NF-κB serum levels in association with significant (p<0.05) reduction in IL-10 serum level when compared to the control rats. However, treatment of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs instigated significant (p<0.05) decline in serum TLR4 and NF-κB levels relative to the untreated obese rats. Simultaneously, the group of obese rats treated with orlistat manifested significant (p<0.05) enhancement in IL-10 serum level in contrary to the group of untreated obese rats. Furthermore, treatment of obese rats with PLGA-CS-PEG nano-void caused significant (p<0.05) elevation in TLR4 and NF-κB serum levels versus the group of obese rats treated with orlistat.
Table 6: Effect of treatment with orlistat, free Qu, Qu NPs as well as free CUR and CUR NPs on inflammation of obese rats.
| Parameters
Groups |
TLR4
(pg/ml) |
NF-κB
(ng/L) |
IL- 10
(ng/L) |
| Control group | 1458.8 ± 36.9 | 372.6± 20.4 | 281.4±13.3 |
| Obese group | 3167.6±237.8a | 687.08±34.3a | 148.9±6.7a |
| Ob + Orlistat | 1671.0± 125.1b | 434.98 ± 9.8b | 234.5±21.4b |
| Ob + PLGA-CS-PEG nano-void | 2970.7±52.0c | 638.3±25.7c | 168.9±7.6 |
| Ob + free Qu | 2052.47 ± 65.5b | 505.4 ±11.0b | 191.1±15.8 |
| Ob + Qu NPs | 1948.1 ±87.9b | 491.5 ± 15.2b | 208.7±18.3 |
| Ob + free CUR | 1970.9± 72.2b | 498.5 ± 21.1b | 201.3±14.6 |
| Ob + CUR NPs | 1897.3 ± 154.9b | 465.1 ± 19.5b | 210.2±16.8 |
Data were depicted as Mean ± S.E for each group of rats n=8.
a: Change of significant at P>0.05 comparing with the control rats.
b: Change of significant at P>0.05 comparing with the obese rats.
c: Change of significant at P>0.05 comparing with the rats treated with orlistat.
Discussion
Phytochemicals encapsulation into polymeric nanoparticles or conjugation to polymers enhance their bioavailability and augment their sustained and controlled release rate 39 in addition to the enhancement of their aqueous solubility, and stability 40. The polymeric nanoparticles with their distinctive surface chemistry can adsorb, entrap, or covalently attaches medicinal phytochemicals to provide a considerable delivery enhancement 41. The synthetic polymers [poly(lactide-coglycolide) (PLGA), poly (lactide) (PLA), poly (ε-caprolactone) (PCL) and poly (ethylene glycol) (PEG)] are the most common used biodegradable polymers. The most common natural polymers include chitosan and alginate 39. PLGA nanoparticles have acquired prominent attention as an effictive delivery vehicle, owing to its ease of preparation, elevated the ability of drug loading, competence of exceptional endocytosis, and controlled drug release manner 42, 43. Another frequently applied biocompatible polymer is PEG, which is extremely hydrophilic, and has trivial immunogenicity 41.
The major problem facing the utilization of nanoparticles made from polymers as drug carriers is their elimination from the blood circulation rapidly via phagocytosis. Thus, the present interest has been directed towards the formulation of NPs coated with various macromolecules that can restrain the process of phagocytosis and elevate of blood drug pharmacokinetics 44. Thus, in this scenario, we have originate Qu and CUR-loaded PLGA NPs, surface coated with PEG and chitosan to boost their therapeutic impact for management of obesity. The modulated, surface coated PEG NPs may avoid activity of macrophage uptake and prolong the encapsulated drug blood circulation time. Furthermore, chitosan coating inspires biocompatible, biodegradable and non-toxic system 45.
A reproducible and simple procedure has been adopted in the current study to fabricate and characterize Qu and CUR-loaded PLGA NPs coated with chitosan and PEG. The drug loaded nanocarriers characterization is physicochemically serve as a crucial merit for acquiring a potent drug delivery vehicle as it considerably monitors the fate of therapeutic carrier system at the site of action. In this situation, the size distribution is a remarkable parameter that promptly influences the drug release profile, physical stability, cellular uptake with in vivo bioavailability and bio-distribution of the drug-loaded nanocargo 47. The dynamic light scattering findings in the current work, revealed that the developed Qu and CURNPs were of nanometer size range. Moreover, TEM images showed that the configuration of Qu NPs and CUR NPs contain core in which Qu or CUR were localized and surrounded by the coat of PLGA-CS-PEG. Furthermore, Qu NPs exhibited negative zeta potential of -8.5 mV, while, CUR NPs displayed positive zeta potential of +0.916 mV. Our findings regarding the characterization of the formulated Qu NPs were in accordance with those of Abd El-Fattah et al. 47 who cited that the prepared quercetin-loaded phytosome nanoparticles were homogeneously spherical with negatively charged zeta potential at -44.6 ± 4.1 mv. Besides, Tefas et al. 48 registered that the prepared samples of Qu-loaded PLGA NPs were negatively charged as a result of the ionized terminal carboxylic groups of PLGA exist on the surface of the NPs with zeta potential ranged between −27.5 ± 0.721 mV and −14.2 ± 1.069 mV. The obtained characterization results of the developed CUR NPs match those of Arya et al. 21. These investigators found that the prepared CUR NPs were of size in nanometer range with an average diameter of 264 nm, a reduced polydispersity index (PDI) value of 0.181 and positive zeta potential of 19.1 mV. Furthermore, they demonstrated that the surface topology of CUR NPs (observed by atomic force microscope (AFM) analysis) was smooth and spherical innature.
The induction of obesity in rats by employment of HFD is considered as one of the most preferred and validated procedure to generate obese animal model for investigation of obesity and its mechanism, obesity-related complications, and screening of unprecedented medicinal interventions owing to its great relevance to the pathophysiology of obesity in human 49.
In this sense, our data revealed that rats fed the HFD for 12 weeks developed obesity characterized by an increase in body weight, body length, TC, AC, BMI and Lee index. Prior studies have shown that the differences in weight and lipid profile in blood can be used to ascertain the successful fulfillment of the obesity in animal model 50. Furthermore, Rocha et al. 51 stated that supplementation of rats with unsaturated HFD yields an increase in weight gain, total body fats and fat pads (retroperitoneal, visceral and epididymal). Also, the findings of Wang et al. 52 study clarified that, the weight of animals fed on HFD for 10 weeks was over 20 % greater than that of the control animals; the Lee’s index was significantly higher than that of the control animals; the waist circumference as well as visceral fat mass were also higher than those in the control one. Consequently, the obesity in the HFD fed animals could be allied to the elevated constitution of fat ingredient due to HFD regime53.
The weight of the internal organs especially the fresh weight of the liver and fat, is important parameter for establishing HFD obesity 54. In this study, the untreated obese rats had a larger liver, right and left kidney, heart, spleen, adrenal, thymus and visceral, gonadal as well as subcutaneous adipose tissue weight in relation to the control rats. These findings echo those of Kabir et al. 55 who mentioned the increased liver weight in the HFD–fed animals than that of the control counterparts. Furthermore, they recorded the increased weight of mesenteric, peritoneal, and epididymal fats in the HFD–fed rats than that of the control one. Also, Tung et al. 56 registered the significant increase in the weight of kidney, liver, fat bodies around kidney and fat bodies around epididymis in the HFD-fed rats. Seo et al. 57 cited that liver, kidney, spleen, thymus, epididyimal and intestinal fat weights were increased in HFD group. Other investigators also stated that the weight of the heart 58 and adrenal gland 59 is increased in HFD group.
It has been proposed that mesenteric fat accumulation may lead to the lipid deposition in the liver owing to the immediate supply of free fatty acids via the portal vein 60. Moreover, obesity-linked inflammation and insulin resistance deteriorate the surplus free fatty acid metabolism in the liver and participate in the evolution of fatty liver which in turn increased the weight of the liver 61. Moreover, the kidney weight increment in obesity may be due to either compensatory hypertrophy of individual nephrons or due to accumulation of fluids and lipids 62. Furthermore, Leopoldo et al. 58 found that obesity led to an increase in the interstitial collagen deposition in the left ventricle. Also, it was verified that a HFD for a 12-week period in rabbits causes fibrosis in coronary vessels, as well as assemblage of collagen in the cardiac interstitium 63.
The outcome of the present work demonstrated that the treatment of HFD-fed rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs caused a reduction in body weight, body length, TC, AC, BMI and Lee index. Furthermore, these treatments triggered a decrease in liver, right and left kidney, heart, spleen, adrenal, thymus and visceral, gonadal as well as subcutaneous adipose tissues weight. Karimi et al. 64 represented that HFD rats treated with orlistat showed lower body weight, body length and BMI than untreated HFD rats. Also, these investigators found that the treatment with orlistat produced a decrease in fat mass, liver, kidney, spleen and heart weights. Furthermore, Othman et al. 53 recorded that the treatment of HFD rats with orlistat induced a decrease in Lee index and weight gain. Previous studies indicated that orlistat intake yields a reduction in body weight by 30% in obese population. The mode of action by which orlistat could reduce body weight is related to the decreasing of leptin level and adipocyte size 65.
The observed decrease in body weight upon treatment of HFD-fed rats with Qu is in congruity with the previous findings of Imessaoudene et al. 66 and Perdicaro et al. 67. Imessaoudene et al. 66 proved that the treatment with Qu induced a significant decrease in relative liver and adipose tissue weights in obese rats. Dong et al. 12 reported that Qu has the ability to suppress fat accumulation in animal models. Furthermore, Rivera et al. 68 pointed out that in obese Zucker rats, chronic administration of Qu markedly reduced body weight gain. Qu could reduce lipid aggregation in 3T3-L1 adipocytes 69 and attenuate lipid cumulation via down-regulation of PPARγ and C/EBPα. Also, Qu could induce adipocyte apotosis via the inhibition of ERK1/2 phosphorylation and enhancement of the mitochondria pathway 70. In Western diet fed mice, Qu diminished fat cumulation in the liver and the expression of steatosis-linked genes 71. Accumulating evidence indicated that Qu acts by modulating hepatic cholesterol absorption and triglyceride assembly and production as well as via suppression of phosphodiesterase in both liver and adipose tissue 68.
Our data indicated that administration of CUR to HFD-fed rats lowered body weight gain and also the weights of the tested organs and tissues. These findings come in line with those of El-Wakf et al. 72. du Preez et al. 73 recorded that CUR-PLGA NPs reduced liver fat deposition in HFD-fed rats. CUR has been found to inhibit adipose tissue angiogenesis, decrease preadipocytes differentiation and reduce lipids accumulation in adipocytes which in all aid in lowering body weight 74. du Preez et al. 73 suggested that CUR-PLGA NPs reduces fat deposition through increasing energy expenditure. The underlying mechanisms by which CUR can decrease the accumulation of fat in adipocytes is the enhancement of AMPK, suppression of ACC enhancement, and down-regulation of the expression of lipogenic genes like SCD1, FAS and SREBP-1c 9. Also, CUR can boost lipolysis and motivate fatty acid β-oxidation via up regulating hormone sensitive lipase (HSL), CPT-1, UCPs and PGC-1α 74. As well it can diminish the proliferation of pre-adipocytes, stimulate their apoptosis, and hearten cell cycle arrest. It also induces G0/G1 and G2/M cell cyclearrest and apoptosis of adipocytes [9]. Additionally, CUR could down-regulate the expression of key genes implicated in adipocyte differentiation, such as C/EBPα, aP2 and PPARγ 75.
