Manuscript accepted on :01-11-2021
Published online on: 11-11-2021
Plagiarism Check: Yes
Reviewed by: Dr. Ana Golez
Second Review by: Dr. Nicolas Padilla
Final Approval by: Dr. Ayush Dogra
Cijoy Jose .P1, V.P. Karthik2 , Vasanthi2 and Punnagai guna2
, Vasanthi2 and Punnagai guna2
1Government taluk hospital, Assistant surgeo, Chalakudy, Thrissur, Kerala
2Department of Pharmacology, Sri Ramachandra Medical College and Research Institute
Corresponding Author E-mail: drkarthikvp@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2319
Abstract
Background: Benign prostatic hypertrophy (BPH) is a common entity among elderly men and is responsible for significant disability. Medical treatment for BPH has played a major role in improving the symptoms associated with bladder outlet obstruction. Recent guidelines recommend the combination of an alpha-1 blocker and a 5-ARI as first-line treatment of men with moderate-to-severe LUTS. There is limited study data to support which is the effective therapy for BPH/LUTS in a tertiary care hospital in India. Methods: A randomized, open labelled, double blind, active control parallel group study comparing the safety and efficacy of tamsulosin versus tamsulosin+finasteride in subjects with lower urinary tract symptoms secondary to benign prostatic hypertrophy. The subjects were randomized after obtaining written informed consent. The subjects were recruited from the patient population that was attending the Out-Patient department of Urology, Sri Ramachandra Medical College & Research Institute, Sri Ramachandra University. Results: A total sample of 60 subjects, 30 into each group was enrolled. In the tamsulosin group, according to IPSS score, 11 patients who had moderate symptoms (36.7%) became mild, and among 19 patients who had severe symptoms (63.3%), 12 patients became mild (40%) and 7 patients became moderate (23.3%) with a p value of 0.021 which is statistically significant. In the tamsulosin+ finasteride group, according to IPSS score, 10 patients who had moderate symptoms (33.3%) became mild, and among 20 patients who had severe symptoms (66.7%), 12 patients became mild (40%) and 8 patients became moderate (26.7%) with a p value of 0.020 which is statistically significant. When comparing between the two groups, 76.7% attained mild symptoms in group 1 and 73.3% patients attained mild symptoms in group 2 with a p value of 0.766 which is statistically not significant. Conclusion: To conclude tamsulosin 0.4mg is as effective as the combination therapy of tamsulosin 0.4mg and finasteride 5mg in the treatment of lower urinary tract symptoms secondary to Benign prostatic hypertrophy.
Keywords
Benign Prostatic Hypertrophy; Finasteride; Lower urinary tract; Tamsulosin
Download this article as:| Copy the following to cite this article: Jose P. C, Karthik V. P, Vasanthi V, Guna P. Comparative Efficacy of Tamsulosin Versus Tamsulosin Plus Finasteride in Patients with Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hypertrophy, a Randomized Open Label Parallel Group Study. Biomed Pharmacol J 2021;14(4). |
| Copy the following to cite this URL: Jose P. C, Karthik V. P, Vasanthi V, Guna P. Comparative Efficacy of Tamsulosin Versus Tamsulosin Plus Finasteride in Patients with Lower Urinary Tract Symptoms Secondary to Benign Prostatic Hypertrophy, a Randomized Open Label Parallel Group Study. Biomed Pharmacol J 2021;14(4). Available from: https://bit.ly/3HcAYri |
Introduction
Benign prostatic hyperplasia/hypertrophy (BPH) is a common entity among elderly men and is responsible for significant disability. In men 20 to 30 years of age, the prostate weighs about 20 g approximately. However, the mean prostatic weight increases after the age of 50. The prevalence of histologically diagnosed prostatic hyperplasia increases from 8% in men aged 31 to 40 years, to 40% to 50% in men aged 51 to 60 years, to more than 80% in men older than age 80 years 1.
The common symptoms of BPH are increased frequency of urination, nocturia, hesitancy, urgency, and weak urinary stream. These symptoms typically appear slowly and progress gradually over a period of years. However, they are not specific for BPH. Furthermore, the correlation between symptoms and the presence of prostatic enlargement on rectal examination is poor. This discrepancy probably results from changes in bladder function that occur with aging and from enlargement of the transitional zone of the prostate that is not always evident on rectal examination. It is critical to exclude causes of lower urinary tract symptom other than BPH before institution of any medical or surgical treatment 2.