In the current experiment, prolonged HFD feeding in yielded an imparment in lipid metabolism, as manifested by greater levels of Chol, TG, and LDL associated with lower levels of HDL. This observation is extremely supported by that of Noeman et al. 76; Kabir et al. 55 and Othman et al. 53. HFD has been reported to trigger the synthesis of phospholipids and cholesterol esters in rats 77. In general, all lipids are absorbed into the blood from the gastrointestinal tract in the form of chylomicrons, composed of TGs, phospholipids, Chol and apolipoprotein B. The TGs in these chylomicrons are then digested as fatty acids and glycerol by lipoprotein lipase. These fatty acids are transported and stored in the liver and adipose tissues in the form of TGs. The remnants of the chylomicrons are fundamently taken up by liver, and then transformed into several lipoproteins containing TGs, phospholipids, Chol and apolipoproteins. Among the lipoproteins, very low-density lipoproteins, composed of TGs, Chol and phospholipids, transport TGs that are formed in the liver to adipose tissues. LDL, which consists of Chol and phospholipids, transports Chol into peripheral tissues. Hence, elevated ingestion of lipids promotes the cumulation of body fat and increases Chol, phospholipids, and free fatty acids in the blood circulation that are transported to peripheral tissues. Furthermore, it has been hypothesized that elevated Chol and TG levels is the pivotal factor in lipoprotein metabolism, and their higher concentrations are ascribed to the elevated LDL formation and deposition, which is potentially atherogenic 78. The homeostasis of cellular Chol is preserved primarily via regulation of the LDL receptor and 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, which respectively influence the uptake and endogenous synthesis of Chol in the liver 79. Long-term consumption of HFD enhanced Chol biosynthesis and reduced expression of LDL receptor sites 80. Emerging data have proved that reactive oxygen species (ROS) interact with protein thiol molecules to generate a variety of sulfur oxidations which impaired the receptor signal of INS and hampers cellular uptake of TG from the blood circulation 81.
Supplementation of obese rats with orlistat provoked a decline in serum Chol, TG and LDL levels along with a rise in serum HDL level. These results echo those of Karimi et al. 64 and Othman et al. 53. Carrière et al. 82 reported that orlistat blocked dietary TGs hydrolysis and thus minimized the subsequent intestinal absorption of the lipolysis products monoglycerides and free fatty acids by covalently blocking the lipase active site. Furthermore, Bougoulia et al. 83 reported the ability of orlistat to decrease serum LDL level because of its action towards suppression of gastric and pancreatic lipase enzymes.
The current data revealed that administration of obese rats with free Qu, Qu NPs, free CUR or CUR NPs lead to a decrease in serum Chol, TG and LDL levels in concomitant with an increase in serum HDL level. These findings match those of Seiva et al. 84, Hu et al. 85, Tong et al. 86 and Shamsi-Goushkiet al. 87. The hypolipidemic effects of Qu could be principally allied to its antioxidant properties 88 and indirectly by affecting the Chol levels. Hence, LDL-Chol and VLDL-Chol would be protected against harmful impacts emerging from oxidative process 89. Other hypothesis includes the role of Qu in decreasing HMG-CoA reductase activity and consequently reducing Chol biosynthesis in the liver 88, 90. Besides, there is growing evidence that Qu and its glycoside act by altering hepatic Chol absorption and TG assembly and secretion as well as via suppression of phosphodiesterases in the liver and adipose tissue 91, 68.
CUR is also recognized to have anti-hyperlipidemic activity by inhibiting the cumulation of TGs and Chol in the liver 92. The study of Shin et al. 93 documented that CUR could inhibit the activity of hepatic HMG-CoA and down-regulate the gene of HMGR that codes for HMG-CoA enzyme in the liver. Rachmawati et al. 92 recorded that the inhibition rate of HMG-CoA reductase by CUR nanoemulsion was greater than free CUR. Furthermore, CUR increases the transcription of liver X receptor alpha (LXRα), which regulates CYP7A1 (encoding cholesterol-7a-hydroxlylase). Cholesterol-7a-hydroxlylase plays role in the conversion of Chol into bile acids [93]. In vitro using 3T3-L1 cells, it has been demonstrated that CUR can decrease the expression of glycerol-3-phosphate acyl transferase-1 (GPAT-1), a crucial enzyme for TG synthesis. In addition, CUR promotes adipocytes fatty acid β-oxidation with a concomitant increase in the expression of carnitine palmitoyl transferase 1 (CPT-1), a key enzyme in transferring acyl-CoA into mitochondria for β-oxidation 74. Also, CUR could suppress the atherosclerotic lesions formation in mice fed with atherogenic food, as indicated by the suppression of the atherogenic indicator and the rise in the HDL/total Chol percentage 93. Importantly, it has been evidenced that nano-CUR is more effective than CUR in reducing the lipid profile 87. This superior effect could be explained the high sensitivity of CUR to the changes in physiological pH. In addition, CUR absorption through the gastrointestinal tract is very weak instead nano-curcumin has high bioavailability and uptake 94.
In the current investigation, obese rats exhibited high serum glucose and INS levels as well as insulin resistance value. This result is comparable with the previous studies which clarified that rats fed a HFD display increased serum glucose concentrations 61, 55. Besides, Xia et al. 95 documented that the feeding of rats with HFD for 8 weeks resulted in notably higher fasting glucose and fasting INS levels, as well as a higher HOMA-IR, which is indicative of increased insulin resistance compared with the control counterparts. These observations could be explained by the action of gram-negative bacterial cell wall contains lipopolysaccharide (LPS), which may transfuse the gut because of the leakiness of intestinal mucosa triggered by a high fat content in the diet 96. Bacterial LPS may cause endotoxemia and inflammation 97. Also, the intestinal permeability may ease the entry of bacterial fragments into the body which interact with the Toll-like receptor to enhance the innate and adaptive immunity and lead to hyperglycemia and insulin resistance 98. Hsieh 61 cited that some pro-inflammatory pathways interrupt INS signaling and play pathological roles in developing obesity-related type 2 diabetes mellitus. A growing body of evidence proposes that oxidative stress and inflammation cause insulin resistance via INS receptor substrate (IRS-1) serine/tyrosine phosphorylation instead of tyrosine/serine phosphorylation. Studies have shown that serine phosphorylation of IRS-1 attenuates the proximal INS signaling which results in insulin resistance 99.
Treatment of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs inspired a drop in serum glucose and INS levels as well as insulin resistance value. Karimi et al. 64 and Lee et al. 100 stated that serum glucose and INS levels are decreased in HFD rats treated with orlistat. The observed reduction in serum glucose and INS levels upon treatment of obese rats with Qu matches with the findings of Seiva et al. 84. Furthermore, Tong et al.86 mentioned that Qu NPs decrease blood glucose level. Ying et al. 101 found that Qu is able to preserve pancreatic beta cell integrity. Qu consumption has been reported to significantly reduce the size of adipocyte, attenuate adipose inflammation, and prevent systemic insulin resistance. These impacts can be ascribed, in part, to the competence of Qu to motivate a healthy adipose tissue expansion, angiogenesis and adipogenesis 67. In addition, the glycemic control could be improved by Qu via the inhibition of glucose absorption from intestine at the level of glucose transporters (GLUT), through enhancing the activity of glucokinase converting glucose into glucose-6-phosphate, a metabolite destined to glycogen synthesis. This action could be done via the increasing of the pancreatic islets number thus boosting INS release, and via metal chelating activity, herewith eliminating a causal factor for the generation of free radicals 68.
The demonstrated drop in serum glucose level and insulin resistance value after treatment of obese rats with free CUR or CUR NPs is in parallel with the results of Shamsi-Goushkiet al. 87. These actions of CUR and nano-CUR are possibly owing to the activation of PPAR-Y, motivation of glucokinase activity in the liver, induction of glucose transporter-4 (GLUT4) expression and attenuation of tumor necrosis factor-alpha (TNF-α) [87]. Na et al.102 recorded that CUR improved INS sensitivity in muscle tissue of the rodent model of insulin resistant by an increase of the cellular glucose uptake as a result of the augmentation of GLUT4 translocation from intracellular compartments to the plasma membrane. In addition, increased glucose oxidation and glycogen synthesis have also been found in the C2C12 mouse skeletal muscle cells upon treatment with CUR 103. In the study of Mantzorou et al. 104, the improvement of insulin resistance and the reduction of plasma glucose levels in diabetic rats have been demonstrated after treatment with CUR. Likewise, the study of Rahimi et al. 105 demonstrated that nano-CUR supplementation significantly reduced fasting blood glucose level compared to the placebo.
The obtained results in the current work demonstrated that obese rats experienced an elevation in serum LH level accompanied with a reduction in serum E2 level. The observed increase in serum LH level and the decrease in serum E2 level are greatly supported by the recent findings of Yi et al. 106. Female fertility and reproductive health are compromised by overweight and obesity 107. The prime factor underlying the adverse metabolic outcomes of obesity is thought to be insulin resistance. The decline in INS sensitivity affects not only glucose metabolism, but also all aspects of INS action 108. INS has been reported to promote the proliferation of theca cells, the release of LH, and the number as well as affinity of LH receptors on granulosa cells 109. Akamine et al. 109 stated the simulateous occurance of systemic insulin resistance and hyperinsulinemia together with the impairment of INS signaling in the ovary, and alteration of estrous cycle and of ovarian morphology in HFD-induced obesity in female rats. Insulin signaling has been involved in the regulation of female reproductive function by acting in both central nervous system and ovaries. The hyperinsulinemia can potentiate gonadotropin-stimulated steroidogenesis in granulosa and thecal cells, by elevating LDL receptor, 3β-hydroxysteroid dehydrogenase, 17α-hydroxylase, and 17,20 lyase expression 110, 111. In addition, INS may act on the pituitary to boost LH release 112. Gonadotropin-releasing hormone (GNRH) stimulates LH secretory response by a calcium-dependent signaling, and motivates mRNA and protein synthesis of LH via the MAPK signaling pathway. In the gonadotropes, both GNRH and INS stimulate the MAPK pathway, which is the point of signaling interaction and may be implicated with the potentiation of gonadotropin release by INS 113. Furthermore, the cumulation of lipid droplets in the stromal and granulosa cells may be a consequence of hyperinsulinemia effect on the expression of the LDL receptor, which would contribute to enhance steroidogenesis [108]. Besides, it has been recorded that, insulin resistance and hyperinsulinemia prompt a serious imbalance in the synthesis of sexual steroid leading to excess androgen and slight estrogen levels and interfere with the effect of estrogen even at the receptor level 114.
Treatment of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs elicited a reduction in serum LH accompanied by an elevation in serum E2 level. Our data support the finding of Salehpour et al. 115 which indicated that the treatment of obese women with polycystic ovary syndrome (PCOS) with orlistat reducesthe serum LH level through reducing body weight and improving hyperinsulinemia. The observed decline in serum LH level upon treatment of obese rats with Qu comes in line with the findings of Khorshidi et al. 116 who recorded that Qu supplementation decreases serum LH concentration in overweight or obese women with PCOS. It has been mentioned that resistin can promptly enhance steroidogenesis by elevation of LH receptor, steroidogenic acute regulatory protein, and the gene expression of INS receptor. Furthermore, resistin, along with LH, stimulates the synthesis of androgens via increasing INS receptor gene expression 117. Khorshidi et al. 116 suggested that Qu can efficiently play a role inthe modulation of serum LH through reducing resistin levels. On the other hand, the observed increase in serum E2 level in obese rats treated with Qu was found inconsistent with the observation of Nna et al. 118 which demonstrated that the improvement in serum concentration of E2 in the Qu-treated group is not far from the decrease in oxidative stress. Furthermore, Wang et al. 119 cited that the antioxidant capacity of the ovary is increased by administration of Qu via upregulating expression of some oxidative stress-related genes. Also, Qu is characterized by its phytoestrogen-like action 120 and van der Woude et al. 121 stated that Qu had the ability to induce cell division in the MCF-7 cell line through the induction of ERα and ERβ expression. Neisy et al. 122 registered that Qu can enhance and increase uterine ERα gene expression. Our findings for diminished serum LH level and elevated serum E2 level after treatment of obese rats with CUR are consistent with previous studies 123, 124. Various studies have established that CUR could stimulate the ovarian functions via its capability to enhance proliferation and reduce apoptosis 125 while promoting folliculogenesis 126 and steroidogenesis in murine ovarian cells 127. A possible explanation for our findingis the ability of CUR to reduce insulin resistance 128. Igbakin and Oloyede 129 stated that CUR may include biomolecules that are capable to modify or stimulate INS receptors, alter glucose transport protein structure, and suppress INS antagonist in the body.
The consumption of a HFD has been found to promote oxidative stress which is apparent in most experimental models and patients with clinical conditions 130, 131. In the present setting, obese rats showed an elevation in the lipid peroxidation product; MDA level and a reduction in TAC. Our results are in accordance with the previous researches showing that tissue antioxidant defenses may be compromised in obese animals due to prolonged consumption of HFD 132, 134. The imbalance between free radical production and antioxidant level results in oxidative stress, manifested by the diminished antioxidant defense system in the untreated obese rats in the current investigation. The depressed antioxidant defense system in obese rats results in damage to so-called lipid peroxidation. Yang and colleagues 134 observed that the HFD-fed animals experienced high circulating concentration of thiobarbituric acid reactive substances, which are known as the indices of lipid peroxidation. The high caloric intake is a key factor in lowering fluidity of the mitochondrial membrane and enhancing ROS formation 135. Therefore, when ROS concentration is raised, the body’s antioxidant defenses may be unable to cope leading to oxidative stress condition 136. Furthermore, it has been mentioned that, ROS stimulate inducible cyclooxygenase 2(COX-2) isoform and promote the biosynthesis of prostaglandin E2 (PGE2), by activating extracellular signal–regulated protein kinase (ERK) 137. It has been demonstrated that the up-regulated expression and activity of COX-2 isoform elevates the lipid peroxidation 138.
Increased oxidative stress leads to attenuation of lipoprotein lipase (LPL) activity; an enzyme implicated in the transformation of dietary lipids into sources of energy for peripheral tissues which leads to disturbance of lipid metabolism 139, 140. The reduced activity LPL may be due to decreased expression of LPL and/or by an immediate effect of ROS on LPL. Yang and colleagues 134 found that LPL activity is positively correlated with TAC. Thus, the observed decrease in antioxidant defense system in the current investigation could be the reason behind the impaired-lipid metabolism in HFD-fed rats due to the decreased activity of LPL. Also, previous studies have correlated both BMI and the oxidative stress status 141. Furthermore, impaired redox balance blights the secretion of INS, which can be considered as a cause of insulin resistance in obese individuals 142.
The data from this study demonstrated the prominence of the suggested treatment in rectifying the altered oxidant/anti-oxidantbalance induced by prolonged consumption of HFD in rats. The recorded effect of orlistat in reducing serum MDA level and elevating TAC value comes in line with the findings of Othman et al. 143. Yesilbursa et al. 144 found remarkable decrease in MDA level in concomitant with orlistat-triggered weight loss. The improvement in the oxidative stress level might be ascribed to the ability of orlistat to prevent pro-atherogenic LDL Chol from oxidation. Also, Audikovszky et al. 145 suggested that, the increase in HDL level by orlistat treatment not only protects the progress of atherosclerosis by reverse Chol transport, but also by its action on hydrolyzing lipid peroxidation product.
Plentiful of studies have indicated that Qu loaded NPs, had more anti-radical and antioxidative activities compared to free Qu. Encapsulating of Qu into PLGA-NPs promoted its activity as lipid oxidation inhibitor and its antioxidative properties 146. In addition, loading Qu into aminoalkyl methacrylate co-polymers)- poly vinyl alcohol NPs enhanced the anti-radical and antioxidant activities of Qu 147. In line with these studies, our data revealed that the efficacy of Qu NPs in restoring the oxidant/anti-oxidant balance in obese rats was superior than free Qu. Imessaoudene et al. 66 and Ting et al. 148 confirmed that the treatment of obese rats with Qu decreases plasma MDA level and increased TAC. Furthermore, Al-Jameel and Abd El-Rahman 149 registered that the treatment of diabetic rats with Qu NPs reduces the serum level of MDA and elevates the level of antioxidant enzymes. Polyphenols have been established as ROS scavengers and pro-oxidant metal chelators 150. Flavonoids antioxidant activities are based on their free-radical scavenging ability and their abilities to strongly chelate redox-active transition metal ions 151. The finding of Imessaoudene et al. 66 demonstrated that the treatment with Qu alleviated oxidative stress in obese rats, by decreasing oxidant markers and elevating antioxidant defense molecules. This increment in antioxidants may be owing to neutralization of ROS as Qu has been recognized to scavenge superoxide anions and other free radicals in vitro 152. Qu is characterized by a strong scavenging activity of oxygen radicals and protection against lipid and protein oxidation which has been mostly ascribed to its flavonoid fraction 153. Qu acts on the leading regulator of fat cumulation and metabolic disorders, particularly, by offsetting mitochondrial dysfunction and oxidative process 71.
Regarding the effect of treatment of obese rats with free CUR or CUR NPs on serum MDA and TAC value is compatible with the previous reports where CUR reversed the diminished total antioxidant status and attenuated the increase of the lipid peroxidation product, MDA 154, 155. It has been reported that CUR displays its protective effect by modulating lipid peroxidation and boosting the antioxidant defense response. This action is attributed to the presence of the hydroxyl groups and methylene group of the β-diketone moiety 156. Prior reports have shown significant reduction of MDA and increased antioxidant enzyme activity in diabetic rats upon CUR treatment 157. Past studies recorded that CUR up-regulated nuclear factor erythroid-2-related factor-2 (Nrf2), a transcription factor that stimulates the expression of several genes, including those that encode for several antioxidant enzymes 158. He et al. 159 has cited that Nrf2 enhancement by CUR in HFD-fed mice thwarts muscular oxidative stress and mitochondrial redox imbalance.
Obesity usually triggers interruption in the immune function of the mature adipocytes, and motivates the release of several pro-inflammatory cytokines and chemokines while suppresses the anti-inflammatory cytokines 160. All these abnormalities result in the generation of obesity-associated chronic low-grade inflammation in adipose tissues that acts as the key to the initiation of different pro-inflammatory pathways 61.
Findings from this study showed that feeding of HFD, potentiates an elevation in serum TLR4 and NF-κB levels in concomitant with a decline in the serum IL-10 level. This finding is greatly supported by that of Zhang et al. 161 and Liu et al. 162. TLR4 could regulate chemokine formation, neutrophil recruitment, and tissue damage 163. Former reports indicate that free fatty acid (FFA), especially saturated fatty acid, can activate TLR4-mediated pro-inflammatory signaling pathways 164. It has been also reported that chronic HFD intake led to TLR4-mediated RIP3 activation in mice and the knockdown of TLR4 down-regulates RIP3 expression and inflammatory response in palmitate-incubated hepatocytes 165. Furthermore, it has been found that TLR4 mediated signaling cascades in inflammatory diseases, such as activation of NF-κB and up-regulation of TNF-α 166. It has been registered that, TLR4 enhanced nuclear translocation of NF-κB by mediating the myeloid differentiation primary response protein (MyD88)-dependent early response pathway, thus initiating a series of inflammatory cascades by producing the pro-inflammatory factors and monocyte chemoattractants 167. Park et al. 168 pointed out that NF-κB is a redox sensitive transcription factor that is activated by oxidative stress and its activation increases the expression of other pro-inflammatory mediators. IL-10 is recognized as one of the most remarkable anti-inflammatory and immunosuppressive cytokines that mainly acts on monocytes. It has been reported that there was a negative correlation between adipose IL-10 and serum TG 162. The effect of IL-10 on TG serum levels is attributable to enhanced hepatic FA synthesis and TG secretion 169. Therefore, dysregulation of IL-10 is directly or indirectly linked with the pathogenesis of obesity-related hypertriglyceridemia 162.
Treatment of obese rats with orlistat, free Qu, Qu NPs, free CUR or CUR NPs yielded a depletion in serum TLR4 andNF-κB associated with an elevation in the serum IL-10 level. The observed decrease in TLR-4 and NF-κB levels upon treatment of obese rats with orlistat is in harmony with the findings of El-Baz et al. 170 and Bansal et al. 171. Also, the elevation in the serum IL-10 level in orlistat treated group is greatly supported by Li et al. 172 findings. On the other hand, Perdicaro et al. [67] found that Qu supplementation attenuates HFD-induced TLR4 increase. Byun et al. 173 mentioned that Qu could suppress lipopolysaccharides induced-TLR4 signaling in macrophages 264.7 through the positive regulation of Tollip expression (a negative regulator of TLR signaling). Furthermore, previous studies showed that Qu impedes NF-κB stimulation and thereby the generation of inflammatory mediators 174, 175. Dias et al. 176 recorded that Qu decreases IκB-α degradation by inhibiting the upregulation of IKK-α and IKK-β, thereby prevented NF-κB activation. Xu et al. 177 stated that gold-Qu NPs can inhibit TLR4/NF-κB signaling in HFD-fed mice. The enhancement of serum IL-10 level in Qu treated rats is in concurrent with the findings of Ahmed et al. 178.
Several studies have shown that CUR administration is implicated in the regulation of various inflammatory cytokines via inhibiting the activation of the TLR4/NF-κB signaling pathways 179, 180. Feng et al. 181 study revealed that CUR administration significantly down-regulated hepatic TLR4 and MyD88 expression, reduced p65 nuclear translocation and NF-κB DNA binding activity, indicating that CUR could suppress HFD-induced the activation of TLR4-MyD88/ NF-κB signaling in the liver, and then prevent liver fat accumulation induced by HFD in ApoE−/− mice. Zhou et al. 182 showed that CUR modulates macrophage polarization by inhibiting TLR4-MAPK/NF-κB signaling pathway. Wang et al. 183 showed that CUR suppresses LPS-induced sepsis in mice via inhibiting TLR4 signaling activation. CUR was also found to exert an anti-inflammatory effect on rat vascular smooth muscle cells through suppressing ROS-related TLR4-MAPK/NF-κB signaling pathway 184. CUR has been shown to inhibit the NF-κB pathway activity, which is mediated by the reduction of IκB kinase and p-IκBα 185. Inhibition of NF-κB activity has been proposed as the most celebrated mechanism for the medicinal properties of CUR. Downregulation of NF-κB enhancement has been recorded to account for the blunting effect of CUR on inflammatory cytokine expression and release in both preadipocytes and differentiated 3T3-L1 adipocytes 186. Yang et al. 187 found that CUR-loaded chitosan-bovine serum albumin NPs induces the lower protein expression levels of TLR4 than free CUR, with concomitant inhibition of NF-κB phosphorylation. These investigators suggested that CUR-loaded chitosan-bovine serum albumin NPs may motivate CUR-induced macrophage phagocytosis by suppressing M1 macrophage polarization via hindering the TLR4-MAPK/NF-κB signaling pathway, thus boosting curcumin’s anti-inflammatory effect. Coinciding with our finding, Mollazadeh et al. 188 cited that CUR can enhance the production of anti-inflammatory IL-10 to counteract inflammatory mediators.