Medical treatment for BPH has played a major role in improving the symptoms associated with bladder outlet obstruction. Although one decade ago, surgical treatment may have been at the forefront of therapy for BPH, medical treatment is now the first line therapy for BPH. Medical therapy focuses on two aspects of the pathophysiology of BPH:
A dynamic (physiologic, reversible) component related to the tension of prostatic smooth muscle in the prostate, prostate capsule, and bladder neck
A fixed (structural) component related to the bulk of the enlarged prostate impinging on the urethra 3
The two classes of drugs, α-adrenergic antagonists (release smooth muscle tension) and 5-α reductase inhibitors (reduce the enlarged prostate size), act on each of these components mentioned 3.
The most common 5-α reductase inhibitor used in the United States is finasteride. It acts by blocking the conversion of testosterone to the more potent androgen, DHT. The decreased serum and per-prostatic levels of DHT lead to reduction in prostatic size over time 4.
α Adrenergic blockers have been shown to be more efficacious than 5-α reductase inhibitors by improving the lower urinary tract symptoms associated with BPH 5.
The approach of combination therapy is theoretically favored by the fact that multiple factors contribute to the enlargement of prostate and achieving control with single agent that acts through one particular mechanism may be unrealistic. Combining the second agent may lead to better control, acting by complimentary mechanism. As many factors contribute to enlarged prostate, the combination of agents with different (and complementary) mechanisms of action provides more benefits with less activation of counter-regulatory mechanisms 6.
Alpha-1 blockers provide symptomatic improvement within weeks, but cannot prevent disease progression and acute urinary retention. 5-ARIs achieve the latter, but take months to improve symptoms. Combining the two achieves both treatment objectives of rapid relief of symptoms and reducing the disease progression. Advantages were seen in improvements of storage and voiding IPSS subscales and flow rates and time taken for symptom progression 3. Studies have shown that urinary retention rates were lower with combination therapy than with tamsulosin monotherapy already after 8 months, and continued to diverge linear to a relative reduction of 68% at 4 years [35, 36]. Patients with larger prostates at baseline clearly profited most, and symptom improvement was superior when comparing tamsulosin monotherapy with combination therapy from month 21 in the lowest volume tertile (30–42 cm3), from month 6 in the middle volume tertile, and from month 3 in the highest volume tertile (>58 cm3) 7.
Recent guidelines recommend the combination of an alpha-1 blocker and an 5-ARI as first-line treatment of men with moderate-to-severe LUTS and an enlarged prostate, although the definition of “enlarged” varies from no precise definition to prostate volumes >30–40 mL [8]. Differing opinions on this issue only exist on the lower cut-off value of prostate volume above which this combination becomes the first-line therapy.
There is limited study data to support which is the effective therapy for BPH/LUTS in a tertiary care hospital in India. Hence, we undertake this study to find out a rationale combination drug therapy which is cost effective too.
Materials and Methods
Aim
To compare the efficacy of Tamsulosin versus Tamsulosin plus Finasteride in patients with lower urinary tract symptoms secondary to Benign Prostatic Hyperplasia
Primary Objective
To find out which is more symptomatically efficacious drug therapy for BPH/LUTS, either tamsulosin given singly or tamsulosin plus Finasteride given in combination.
Secondary Objective
To compare the efficacy in Tamsulosin alone group and Tamsulosin plus Finasteride group through various investigations.
This was a randomized, open labelled, double blind, active control parallel group study comparing the safety and efficacy of tamsulosin versus tamsulosin+finasteride in subjects with lower urinary tract symptoms secondary to benign prostatic hypertrophy. The study commenced after getting written approval from The Institutional Ethics Committee of Sri Ramachandra Medical College & Research Institute, Sri Ramachandra University (REF: CSP-MED/13/JUN/07/39, dated 17.06.2013). The subjects were randomized after obtaining written informed consent.
Study Site
The study was conducted at Department of Urology, Sri Ramachandra Hospital in collaboration with department of Pharmacology, Sri Ramachandra Medical College and Research Institute, Sri Ramachandra University.
Study Design
Randomized, comparative, open labelled parallel group study.
Patient Selection
The trial was designed and conducted in accordance with Good Clinical Practice guidelines. Written informed consent was obtained from each participating subject before entry into the study. A total sample of 60 subjects, 30 into each group was enrolled. The subjects were recruited from the patient population that was attending the Out-Patient department of Urology, Sri Ramachandra Medical College & Research Institute, Sri Ramachandra University.