Conclusion
Conclusively, the data presented herein highlighted the prominent role of Qu and CUR in combating obesity and its related metabolic alterations via their hypolipidemic, hypoglycemic, antioxidative and anti-inflammatory mechanisms. Interestingly, both Qu and CUR NPs showed a preferable effect than their free counterparts probably due to increased solubility and bioavailability of nano-formulations. The promising therapeutic effect of Qu and CUR modified nanoencapsulation against obesity may have a suitable impact on achieving clinically convenient approaches to treat patients with obesity in the future.
Acknowledgment
NA
Conflict of interest
The authors declare that there is no any conflict of interest
Funding sources
No funding source
References
- Chen HH, Tseng YJ, Wang SY. et al. () The metabolome profiling and pathwayanalysis in metabolic healthy and abnormal obesity. Int J Obes. 2015; 39: 1241-1248.doi: 10.1038/ijo.2015.65. Epub 2015 Apr 24.
CrossRef - Ghaben AL, Scherer PE. Adipogenesis and metabolic health. Nature Reviews Molecular Cell Biology 2019; 20(4): 242-258. doi: 10.1038/s41580-018-0093-z.
CrossRef - Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obesity reviews: an official journal of the International Association for the Study of Obesity 2011; 12: 50–61. doi: 10.1111/j.1467-789X.2009.00708.x.
CrossRef - Engin A. The definition and prevalence of obesity and metabolic syndrome. Adv. Exp. Med. Biol. 2017; 960: 1–17. doi: 10.1007/978-3-319-48382-5_1
CrossRef - Kumar P, Dubey KK. () Current Trends and Future Prospects of Lipstatin: A Lipase Inhibitor and Pro-Drug for Obesity. RSC Adv. 2016; 46: 86954. https://doi.org/10.1039/C5RA14892H
CrossRef - Shah AS, D’Alessio D, Ford-Adams ME, Desai AP, Inge TH. Bariatric Surgery: A Potential Treatment for Type 2 Diabetes in Youth. Diabetes Care 2016; 39: 934-940. doi: 10.2337/dc16-0067.
CrossRef - Tsou YH, Wang B, Ho W, Hu B, Tang P, Sweet S, Zhang XQ, Xu X. Nanotechnology-Mediated Drug Delivery for the Treatment of Obesity and Its Related Comorbidities. Adv Healthc Mater. 2019; 8(12): e1801184. doi: 10.1002/adhm.201801184.
CrossRef - Goktas Z, Zu Y, Abbasi M, Galyean S, Wu D, Fan Z, Wang S. Recent advances in nano-encapsulation of phytochemicals to combat obesity andits comorbidities. Agric. Food Chem.2020; 68(31): 8119–8131.doi: 10.1021/acs.jafc.0c00131.
CrossRef - Wang S, Moustaid-Moussa N, Chen L, Mo H, Shastri A, Su R, Bapat P, Kwun I, Shen CL. Novel insights of dietary polyphenols and obesity. J Nutr Biochem. 2014; 25 (1): 1-18. doi: 10.1016/j.jnutbio.2013.09.001.
CrossRef - Labuschagne P. Impact of wall material physicochemical characteristics on thestability of encapsulated phytochemicals: A review. Food Res Int. 2018; 107: 227-247. doi: 10.1016/j.foodres.2018.02.026.
CrossRef - Zhou M, Wang S, Zhao A, Wang K, Fan Z, Yang H, Liao W, Bao S, Zhao L, Zhang Y, Yang Y, Qiu Y, Xie G, Li H, Jia W. Transcriptomic and metabonomic profil-ing reveal synergistic effects of quercetin and resveratrolsupplementation in high fat diet fed mice. J. Proteome Res. 2012; 11(10):4961-71. doi: 10.1021/pr3004826.
CrossRef - Dong J, Zhang X, Zhang L, Bian HX, Xu N, Bao B, Liu J. Quercetin reduces obesity-associated ATM infiltration and inflammation in mice: a mechanism including AMPKα1/SIRT1. J. Lipid Res. 2014; 55: 363–374. doi: 10.1194/jlr.M038786.
CrossRef - Peredo-Escárcega E, Guarner-Lans V, Pérez-Torres I, Ortega-Ocampo S, Carreón-Torres E, Castrejón-Tellez V, Díaz-Díaz E, Rubio-Ruiz ME. The Combination of Resveratrol and Quercetin Attenuates Metabolic Syndromein Rats by Modifying the Serum Fatty Acid Compositionand by Upregulating SIRT 1 and SIRT 2 Expression in White Adipose Tissue. J. Evidence-Based Complementary Altern. Med. 2015; 2015: 474032. doi: 10.1155/2015/474032.
CrossRef - Etxeberria U, Arias N, Boqué N, Macarulla MT, Portillo MP, Martínez JA, Milagro FI. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015; 26: 651–660. doi: 10.1016/j.jnutbio.2015.01.002.
CrossRef - Alappat L, Awad AB. Curcumin and obesity: evidence and mechanisms. Nutrition reviews 2010; 68: 729–738. doi: 10.1111/j.1753-4887.2010.00341.x.
CrossRef - Shishodia S, Sethi G, Aggarwal BB. Curcumin: getting back to the roots. Annals of the New York Academy of Sciences. 2005; 1056: 206–217. doi: 10.1196/annals.1352.010.
CrossRef - Meydani M, Hasan Dietary polyphenols and obesity. Nutrients 2010; 2: 737–751. doi: 10.3390/nu2070737.
CrossRef - Shao W, Yu Z, Chiang Y, Yang Y, Chai T, Foltz W, et al. Curcumin prevents high fat dietinduced insulin resistance and obesity via attenuating lipogenesis in liver and inflammatory pathway in adipocytes. PloS one. 2012; 7:e28784. doi: 10.1371/journal.pone.0028784.
CrossRef - Xie XY, Kong PR, Wu JF, Li Y, Li YX. Curcumin attenuates lipolysis stimulated by tumornecrosis factor-alpha or isoproterenol in 3T3-L1 adipocytes. Phytomedicine international journal of phytotherapy and phytopharmacology 2012; 20: 3–8. doi: 10.1016/j.phymed.2012.09.003.
CrossRef - Abd-Rabou AA, Ahmed HH. CS-PEG decorated PLGA nano-prototype for delivery of bioactive compounds: A novel approach for induction of apoptosis in HepG2 cell line. Advances in Medical Sciences 2017; 62: 357–367. doi: 10.1016/j.advms.2017.01.003.
CrossRef - Arya G, Das M, Sahoo Evaluation of curcumin loaded chitosan/PEG blended PLGA nanoparticles for effective treatment of pancreatic cancer. Biomedicine & Pharmacotherapy 2018; 102: 555-566. doi: 10.1016/j.biopha.2018.03.101.
CrossRef - Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, Bollheimer LC. Defining high-fat diet rat models: metabolic and molecular effects of different fat types. J Mol with an improved serum lipid profile. Diab Obes Metab. 2006; 7: 65-72. doi: 10.1677/jme.1.01909.
CrossRef - Soliman MM, Attia HF, El-Shazly SA, Saleh OM. Biomedical effects of cinnamon extract on obesity and diabetes relevance in Wistar rats. Am J BiochemMol Biol. 2012; 2: 133-145. doi: 3923/ajbmb.2012.133.145
CrossRef - Nishioka T, Hafkamp AM, Havinga R, Vanlierop PE, Velvis H, Verkade HJ. Orlistat treatment increases fecal bilirubin excretion and decreases plasma bilirubin concentrations in hyperbiliruninemicgunn rats. The Journal of Pediatrics. 2003; 143: 327-334. doi: 10.1067/s0022-3476(03)00298-1.
CrossRef - da Silva EL, Piskula MK, Yamamoto N, Moon JH, Terao J. Quercetin metabolites inhibit copper ion-induced lipid peroxidation in rat plasma. FEBS Letters 1998; 430: 405-408. doi: 10.1016/s0014-5793(98)00709-1.
CrossRef - El-Habibi EM, El-Wakf AM, Mogall A. Efficacy of Curcumin in Reducing Risk of Cardiovascular Disease in High Fat Diet-Fed Rats. J Bioanal Biomed. 2013; 5(3): 066-070. doi : 10.4172/1948-593X.1000082
CrossRef - Bernardis LL, Patterson BD. Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. J. Endocrinol 1968; 40: 527-528. doi: 10.1677/joe.0.0400527.
CrossRef - Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, et al. Anthropometrical parameters and markers of obesity in rats. Lab Anim. 2007; 41(1): 111-119. doi: 10.1258/002367707779399518.
CrossRef - Meiattini F, Prencipe L, Bardelli F, Giannini G, Tarli P. The 4-hydroxybenzoate/4-aminophenazone chromogenic system used in the enzymic determination of serum cholesterol. Clin Chem 1978; 24(12): 2161-2165. PMID: 719864
CrossRef - Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973; 19(5): 476-482. PMID: 4703655
CrossRef - Naito HK, Cholesterol HD. Clinical Chemistry. St. Louis, Toronto, Princeton: The C.V. Mosby Co. 1984; p. 1207-13, 437.
CrossRef - Trinder P. Enzymatic determination of glucose in blood serum. Annals of Clinical Biochemistry 1969; 6: 24.
CrossRef - Ohkawa H, Ohishi W, and Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. biochem. 1979; 95: 351. doi: 10.1016/0003-2697(79)90738-3.
CrossRef - National committee for clinical laboratory standards procedures for the collection of diagnostic blood specimens by venipuncture: Approved standards 4thed NCC LS Document H3-A4, Wayne, PA 1998.
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. 1985; 28(7): 412–419.doi: 10.1007/BF00280883.
CrossRef - Uotilla M, Ruoslahti E, Egvall E. Two-site sandwich enzyme immunoassay with monoclonal antibodies to human alpha-fetoprotein. J immunolmethods 1981; 42: 11-15. doi: 10.1016/0022-1759(81)90219-2.
CrossRef - Tietz NW ed., Clinical guide to laboratory tests, 3rd edition, W.B. Saunders , Co., Philadelphia 1995; 216-217.
- Armitage P, Berry G. Comparison of several groups. In: Statistical Method in Medical Research. 2nd ed. Oxford: Block well Significant Publication 1987; p. 186-213. doi:1002/9780470773666
CrossRef - Banik BL, Fattahi P, Brown JL. Polymeric nanoparticles: the future of nanomedicine. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology 2016; 8(2): 271-299. doi: 10.1002/wnan.1364.
CrossRef - Mukhopadhyay P, Maity S, Mandal S, Chakraborti AS, Prajapati AK, Kundu PP. Preparation, characterization and in vivo evaluation of pH sensitive, safequercetin-succinylated chitosan-alginate core-shell-corona nanoparticle for diabetestreatment. Carbohydrate Polymers 2018; 182: 42-51. doi: 10.1016/j.carbpol.2017.10.098.
CrossRef - Nigam K, Kaur A, Tyagi A, Nematullah M, Khan F, Gabrani R, Dang S. Nose-to-brain delivery of lamotrigine-loaded PLGA nanoparticles. Drug Deliv Transl Res. 2019; 9 (5): 879-890. doi: 10.1007/s13346-019-00622-5.
CrossRef - Mathew A, Fukuda T, Nagaoka Y, Hasumura T, Morimoto H, Yoshida Y, Maekawa T, Venugopal K, Kumar DS. Curcumin loaded-PLGA nanoparticlesconjugated with Tet-1 peptide for potential use in Alzheimer’s disease. PLoS One 2012; 7(3): e32616 doi: 10.1371/journal.pone.0032616.
CrossRef - Shome S, Talukdar AD, Choudhury MD, Bhattacharya MK, Upadhyaya H. Curcumin as potential therapeutic natural product: a nanobiotechnological perspective. J. Pharm. Pharmacol. 2016; 68 (12): 1481–1500.
CrossRef - Perrault SD, Walkey C, Jennings T, Fischer HC, Chan WC. Mediating tumortargeting efficiency of nanoparticles through design. Nano Lett. 2009; 9 (5): 1909–1915. doi: 10.1021/nl900031y.
CrossRef - Ilk S, Saglam N, Ozgen M, Korkusuz F. Chitosan nanoparticles enhances the antiquorumsensing activity of kaempferol. Int. J. Biol. Macromol. 2016; 94 (Pt. A): 653–662. doi: 10.1016/j.ijbiomac.2016.10.068.
CrossRef - Win KY, Feng Effects of particle size and surface coating on cellular uptake ofpolymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005; 26 (15): 2713–2722. doi: 10.1016/j.biomaterials.2004.07.050.
CrossRef - Abd El-Fattah AI, Fathy MM, Ali ZY, El-Garawany AERA, Mohamed EK. Enhanced therapeutic benefit of quercetin-loaded phytosome nanoparticles in ovariectomized rats. Chemico-Biological Interactions 2017; 271: 30-38. doi: 10.1016/j.cbi.2017.04.026.
CrossRef - Tefas LR, Tomuţă I, Achim M, Vlase L. Development and optimization of quercetin-loaded PLGA nanoparticles by experimental design. Clujul Med. 2015; 88(2): 214–223. doi: 10.15386/cjmed-418.
CrossRef - Bais S, Singh GS, Sharma R. Antiobesity and hypolipidemic activityof Moringa oleifera leaves against high fat diet-induced obesityin rats. Adv Biol 2014; 2014: 162914. https://doi.org/10.1155/2014/162914
CrossRef - Gaur A, Pal GK, Ananthanarayanan PH et al. Role of Ventromedial hypothalamus in high fat diet induced obesity in male rats: association with lipid profile, thyroid profile and insulin resistance. Annals of Neurosciences 2014; 21(3): 104–107. doi: 10.5214/ans.0972.7531.210306.
CrossRef - Gaur A, Pal GK, Ananthanarayanan PH et al. Role of Ventromedial hypothalamus in high fat diet induced obesity in male rats: association with lipid profile, thyroid profile and insulin resistance. Annals of Neurosciences 2014; 21(3): 104–107. doi: 10.5214/ans.0972.7531.210306.
CrossRef - Wang C, Ha X,Li W, Xu P, Gu Y, Wang T, Wang Y, Xie J, Zhang J. Correlation of TLR4 and KLF7 in Inflammation Inducedby Obesity. Inflammation. 2017; 40(1): 42-51. doi: 10.1007/s10753-016-0450-z.
CrossRef - Othman ZA, Ghazali WSW, Noordin L, Yusof NAM, Mohamed M. Phenolic Compounds and the Anti-Atherogenic Effect of Bee Bread in High-Fat Diet-Induced Obese Rats. Antioxidants 2020; 9: 33. doi: 10.3390/antiox9010033.
CrossRef - Lasker S, Rahman MM, Parvez F et al. High-fat diet-induced metabolic syndrome and oxidative stress in obese rats are ameliorated by yogurt supplementation. Sci Rep 2019; 9: 20026. https://doi.org/10.1038/s41598-019-56538-0
CrossRef - Kabir F, Nahar K, Rahman MM, Mamun F, Lasker S, Khan F, Yasmin T, Ayesha K, Subhan N, Alam A. Etoricoxib treatment prevented body weight gain and amelioratedoxidative stress in the liver of high-fat diet–fed rats. Naunyn-Schmiedeberg’s Archives of Pharmacology 2020; 394(1): 33-47. Doi: 1007/s00210-020-01960-9
CrossRef - Tung YT, Chen HL, Wu HS, Ho MH, Chong KY,Chen CM. Kefir Peptides Prevent Hyperlipidemia and Obesity in High-Fat-Diet-Induced Obese Rats via Lipid Metabolism Modulation. Mol Nutr Food Res. 2018; 62(3): doi: 10.1002/mnfr.201700505.
CrossRef - Seo EY, Ha AW, Kim WK. α-Lipoic acid reduced weight gain and improved the lipid profile in rats fed with high fat diet.Nutrition Research and Practice (Nutr Res Pract) 2012; 6(3): 195-200. https://doi.org/10.4162/nrp.2012.6.3.195
CrossRef - Leopoldo AS, Sugizaki MM, Lima-Leopoldo AP, Nascimento AF, Luvizotto RAM, de Campos DHS, Okoshi K, Pai-Silva MD, Padovani CR, Cicogna Cardiac remodeling in a rat model of diet-induced obesity. Can J Cardiol. 2010; 26(8): 423–429. https://doi.org/10.3390/antiox8090368
CrossRef - Ramalho L, da Jornada MN, Antunes LC, Hidalgo MP. Metabolic disturbances due to a high-fat diet in a non-insulin-resistant animal model. Nutrition & Diabetes 2017; 7: e245. doi: 10.1038/nutd.2016.47.
CrossRef - Hung CS, Lee JK, Yang CY, Hsieh HR, Ma WY, Lin MS, Liu PH, Shih SR, Liou JM, Chuang LM, Chen MF, Lin JW, Wei JN, Li HY () Measurement of visceral fat: should we include retroperitoneal fat? PLoS One 2014; 9:e112355. https://doi.org/10.1371/journal.pone.0112355
CrossRef - Hsieh PS. Obesity-induced adipose tissue inflammation and insulin resistance: role of the adipocyte in development of type 2 diabetes.In: Croniger C (ed). Intech Open, 2011. https://doi.org/10.5772/20561.
CrossRef - Tsuboi N, Okabayashi Y, Shimizu A, Yokoo The renal pathology of obesity. Kidney international reports 2017; 2 (2): 251–260. doi: 10.1016/j.ekir.2017.01.007.
CrossRef - Carroll JF, Tyagi Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbits. Am J Hypertens 2005; 18: 692–698.doi: 10.1016/j.amjhyper.2004.11.035.
CrossRef - Karimi G, Sabran MR, Jamaluddin R, Parvaneh K, Mohtarrudin N, Ahmad Z, Khazaai H, Khodavandi A. The anti-obesity effects of Lactobacillus casei strain Shirota versusOrlistat on high fat diet-induced obese rats. Food & Nutrition Research 2015; 59: 29273. doi:3402/fnr.v59.29273
CrossRef - Blackburn GL, Waltman Pharmacotherapy to reducevisceral fat. Clin Cornerstone 2005; 7: 52_60. doi: 10.1016/s1098-3597(05)80068-7.
CrossRef - Imessaoudene A, Merzouk H, Berroukeche F, Mokhtari N, Bensenane B, Cherrak S, Merzouk SA, Elhabiri M. Beneficial effects of quercetin-iron complexes on serum and tissue lipids and redox status in obese rats. J NutrBiochem. 2016. 29: 107-115. doi: 10.1016/j.jnutbio.2015.11.011.
CrossRef - Perdicaro DJ, Lanzia CR, Tudelab JG, Miatello RM, Oteiza PI, Prieto MAV. Quercetin attenuates adipose hypertrophy, in part through activation of adipogenesisin rats fed a high-fat diet. Journal of Nutritional Biochemistry 2020; 79: 108352. doi: 10.1016/j.jnutbio.2020.108352.
CrossRef - Rivera L, Morón R, Sánchez M, Zarzuelo A, Galisteo M (2008) Quercetinameliorates metabolic syndrome and improves the inflammatory status inobese Zucker rats. Obesity 16: 2081–2087. doi: 10.1038/oby.2008.315.
CrossRef - Ahn J, Lee H, Kim S, Park J, Ha T () The anti-obesity effect of quercetin is mediated bythe AMPK and MAPK signaling pathways. Biochem Biophys Res Commun 2008; 373:545–549. doi: 10.1016/j.bbrc.2008.06.077.
CrossRef - Yang JY, Della-Fera MA, Rayalam S, et al. Enhanced inhibition of adipogenesis andinduction of apoptosis in 3T3-L1 adipocytes with combinations of resveratrol and quercetin. Life Sci. 2008; 82: 1032–1039. doi: 10.1016/j.lfs.2008.03.003.
CrossRef - Kobori M, Masumoto S, Akimoto Y, Oike H. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western stylediet in C57/BL6J mice. Mol. Nutr. Food Res 2011; 55: 530–540. doi: 10.1002/mnfr.201000392.
CrossRef - El-Wakf AM, El-Habibi EM, Mogalli A. Curcumin acts as cardiovascular protector via improving leptin and insulin resistance in obese male rats. Journal of American Science 2013; 9(3): 397-405.
- du Preez R, Pahl J, Arora M, Kumar MNVR, Brown L, Panchal SK. Low-dose curcumin nanoparticles normalize blood pressure in male wistar rats with diet-induced metabolic syndrome. Nutrients 2019; 11: 1542. https://doi.org/10.3390/nu11071542
CrossRef - Ejaz A, Wu D, Kwan P, Meydani M. Curcumin inhibits adipogenesis in 3T3-L1 adipocytes and angiogenesis and obesity in C57/BL mice. The Journal of nutrition 2009; 139: 919–925. doi: 10.3945/jn.108.100966.
CrossRef - Ahn J, Lee H, Kim S, Ha T. Curcumin-induced suppression of adipogenic differentiation isaccompanied by activation of Wnt/beta-catenin signaling. American journal of physiology Cell physiology 2010; 298: C1510–C1506. doi: 10.1152/ajpcell.00369.2009.
CrossRef - Noeman SA, Hamooda HE, Baalash AA. Biochemical Study of Oxidative Stress Markers in the Liver, Kidney and Heart of High Fat Diet Induced Obesity in Rats. Diabetol. Metab. Syndr. 2011; 3: 17. doi: 10.1186/1758-5996-3-17.
CrossRef - Han LK, Xu BJ, Kimura Y et al. () Anti-obesity effects in rodents of dietary tea saponin, a lipase inhibitor. Int J obes relat metab disord 2001; 25: 1459–1564. doi: 10.1038/sj.ijo.0801747.