Inclusion Criteria
Newly diagnosed cases of BPH/LUTS without any complications are included in the study.
BPH/LUTS patients who are under first line of therapy of tamsulosin also will be included in the study.
Males of age between 50 to 65yrs.
Patients with ultrasonographic findings of post voidal residual urine<100 ml.
Patients with IPSS score >8
Patients with PSA value <4
Uroflowmetry with peak flow rate between 10- 15 ml/sec
Patients with no evidence of prostatic malignancy.
Subject is willing and able to give written informed consent.
Exclusion Criteria
Males of age below 50 and above 65 yrs.
BPH patients undergone surgery.
History of hypersensitivity to the study drugs.
Patients with ultrasonographic findings of post voidal residual urine>100 ml.
Patients with definitive indication of invasive treatment such as history of urinary retention, previous history of catheterization to relieve retention, associated complications e.g., hydronephrosis, impaired kidney function, bladder stone, recurrent gross haematuria;
Those who took finasteride tablet at the day before visit.
Patients with history of severe heart failure or previous MI.
History of Drug abuse or Alcohol addiction.
Study Groups
each group 30 patients each.
Study Group 1 – Tamsulosin 0.4mg
Study Group 2 – Tamsulosin 0.4mg + Finasteride 5mg
Study Duration
Total duration of study treatment was for 12 weeks with a 4-week follow-up. Subjects came 3 days before randomization, at randomization and 4 weeks after starting study medication, at 12 weeks i.e. at the end of study medication use and 2 weeks post study medication use as a follow-up. The study was done between February and June 2013.
Method of Generating Random Sequence
Computer Generated Random Number Table
 |
Table 1 |
Procedure at Each Visit
Visit 1- Screening and Baseline Characters (before initiating drug therapy)
CRF was filled, Informed Consent of the patient was obtained and documented.
Visit 2 (randomization visit)
Gathered information and assessed the subject’s inclusion & exclusion criteria and randomized to treatment groups. Drugs were dispensed according to group randomized in a plastic bottle. Subjects were advised to take drug according to protocol, and were told to contact the physician if any adverse event occurs.
Visit 3 (+ 4 weeks of treatment.)
Adverse events were enquired. After assessing the safety, efficacy of the treatment and compliance drugs were given for another 8 weeks.
Visit 4 (+ 12 weeks of treatment.)
Adverse event was enquired. Assessed the compliance by pill count at the end of the study when participants returned the drug bottles. Subjects were told about the study termination.
Visit 5 (+ 2 weeks of end of treatment.)
Follow up visit
Efficacy Variables
Primary Outcome
To compare the reduction of LUTS secondary to BPH using IPSS (International prostate scoring system) and IPSS-QOL (Quality of Life) parameters.
Secondary Outcome
To compare the improvement through investigations like uroflowmetry and ultrasonographic findings.
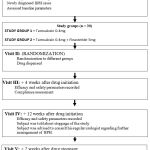 |
Diagram 1: Flow Diagram of the study |
Results
Out of the total 85 patients screened, 60 patients were included in the study and the rest were excluded as they were not meeting the eligibility criteria. The patients selected contained newly diagnosed cases of BPH and were randomized to the two treatment groups. There were no dropouts in the study.
The baseline values of the subjects who took part in this study are given. There was no significant difference in baseline values among the two groups. In the tamsulosin group with a sample size of 30, according to IPSS score 19 patients had severe symptoms (63.3%) and 11 patients had moderate symptoms (36.7%) while in the tamsulosin+ finasteride group,
20 patients had severe symptoms (66.7%) and 10 patients had moderate symptoms (33.3%) showing a p value of 0.787 which is not at all significant.
The baseline values regarding quality of life (QOL) were also similar with the tamsulosin group showing an average value of 4.13 with a standard deviation of 1.306 and tamsulosin and finasteride group showing a mean value of 4.20 with a standard deviation of 1.243 and p value of 0.840 which is statistically not significant.
The baseline values of uroflowmetry in both the groups were also similar with the flow rate falling below 12ml/sec in all the sixty subjects. The baseline values of residual urine for all the sixty subjects were similar with the quantity of residual urine being > 50 ml and < 100 ml. The baseline values for prostate size were also similar with the size being > 50g in all the sixty subjects.