CrossRef - Sacks FM. The crucial roles of apolipoproteins E and C-III in apoB lipoprotein metabolism in normolipidemia and hypertriglyceridemia. Curr. Opin. Lipidol 2015; 26: 56–63. doi: 10.1097/MOL.0000000000000146.
CrossRef - Russell Cholesterol biosynthesis and metabolism. Cardiovasc Drugs Ther 1992; 6: 103–110. doi: 10.1007/BF00054556.
CrossRef - Jones PJ. Regulation of cholesterol biosynthesis by diet inhumans. Am J Clin Nutr 1997; 66: 438–46. doi: 10.1093/ajcn/66.2.438.
CrossRef - Chen K, Thomas SR, Keaney JF. Beyond LDL oxidation: ROS in vascularsignal transduction. Free Radic. Biol. Med. 2003; 35: 117–132. doi: 10.1016/s0891-5849(03)00239-9.
CrossRef - CarrièreF , Renou C, Ransac S, Lopez V, De Caro J, Ferrato F, De Caro A, Fleury A, Sanwald-Ducray P, Lengsfeld H, Beglinger C, Hadvary P, Verger R, Laugier Inhibition of gastrointestinal lipolysis by Orlistat duringdigestion of test meals in healthy volunteers. Am J Physiol Gastrointest Liver Physiol 2001; 281:G16–G28,. doi: 10.1152/ajpgi.2001.281.1.G16.
CrossRef - Bougoulia M, Triantos A, Koliakos G. Effect of weight loss with or without orlistattreatment on adipocytokines, inflammation, and oxidative markers in obese women. Hormones 2006; 5: 259–269. doi: 10.14310/horm.2002.11190.
CrossRef - Seiva FR, Chuffa LG, Braga CP, Amorim JP, Fernandes AA. Quercetin ameliorates glucose and lipid metabolism and improves antioxidant status in postnatally monosodium glutamate-induced metabolic alterations. Food Chem Toxicol. 2012; 50(10): 3556-3561. doi: 10.1016/j.fct.2012.07.009.
CrossRef - Hu GX, Lin H, Lian QQ, Zhou SH, Guo J, et al. Curcumin as a Potent and SelectiveInhibitor of 11b-Hydroxysteroid Dehydrogenase 1: Improving Lipid Profiles in High-Fat-Diet-Treated Rats. PLoS ONE 2013; 8(3): e49976. doi: 10.1371/journal.pone.0049976.
CrossRef - Tong F, Liu S, Yan B, Li X, Ruan S, Yang S. Quercetin nanoparticle complex attenuated diabetic nephropathy via regulating the expression level of ICAM-1 on endothelium. Int J Nanomedicine. 2017; 12: 7799-7813. doi: 10.2147/IJN.S146978.
CrossRef - Shamsi-Goushki A, Mortazavi Z, Mirshekar MA, Mohammadi M, Moradi-Kor N, Jafari-Maskouni S, Shahraki M. Comparative Effects of Curcumin versus Nano-Curcumin on Insulin Resistance, Serum Levelsof Apelin and Lipid Profile in Type 2 Diabetic Rats. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 2020; 13: 2337–2346. doi: 10.2147/DMSO.S247351.
CrossRef - Arai Y, Watanabe S, Kimira M, Shimoi K, Mochizuki R, Kinae N. Dietaryintakes of flavonols, flavones and isoflavones by Japanese women and theinverse correlation between quercetin intake and plasma LDL cholesterol concentration. J. Nutr. 2000; 130: 2243–2250. doi: 10.1093/jn/130.9.2243.
CrossRef - Carrero P, Ortega H, Martínez-Botas J, Gómez-Coronado D, Lasunción MA. Flavonoid-induced ability of minimally modified low-densitylipoproteins to support lymphocyte proliferation. Biochem. Pharmacol 1998; 55:1125–1129. doi: 10.1016/s0006-2952(97)00635-7.
CrossRef - Lapointe A, Couillard C, Lemieux S. Effects of dietary factors on oxidation oflow-density lipoprotein particles. J. Nutr. Biochem. 2006; 17: 645–658. doi: 10.1016/j.jnutbio.2006.01.001.
CrossRef - Peluso MR. Flavonoids attenuate cardiovascular disease, inhibit phosphodiesterase, and modulate lipid homeostasis in adipose tissue andliver. Exp. Biol. Med. 2006; 231: 1287–1299. doi: 10.1177/153537020623100802.
CrossRef - Rachmawati H, Soraya IS, Kurniati NF, Rahma A. In Vitro Study on Antihypertensive and Antihypercholesterolemic Effects of a Curcumin Nanoemulsion. Sci Pharm. 2016; 84(1): 131-140. doi: 10.3797/scipharm.ISP.2015.05.
CrossRef - Shin SK, Ha TY, McGregor RA, Choi MS. Long-term Curcumin Administration Protects Against Atherosclerosis via Hepatic Regulation of Lipoprotein Cholesterol Metabolism. Mol Nutr Food Res. 2011; 55: 1829–1840. doi: 10.1002/mnfr.201100440.
CrossRef - Xie X, Tao Q, Zou Y, et al. PLGA nanoparticles improve the oralbioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem. 2011; 59(17): 9280– doi: 10.1021/jf202135j.
CrossRef - Xia HM, Wang J, Xie XJ, Xu LJ, Tang SQ. Green tea polyphenols attenuate hepatic steatosis, and reduce insulin resistance and inflammation in high-fat diet-induced rats. International Journal of Molecular Medicine 2019; 44(4): 1523-1530. doi: 10.3892/ijmm.2019.4285.
CrossRef - Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesityand diabetes in mice. Diabetes 2008; 57: 1470–1481. https://doi.org/10.2337/db07-1403
CrossRef - Naito E, Yoshida Y, Makino K, Kounoshi Y, Kunihiro S, Takahashi R, Matsuzaki T, Miyazaki K, Ishikawa Beneficial effect of oral administration of Lactobacillus casei strain Shirota on insulin resistance in diet-inducedobesity mice. J. Applied Microbiol. 2011; 110: 650–657. doi: 10.1111/j.1365-2672.2010.04922.x.
CrossRef - Amar J, Chabo Cl, Waget A, Klopp P, Vachoux C, Bermúdez-Humarán LG, Smirnova N, Bergé M, Sulpice T, Lahtinen S, Ouwehand A, Langella P, Rautonen N, Sansonetti PJ, Burcelin Intestinal mucosal adherence and translocation of commensal bacteria at the early onset of type 2 diabetes: molecularmechanisms and probiotic treatment. EMBO Mol. Med 2011; 3: 559–572. doi: 10.1002/emmm.201100159.
CrossRef - Draznin B. Molecular mechanisms of insulin resistance: serinephosphorylation of insulin receptor substrate-1 and increasedexpression of p85α the two sides of a coin. Diabetes 2006; 55: 2392–2397. doi: 10.2337/db06-0391.
CrossRef - Lee YH, Jin B, Lee SH, Song M, Bae H, Min BJ, Park J, Lee D, Kim H. Herbal Formula HT048 Attenuates Diet-Induced Obesity by Improving Hepatic Lipid Metabolism and Insulin Resistance in Obese Rats. Molecules. 2016; 21(11): 1424. https://doi.org/10.3390/molecules21111424
CrossRef - Ying HZ, Liu YH, Yu B, Wang ZY, Zang JN, Yu CH. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Tox. 2013; 52: 53–60. doi: 10.1016/j.fct.2012.10.030.
CrossRef - Na LX, Zhang YL, Li Y, et al. Curcumin improves insulin resistance in skeletal muscle of rats. NutrMetab Cardiovasc Dis. 2011; 21 (7): 526– doi: 10.1016/j.numecd.2009.11.009.
CrossRef - Deng YT, Chang TW, Lee MS, et al. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J Agric Food Chem 2012; 60(4): 1059– doi: 10.1021/jf204496f.
CrossRef - Mantzorou M, Pavlidou E, Vasios G, et al. Effects of curcuminconsumption on human chronic diseases: a narrative review of themost recent clinical data. Phytother Res. 2018; 32(6): 957– doi: 10.1002/ptr.6037.
CrossRef - Rahimi HR, Mohammadpour AH, Dastani M, et al. The effect ofnano-curcumin on HbA1c, fasting blood glucose, and lipid profile indiabetic subjects: a randomized clinical trial. Avicenna J Phytomed. 2016; 6(5): 567–
- Yi W, Li X, Chen K, Zhu M, Cai X, Pan A. Effects of CangfuDaotan Decoction on obese polycystic ovary syndrome and its mechanism. Steroids 2021; 165: 108740. doi: 1016/j.steroids.2020.108740
CrossRef - GesinkLaw DC, Machehose RF, Longnecker MP. Obesity and time topregnancy. Human Reproduction 2007; 22: 414–420. doi: 10.1093/humrep/del400.
CrossRef - Akamine EH, Marcal AC, Camporez JP, Hoshida MS, Caperuto LC, Bevilacqua E, Carvalho CRO. Obesity induced by high-fat diet promotes insulin resistance in the ovary. Journal of Endocrinology 2010; 206: 65–74. doi: 10.1677/JOE-09-0461.
CrossRef - Kuscu NK, Koyuncu F, Ozbilgin K. Insulin: does it induce follicular arrest in the rat ovary. Gynecol Endocrinol 2006; 16: 5-36. doi: 10.1080/gye.16.5.361.364.
CrossRef - Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulinrelatedovarian regulatory system in health and disease. Endocrine Reviews 1999; 20 (4): 535–582. doi: 10.1210/edrv.20.4.0374.
CrossRef - Zhang G, Garmey JC, Veldhuis D. Interactive stimulation byluteinizing hormone and insulin of the steroidogenic acute regulatory (StAR) protein and 17a-hydroxylase/17,20-lyase (CYP17) genes inporcine theca cells. Endocrinology 2000; 141: 2735–2742. doi: 10.1210/endo.141.8.7595.
CrossRef - Moret M, Stettler R, Rodieux F, Gaillard RC,Waeber G, Wirthner D, GiustiV, Tappy L, Pralong FP. Insulin modulation of luteinizing hormonesecretion in normal female volunteers and lean polycystic ovary syndromepatients. Neuroendocrinology 2009; 89: 131–139. doi: 10.1159/000160911.
CrossRef - Lawson MA, Jain S, Sun S, Patel K, Malcolm PJ, Chang RJ. Evidence for insulin suppression of baseline luteinizing hormone in women withpolycystic ovarian syndrome and normal women. Journal of Clinical Endocrinology and Metabolism 2008; 93: 2089–2096. doi: 10.1210/jc.2007-2656.
CrossRef - Moghetti P, Castello R, Negri C, Tosi F, Spiazzi GG, Brun E, Balducci R, Toscano V, Muggeo M. Insulin infusion amplifies 17-(alpha)-hydroxy- corticosteroid intermediates response to adrenocorticotropin in hyperandrogenicwomen: apparent relative impairment of 17, 20-lyase activity. J Clin Endocrinol Metab. 1996; 81: 881–886. doi: 10.1210/jcem.81.3.8772544.
CrossRef - Salehpour S, Hosseini S, Nazari L, Saharkhiz N, Zademodarres S. Effects of orlistat on serum androgen levels among iranian obese women with polycystic ovarian syndrome. JBRA Assist Reprod. 2018; 22(3): 180–184. doi: 10.5935/1518-0557.20180033.