Efficacy Profile
The reduction in the baseline values of all the given parameters like IPSS score, QOL grading, residual urine, uroflowmetry and prostate size after 12 weeks of therapy were clinically very significant for both the groups though few were statistically not significant.
In the tamsulosin group, according to IPSS score, 11 patients who had moderate symptoms (36.7%) became mild, and among 19 patients who had severe symptoms(63.3%), 12 patients became mild (40%) and 7 patients became moderate (23.3%) with a p value of 0.021 which is statistically significant.
In the tamsulosin+ finasteride group, according to IPSS score, 10 patients who had moderate symptoms (33.3%) became mild, and among 20 patients who had severe symptoms (66.7%), 12 patients became mild (40%) and 8 patients became moderate (26.7%) with a p value of 0.020 which is statistically significant. When comparing between the two groups, 76.7% attained mild symptoms in group 1 and 73.3% patients attained mild symptoms in group 2 with a p value of 0.766 which is statistically not significant.
The mean QOL grading of 4.13 of group 1 was reduced to 1.17 at the end of 12 weeks of treatment and the mean value of 4.20 in group 2 was reduced to 1.23 and the comparison between the two groups showed a p value of 0.719 which is statistically not significant.
The urine flow rate recorded in uroflowmetry was increased to >12 ml/sec in more than 80% of the patients in both group1 and group2 at the end of 12 weeks of treatment which was clinically very significant while the difference between the two were not at all significant.
The residual urine recorded in USG was reduced to <50 ml in more than 80% of the patients in both group1 and group2 at the end of 12 weeks of treatment which was clinically very significant while the difference between the two were not at all significant.
The prostate size recorded in USG was reduced to <40 gm in more than 80% of the patients in both group1 and group2 at the end of 12 weeks of treatment which was clinically very significant while the difference between the two were not at all significant.
During the follow up period there is a reversal in the values of all the parameters seen at the end of 12 weeks with IPSS score showing an increase in symptom score to become severe by 46.7% in both the groups which is clinically very significant. There was change in mean value of QOL grading with the value changing to 3.53 in group 1 and 3.47 in group 2 which is clinically very significant. The urine flow rate recorded in uroflowmetry was reduced to <12 ml/sec in 66.7% of patients of both groups which was clinically significant. The residual urine recorded in USG was increased to >50 ml in 56.7% of the patients in both group1 and group2 which was clinically very significant while the difference between the two were not at all significant. There was no significant difference in the prostate size recorded in USG in both groups in the follow up period.
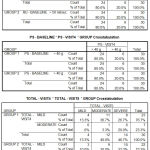 |
Table 2 |
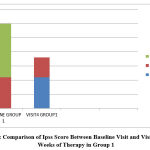 |
Figure 1: Comparison of Ipss Score Between Baseline Visit and Visit after 12 Weeks of Therapy in Group 1 |
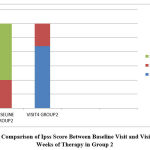 |
Figure 2: Comparison of Ipss Score Between Baseline Visit and Visit after 12 Weeks of Therapy in Group 2 |
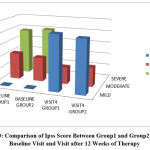 |
Figure 3: Comparison of Ipss Score Between Group1 and Group2 During Baseline Visit and Visit after 12 Weeks of Therapy. |
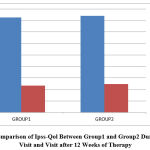 |
Figure 4: Comparison of Ipss-Qol Between Group1 and Group2 During Baseline Visit and Visit after 12 Weeks of Therapy. |
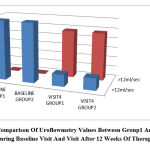 |
Figure 5: Comparison Of Uroflowmetry Values Between Group1 And Group 2 During Baseline Visit And Visit After 12 Weeks Of Therapy. |
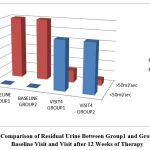 |
Figure 6: Comparison of Residual Urine Between Group1 and Group2 During Baseline Visit and Visit after 12 Weeks of Therapy |
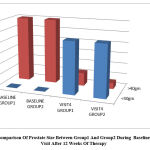 |
Figure 7: Comparison Of Prostate Size Between Group1 And Group2 During Baseline Visit And Visit After 12 Weeks Of Therapy. |
Discussion
BPH is the most common urological problem of ageing men, manifested as severe obstruction in urinary flow with discomfort and pain. BPH is a complex disease from the etiological and pathogenesis point of view 9. A recent AUA guideline (2003) suggests an increase in the incidence of BPH worldwide and predicts by the age of 60 years, more than 50% of men will have microscopic evidence of the disease and by the age of 85 years, as many as 90% of men will be affected 10. Worldwide investigations for incidence of BPH are scanty and at times difficult to compare due to uneven definition of BPH based on different clinical parameters. There is also great geographical disparity in prevalence and degree of severity of symptoms of BPH.