CrossRef - Khorshidi M, Moini A, Alipoor E, Rezvan N, Gorgani‐Firuzjaee S, Yaseri M, Hosseinzadeh‐Attar MJ. The effects of quercetin supplementation on metabolic andhormonal parameters as well as plasma concentration and geneexpression of resistin in overweight or obese women withpolycystic ovary syndrome. Phytotherapy Research 2018; 32(11): 2282-2289. doi: 10.1002/ptr.6166.
CrossRef - Singh A, Suragani M, Ehtesham NZ, Krishna A. Localizationofresistin and its possible roles in the ovary of a vespertilionid bat, Scotophilusheathi. Steroids 2015; 95: 17– doi: 10.1016/j.steroids.2014.12.018.
CrossRef - Nna VU, Usman UZ, Ofutet EO, Owu DU. Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride – induced oxidative stress in the uterus and ovariesof female Wistar rats. Food and Chemical Toxicology 2017; 102: 143e155. doi: 10.1016/j.fct.2017.02.010.
CrossRef - Wang J, Qian X, Gao Q, Lv C, Xu J, Jin H, Zhu H. Quercetin increases the antioxidantcapacity of the ovary in menopausal ratsand in ovarian granulosa cell culture in vitro. Journal of Ovarian Research 2018; 11: 51. doi: 10.1186/s13048-018-0421-0.
CrossRef - Mueller SO, Simon S, Chae K, Metzler M, Korach KS. Phytoestrogens and their human metabolites show distinct agonistic andantagonistic properties on estrogen receptor α (ERα) and ERβ in humancells. Toxicol. Sci. 2004; 80(1): 14–25. doi: 10.1093/toxsci/kfh147.
CrossRef - van der Woude, H., ter Veld MGR, Jacobs N, van der Saag PT, Murk AJ, Rietjens IMCM. The stimulation ofcell proliferation by quercetin is mediated by the estrogen receptor. Mol. Nutr. Food Res. 2005; 49(8): 763–771. doi: 10.1002/mnfr.200500036.
CrossRef - Neisy A, Zal F, Seghatoleslam A, Alaee S. Amelioration by quercetin of insulin resistance and uterineGLUT4 and ERα gene expression in rats with polycysticovary syndrome (PCOS). Reproduction, Fertility and Development. Reprod Fertil Dev 2019; 31(2): 315-323. doi: 10.1071/RD18222.
CrossRef - Reddy PS, Begum N, Mutha S, Bakshi V. Beneficial effect of Curcumin in Letrozole induced polycystic ovary syndrome. Asian Pacific Journal of Reproduction 2016; 5(2): 116–122. https://doi.org/10.1016/j.apjr.2016.01.006
CrossRef - Melekoglu R, Ciftci O, Eraslan S, Cetin A and Basak N. Beneficial effects of curcumin and capsaicinon cyclophosphamide-induced prematureovarian failure in a rat model. Journal of Ovarian Research 2018; 11:33. doi: 10.1186/s13048-018-0409-9.
CrossRef - Aktas C, Kanter M, Kocak Z. Antiapoptotic and proliferative activityof curcumin on ovarian follicles in mice exposed to wholebody ionizing radiation. Toxicol Ind Health 2012; 28: 852–8 doi: 10.1177/0748233711425080.
CrossRef - Alekseyeva IN, Makogon NV, Bryzgina TM, Voznesenskaya TY, SukhinaVS. Effects of NF-κB blocker curcumin on oogenesis andimmunocompetent organ cells in immune ovarian injury in mice. B Exp Biol Med 2011; 151: 432–43 doi: 10.1007/s10517-011-1349-1.
CrossRef - Tiwari-Pandey R, Ram-Sairam M. Modulation of ovarian structureand abdominal obesity in curcumin- and flutamide-treatedaging FSH-R haploinsufficient mice. Reprod Sci. 2009; 16: 539–5 doi: 10.1177/1933719109332822.
CrossRef - Youn JY, Park HY, Cho KH. Antihyperglycemic activity of Commelina Communis L. inhibition of a-glucosidase. Diabetes Res Clin Pract 2004; 66: S149– doi: 10.1016/j.diabres.2003.08.015.
CrossRef - Igbakin AP, Oloyede OB. Comparative studies on the hypoglycaemic,hypoproteinaemic, hypocholesterolaemic and hypolipidaemic properties ofethanolic and normal saline extracts of the root of Vernonia amygdalina in diabetic rats. Adv Environ Biol 2009; 3(1): 33– doi: 10.1016/j.fct.2007.12.012.
CrossRef - Yadav H, Jain S, Sinha PR. Oral administration of dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei delayed the progression of streptozotocin-induced diabetes in rats. J. Dairy Res. 2008; 75: 189–195. doi: 10.1017/S0022029908003129.
CrossRef - Mohammadi AA, Jazayeri S, Khosravi-Darani K, Solati Z, Mohammadpour N, Asemi Z, AdabZ, Djalali M, Tehrani-Doost M, Hosseini M, Eghtesadi S. Effects of probiotics on biomarkers of oxidative stress and inflammatory factors in petrochemical workers:a randomized, double-blind, placebo-controlled trial. Int. J. Prev. Med. 2015; 6: 82. doi: 4103/2008-7802.164146.
CrossRef - Lee JH, Son CW, Kim MY, Kim MH, Kim HR, Shil Kwak E, Kim S, Kim Red beet (Beta vulgaris L.) leaf supplementation improves antioxidant status in C57BL/6J mice fed high fat high cholesterol diet. Nutr. Res. Pract. 2009; 3: 114–121. doi: 10.4162/nrp.2009.3.2.114.
CrossRef - Ulla, A. et al. Supplementation of Syzygiumcumini seed powder prevented obesity, glucose intolerance, hyperlipidemia and oxidative stress in high carbohydrate high fat diet induced obese rats. BMC Complement. Altern.Med. 2017; 17: 289.doi: 10.1186/s12906-017-1799-8.
CrossRef - Yang R, Le G, Li A, Zheng J, Shi Y. Effect of antioxidant capacity on blood lipid metabolism and lipoprotein lipase activity of rats fed a high-fat diet. Nutrition 2006; 22: 1185-91. doi: 10.1016/j.nut.2006.08.018.
CrossRef - Esposito LA, Melov S, Panov A. Mitochondrial disease in mouse results in increased oxidative stress. Proceedings of the National Academy of Sciences 1999; 96: 4820–4825.
CrossRef - Nishiyama Y, Ikeda H, Haramaki N, Yoshida N, Imaizumi T. Oxidative stress is related to exercise intolerance in patients with heart failure. American Heart Journal 1998; 135: 115–20 doi: 10.1016/s0002-8703(98)70351-5.
CrossRef - Hu YP, Peng YB, Zhang YF, Wang Y, Yu WR, Yao M, Fu XJ. Reactive Oxygen Species Mediated Prostaglandin E2 Contributes to Acute Response of Epithelial Injury. Oxid Med Cell Longev 2017; 2017: 4123854. doi: 10.1155/2017/4123854.
CrossRef - Chávez E, Castro-Sánchez L, Shibayama M, Tsutsumi V, Pérez SalazarE, Moreno MG, Muriel P. Effects of acetyl salicylic acid andibuprofen in chronic liver damage induced by CCl4. J Appl Toxicol 2012; 32: 51–59. doi: 10.1002/jat.1638.
CrossRef - Allen RG, Tresini Oxidative stress and gene regulation. Free Radic Biol Med 2000; 28: 463–99. doi: 10.1016/s0891-5849(99)00242-7.
CrossRef - Hensley K, Robinson KA, Gabbita SP, Salsman S, Floyd RA. Reactive oxygen species, cell signaling, and cell injury. Free Radic Biol Med 2000; 28:1456–62. doi: 10.1016/s0891-5849(00)00252-5.
CrossRef - Pahwa R, Adams-Huet B, Jialal I. The effect of increasing body mass index oncardio-metabolic risk and biomarkers of oxidative stress and inflammation in nascentmetabolic syndrome. J. Diabetes Complicat. 2017; 31: 810–813. doi: 10.1016/j.jdiacomp.2017.02.010.
CrossRef - McEvoy B, Sumayao R, Slattery C, McMorrow T, Newsholme P. Cystine accumulation attenuates insulin release from the pancreatic beta-cell due to elevatedoxidative stress and decreased atp levels. J. Physiol. (Lond.) 2015; 593: 5167–5182.doi: 10.1113/JP271237.
CrossRef - Othman ZA, Zakaria Z, Suleiman JB, Ghazali WSW, Mohamed M. Anti-Atherogenic Effects of Orlistat on Obesity-InducedVascular Oxidative Stress Rat Model.Antioxidants. 2021; 10: 251. doi: 10.3390/antiox10020251.
CrossRef - Yesilbursa D, Serdar Z, Serdar A, Sarac M, Coskun S, Jale C. Lipid peroxides in obese patients and ef-fects of weight loss with orlistat on lipid peroxides levels. Int. J. Obes. Relat. Metab. Disord. 2005; 29: 142-145. doi: 10.1038/sj.ijo.0802794.
CrossRef - Audikovszky M, Pados G, Seres I, Harangi M, Fülöp P, Katona E, Illyés L, Winkler G, Katona ÉM, Paragh G. Orlistat increases serum paraoxonase activity in obese patients. Nutr. Metab. Cardiovasc. Dis 2007; 17: 268–273. doi: 10.1016/j.numecd.2006.03.004.
CrossRef - Pool H, Quintanar D, Figueroa JD, et al. Antioxidant effects ofquercetin and catechin encapsulated into PLGA nanoparticles. J Nanomater. 2012; 2012:1–12. https://doi.org/10.1155/2012/145380
CrossRef - Wu TH, Yen FL, Lin LT, Tsai TR, Lin CC, Cham TM. Preparation, physicochemical characterization, and antioxidant effects of quercetin nanoparticles. Int J Pharm. 2008; 342: 160–168. doi: 10.1016/j.ijpharm.2007.06.036.
CrossRef - Ting Y, Chang WT, Shiau DK, Chou PH, Wu MF, Hsu Antiobesity efficacy of quercetin-rich supplement on diet-induced obese rats: Effects on body composition, serum lipid profile, and gene expression. J. Agric. Food Chem. 2018; 66(1): 70–80. doi: 10.1021/acs.jafc.7b03551.
CrossRef - Al-Jameel SS, Abd El-Rahman SN. Effect of quercetin nanoparticles on the kidney of the streptozotocin-induced diabetes in male rats: A histological study and serum biochemical alterations. African Journal of Biotechnology 2017; 16(39): 1944-1952. https://doi.org/10.5897/AJB2017.15999
CrossRef - Blache D, Durand P, Prost M, Loreau N. (+)-Catechin inhibits platelet hyperactivity induced by an acute iron load in vivo. Free Rad. Biol. & Med 2002; 33: 1670-1680. doi: 10.1016/s0891-5849(02)01139-5.
CrossRef - Liu ZD, Hider RC. Design of iron chelators with therapeutic application. Coord. Chem. Rev. 2002; 232: 151-171. doi: https://doi.org/10.1039/C2DT12159J.
CrossRef - Satyendra SB, Nikhil S, Rajendra SB, Preeti A, Sarlesh R. A review of quercetin: Antioxidant and anticancer properties. World J. Pharm. Pharm. Sci. 2012; 1: 146-160.