Asian, particularly vegetarian, men consume low-fat, high-fiber diets rich in weak dietary phytoestrogens, which have been proposed as chemopreventive agents. But the very few studies conducted on BPH patients from India suggest BPH as the most common pathological condition with an incidence of 92.97% (n = 185) and (93.3% (n = 200) 11.
Clinical efficacy of either 5α-reductase inhibitor or α1-AR antagonist has been further improved by using combination therapy; however, long-term outcomes are still awaited. Many more potential new therapies are under development that may improve the treatment of BPH 12.
The present study evaluated the efficacy and safety of tamsulosin 0.4 mg and a fixed dose combination of tamsulosin 0.4mg + finasteride 5mg in reducing lower urinary tract symptoms according to IPSS scoring system and IPSS-QOL secondary to BPH13.
The results demonstrated a comparable efficacy of tamsulosin with tamsulosin + finasteride in controlling LUTS secondary to BPH. The difference in mean percent change of symptoms between both the groups was statistically not significant. The use of α-adrenergic blockade to treat men with symptomatic benign prostatic hyperplasia is based on the hypothesis that the disorder arises from bladder-outlet obstruction and that 40 percent of the cellular volume of the hyperplastic prostate is made up of smooth muscle,20 whose tension is mediated by α1-adrenoceptors. Therapy with α1-adrenergic–antagonist drugs (such as terazosin, doxazosin, and tamsulosin) has been found to be safe and effective in men with benign prostatic hyperplasia, a finding that our study confirmed 14.
The idea for addition of 5α-reductase inhibitor with α-adrenergic blockade in the treatment of benign prostatic hyperplasia is that the development of the condition is an androgen-dependent event. Castration during childhood or puberty prevents it from developing, and the suppression of androgens in adult men promotes its regression 15.
In group 1 subjects who were treated with tamsulosin 0.4 mg, there was significant reduction in both the primary outcome measures like IPSS score and IPSS- QOL and secondary outcome measures like uroflowmetry and USG findings like residual urine and prostate size after 12 weeks of treatment. The patients who had severe (63.3%) and moderate (36.7%) symptoms according to IPSS score were shifted to mild (76.7%) and moderate (23.3%) symptoms and QOL grade was reduced for almost all the patients. There was also significant improvement in all the other parameters in more than 80% of subjects which shows that tamsuloin 0.4 mg has significant effect on LUTS due to BPH. This is in accordance with the study conducted by Alvin lee et al[16]. On European tamsulosin study group which proved that tamsulosin 0.4mg was clinically safe and effective in controlling LUTS due to BPH.
Similarly in group 2 subjects who were treated with tamsulosin 0.4 mg + finasteride 5mg, there was significant reduction in both the primary and secondary outcome measures after 12 weeks of treatment. The patients who had severe (66.7%) and moderate (33.3%) symptoms according to IPSS score were shifted to mild (73.3%) and moderate (26.7%) symptoms and QOL grade was reduced for almost all the patients. There was also significant improvement in all the other parameters in more than 80% of subjects which shows that tamsuloin 0.4 mg+ finasteride 5mg has significant effect on LUTS due to BPH. This is in accordance with the Medical Therapy of Prostatic Symptoms study (MTOPS) conducted by Herbert Lepor, et al., which proves that combination therapy significantly reduced the incidence of the composite measure of BPH progression compared with α-blocker/5-ARI monotherapies or placebo over the study duration 17. But this study was a 4.5year long study with a study population of over 3000 subjects which are some of our study limitations
While comparing between the two groups in our study, it was evident that there was no significant difference between the primary and secondary outcome measures after 12 weeks of therapy both clinically and statistically. This is in accordance with the study conducted by Lepor H, Williford WO, Barry MJ, et al [17], for the Veterans Affairs Cooperative Studies which compared the efficacy of terazosin, finasteride, or both in benign prostatic hyperplasia. According to this study, the differences between terazosin and finasteride were statistically significant in favor of terazosin with regard to both primary outcome measures — symptom scores and urinary-flow rate — and the combination of terazosin and finasteride were no more effective than terazosin alone.