- Lopez-Revuelta A, Sanchez-Gallego JI, Hernandez-Hernandez A, Sanchez-Yaque J, Llanillo M. Membrane cholesterol contents influence the protective effects ofquercetin and rutin in erythrocytes damaged by oxidative stress. Chem. Biol. Interact. 2006; 161:79-91. doi: 10.1016/j.cbi.2006.03.004.
CrossRef - Selvi NMK, Sridhar MG, Swaminathan RP, Sripradha R. Curcumin Attenuates Oxidative Stress and Activation of Redox-Sensitive Kinases in High Fructose- and High-Fat-Fed Male Wistar Rats. Sci Pharm. Jan-Mar; 2015; 83(1): 159–175. doi: 10.3797/scipharm.1408-16.
CrossRef - Abu-Taweel GM, Attia MF, Hussein J, Mekawi EM, Galal HM, Ahmed EI, Allam AA, El-Naggar ME. Curcumin nanoparticles have potential antioxidant effect and restore tetrahydrobiopterin levels in experimental diabetes. Biomedicineand Pharmacotherapy 2020; 131: 110688. https://doi.org/10.1016/j.biopha.2020.110688.
CrossRef - Dinkova-Kostova AT, Talalay Relation of structure of curcumin analogs to their potencies as inducers of Phase 2 detoxification enzymes. Carcinogenesis 1999; 20: 911–914.doi: 10.1093/carcin/20.5.911.
CrossRef - Khattab HAH, Al-Amoudi NS, Al-Faleh AA. Effect of ginger, curcumin and their mixture on blood glucose and lipids in diabetic rats. Life Sci J 2013; 10: 428–442.
- Scapagnini G, Vasto S, Abraham NG, Caruso C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 2011; 44:192–201. doi: 10.1007/s12035-011-8181-5.
CrossRef - He HJ, Wang GY, Gao Y, Ling WH, Yu ZW, Jin TR. Curcumin attenuates Nrf2 signaling defect, oxidative stress in muscle and glucose intolerance in high fat diet-fed mice. World J Diabetes 2012; 15:94–104. doi: 10.4239/wjd.v3.i5.94.
CrossRef - Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of adipose tissue inflammation to the development oftype 2 diabetes mellitus. Compr. Physiol 2018; 9: 1–58. doi: 10.1002/cphy.c170040.
CrossRef - Zhang W, Wang LW, Wang LK, Li X, Zhang H, Luo LP, Song JC, Gong ZJ. Betaine Protects Against High-Fat-Diet-Induced Liver Injury by Inhibition of High-Mobility Group Box 1 and Toll-Like Receptor 4 Expression in Rats. Dig Dis Sci 2013; 58(11): 3198-206. doi: 10.1007/s10620-013-2775-x.
CrossRef - Liu Y, Xu D, Yin C, Wang S, Wang M, Xiao IL-10/STAT3 is reduced in childhood obesity with hypertriglyceridemia and is related to triglyceride level in diet-induced obese rats. BMC Endocr Disord. 2018; 18: 39. doi: 10.1186/s12902-018-0265-z
CrossRef - Awla D, Abdulla A, Regner S, Thorlacius H. TLR4 but not TLR2 regulates inflammation and tissue damage in acute pancreatitis induced by retrograde infusion of taurocholate. Inflammation Research 2011; 60(12): 1093–1098. doi: 10.1007/s00011-011-0370-1.
CrossRef - Sharifnia T, Antoun J, Verriere TG et al. Hepatic TLR4 signaling in obese NAFLD. American Journal of Physiology Gastrointestinal and Liver Physiology. 2015; 309(4): G270–G278. doi: 10.1152/ajpgi.00304.2014.
CrossRef - Chenxu G, Minxuan X, Yuting Q et al. Loss of RIP3 initiates annihilation of high-fat diet initialized nonalcoholic hepatosteatosis: a mechanism involving toll-like receptor 4 and oxidative stress. Free Radical Biology & Medicine 2019; 134: 23–41. doi: 10.1016/j.freeradbiomed.2018.12.034.
CrossRef - Xu M, Wang KN, Wu K, Wang XP. Pyrrolidine dithiocarbamate inhibits nuclear factor κB and toll-like receptor 4 expression in rats with acute necrotizing pancreatitis. Gut and Liver. 2015; 9(3): 411–416. doi: 10.5009/gnl14050.
CrossRef - Kutikhin AG, Ponasenko AV, Khutornaya MV, Yuzhalin AE, Zhidkova, Salakhov RR, Golovkin AS, Barbarash OL, Barbarash LS. Association of TLR and TREM-1 gene polymorphisms with atherosclerosis severity in a Russian population. Meta Gene 2016; 9: 76–89.doi: 10.1016/j.mgene.2016.04.001
CrossRef - Park HJ, Lee JY, Chung MY, Park YK, Bower AM, Koo SI, Giardina C, Bruno Green tea extract suppresses NFkB activation andinflammatory responses in diet-induced obese rats with nonalcoholic steatohepatitis. The journal of nutrition. 2012; 142 (1): 57–63. doi: 10.3945/jn.111.148544.
CrossRef - Feingold KR, Soued M, Adi S, Staprans I, Neese R, Shigenaga J, Doerrler W, Moser A, Dinarello CA, Grunfeld C. Effect of Inteleukin-1 on lipid-metabolism in the rat – similarities to and differences from tumor-necrosis-Factor. Arterioscler Thromb 1991; 11(3): 495–500.doi: 10.1161/01.atv.11.3.495.
CrossRef - EL-baz FK, Aly HF, Fayed DB Dunaliella salina improves obesity-associated inflammation and oxidative damage in animals , rodents. Asian Journal of Pharmaceutical and Clinical Research, Asian J Pharm Clin Res, 2018, 11(5): 240-246. doi: https://doi.org/10.22159/ajpcr.2018.v11i5.24622.
CrossRef - Bansal P, Bhandari U, Sharma K, Arya P. Embelin modulates metabolicendotoxemia and associated obesity in high fat diet fed C57BL/6 mice. Human and Experimental Toxicology 2021; 40(1): 60–70. https://doi.org/10.1177/0960327120934522.
CrossRef - Li X, Yang L, Xu M, Qiao G, Li C, Lin L, Zheng G, Smilaxchina L. polyphenols alleviates obesity and inflammation by modulating gut microbiota in high fat/high sucrose diet-fed C57BL/6J mice. Journal of Functional Foods 2021; 77: 104332. https://doi.org/10.1016/j.jff.2020.104332
CrossRef - Byun EB, Yang MS, Choi HG, Sung NY, Song DS, Sin SJ, et al. Quercetin negativelyregulates TLR4 signaling induced by lipopolysaccharide through Tollip expression. Biochem Biophys Res Commun 2013; 431(4): 698–705. doi: 10.1016/j.bbrc.2013.01.056.
CrossRef - Mahmoud MF, Hassan NA, El Bassossy HM, Fahmy A. Quercetin protects against diabetes-induced exaggerated vasoconstriction in rats: effect on low grade inflammation. PLoS One 2013; 8: e63784. doi: 10.1371/journal.pone.0063784.
CrossRef - Eitah HE, Maklad YA, Abdelkader NF, el Din AAG, Badawi MA, Kenawy SA. Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats, Toxicol. Appl. Pharmacol. 2019; 365: 30–40. doi: 10.1016/j.taap.2018.12.011.
CrossRef - Dias AS, Porawski M, Alonso M, Marroni N, Collado PS, Gonzalez-Gallego J. Quercetin decreases oxidative stress, NF-κB activation, and iNOS overexpression inliver of streptozotocin-induced diabetic rats. J. Nutr. 2005; 135: 2299–2304.
CrossRef - Xu MX, Wang M, Yang WW. Gold-quercetin nanoparticles prevent metabolic endotoxemia-induced kidney injury by regulating TLR4/NF-κB signaling and Nrf2 pathway in high fat diet fed mice. Int J Nanomedicine. 2017; 12: 327–345. doi: 10.2147/IJN.S116010.
CrossRef - Ahmed OM, Mohamed T, Moustafa H, Hamdy H, Ahmed RR, Aboud E. Quercetin and low level laser therapy promote wound healing process indiabetic rats via structural reorganization and modulatory effects oninflammation and oxidative stress. Biomedicine & Pharmacotherapy 2018; 101: 58–73. doi: 10.1016/j.biopha.2018.02.040.
CrossRef - Singh AK, Vinayak Curcumin attenuates CFA induced thermal hyperalgesia by modulation of antioxidant enzymes and down regulation of TNF-alpha, IL-1beta and IL-6. Neurochem Res. 2015; 40: 463–472.doi: 10.1007/s11064-014-1489-6.
CrossRef - Kong F, Ye B, Cao J, Cai X, Lin L, Huang S, et al. Curcumin represses NLRP3 Inflammasome activation via TLR4/MyD88/NF-kappaB and P2X7R signaling in PMA-induced macrophages. Front Pharmacol 2016; 7: 369.doi: 10.3389/fphar.2016.00369.
CrossRef - Feng D, Zou J, Su D, Mai H, Zhang S, Li P, Zheng X. Curcumin prevents high-fat diet-induced hepatic steatosis in ApoE−/− mice by improvingintestinal barrier function and reducing endotoxin and liver TLR4/NF-κB inflammation. Nutr Metab (Lond); 2019; 16: 79. doi: 10.1186/s12986-019-0410-3.
CrossRef - Zhou Y, Zhang T, Wang X, Wei X, Chen Y, Guo L, et al. () Curcumin modulates macrophage polarization through the inhibition of the toll-like receptor 4 expression and its signaling pathways. Cell Physiol Biochem 2015; 36: 631–641.doi: 10.1159/000430126.
CrossRef - Wang Y, Shan X, Dai Y, Jiang L, Chen G, Zhang Y, et al. Curcumin analog L48H37 prevents lipopolysaccharide-induced TLR4 signaling pathway activation and Sepsis via targeting MD2. J Pharmacol Exp Ther. 2015; 353: 539–550.doi: 10.1124/jpet.115.222570.
CrossRef - Meng Z, Yan C, Deng Q, Gao DF, Niu XL. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitrovia ROS-relative TLR4-MAPK/NF-kappaB pathways. Acta Pharmacol Sin. 2013; 34: 901–911.doi: 10.1038/aps.2013.24.
CrossRef - Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol 2006; 69(1): 195–206. doi: 10.1124/mol.105.017400.
CrossRef - Gonzales AM, Orlando RA () Curcumin and resveratrol inhibit nuclear factor-kappaBmediated cytokine expression in adipocytes. Nutrition and Metabolism 2008; 5(1):17. doi: 10.1186/1743-7075-5-17.
CrossRef - Yang R, Zheng Y, Wang Q, Zhao L. Curcumin-loaded chitosan–bovine serum albumin nanoparticles potentially enhanced Aβ 42 phagocytosis and modulated macrophage polarization in Alzheimer’s disease. Nanoscale Res Lett. 2018; 13: 330. doi: 10.1186/s11671-018-2759-z.
CrossRef - Mollazadeh H, Cicero AFG, Blesso CN, Pirro M, Majeed M, Sahebkar A. Immune modulation by curcumin: The role of interleukin-10. Crit. Rev. Food Sci. Nutr. 2019; 59: 89–101. doi: 10.1080/10408398.2017.1358139.
CrossRef