According to the Prospective European Doxazosin and Combination Therapy (PREDICT) trial done by David Wofsy, et al [18]. Doxazosin was effective in improving urinary symptoms and urinary flow rate in men with benign prostatic hyperplasia, and was more effective than finasteride alone or placebo. The addition of finasteride did not provide further benefit to that achieved with doxazosin alone. According to ALFIN Study trial conducted by F M Debruyne et al 19 done to compare sustained-release alfuzosin, an alpha blocker with finasteride and the combination of both in the treatment of benign prostatic hyperplasia, SR alfuzosin was more effective than finasteride, with no additional benefit in combining both drugs which again supports our study.
There was clinically significant difference in the primary and secondary outcomes in both the groups between the end of treatment after 12 weeks and the follow up period during which the drugs were not given though they were statistically not significant. There was not much increase in the prostate size in both the groups during the follow up period. This is also in accordance with previous studies which suggests that the drugs whether alone or combined if stopped, leads to recurrence of LUTS and so must continue for a longer period 9.
The study drugs were well tolerated in both the groups. The incidence of adverse events was comparatively very less in both the groups. This can be due to reduced duration of the study after which the adverse events might appear 20.
Both the groups exhibited a good patient compliance to drug therapy and there was no significant difference observed in treatment compliance of both the groups. This might due to the short duration of treatment period (12 weeks) and frequent reminders given to the study participants 21.
Conclusion
This study has revealed that there is no significant difference in daily therapy with tamsulosin and tamsulosin+ finasteride in controlling LUTS secondary to BPH. The study has also revealed that there is a tendency of worsening of symptoms on stopping medications in both the groups. At the same time, the therapy in both the groups is associated with fewer incidence of adverse events.
To conclude tamsulosin 0.4mg is as effective as the combination therapy of tamsulosin 0.4mg and finasteride 5mg in the treatment of lower urinary tract symptoms secondary to Benign prostatic hypertrophy.
References
- Deepak Sundaram, Ponnusamy Kasirajan Sankaran, Gunapriya Raghunath, S Vijayalakshmi, J Vijayakumar, Maria Francis Yuvaraj, Munnusamy Kumaresan, and Zareena begum. Correlation of Prostate Gland Size and Uroflowmetry in Patients with Lower Urinary Tract Symptoms; J Clin Diagn Res. 2017 May; 11(5): AC01–AC04.
CrossRef - Kevin T McVary, Clinical Evaluation of Benign Prostatic Hyperplasia; Rev Urol. 2003; 5(Suppl 4): S3–S11.
CrossRef - Herbert Lepor. Pathophysiology of Benign Prostatic Hyperplasia in the Aging Male Population; Rev Urol. 2005; 7(Suppl 4): S3–S12.
- Jason M. Hirshburg, Petra A. Kelsey, A. Therrien, A. Carlo Gavino, Jason S. Reichenberg, Adverse Effects and Safety of 5-alpha Reductase Inhibitors (Finasteride, Dutasteride): A Systematic Review. J Clin Aesthet Dermatol. 2016 Jul; 9(7): 56–62.
- Eric H. Kim, John A. Brockman, and Gerald L. Andriole; The use of 5-alpha reductase inhibitors in the treatment of benign prostatic hyperplasia; Asian J Urol. 2018 Jan; 5(1): 28–32.
CrossRef - Neelima Dhingra and Deepak Bhagwat; Benign prostatic hyperplasia: An overview of existing treatment; Indian J Pharmacol. 2011 Feb; 43(1): 6–12.
CrossRef - Curtis Nickel; Benign Prostatic Hyperplasia: Does Prostate Size Matter?; Rev Urol. 2 S. Gravas (Chair), J.N. Cornu, M. Gacci, C. Gratzke,
- R.W. Herrmann, C. Mamoulakis, M. Rieken, M.J. Speakman, K.A.O. Tikkinen Guidelines Associates: M. Karavitakis, I. Kyriazis, S. Malde, V. Sakalis, R. Umbach Management of Non-Neurogenic Male Lower Urinary Tract Symptoms (LUTS), incl.Benign Prostatic Obstruction (BPO) 003; 5(Suppl 4): S12–S17.
- Claus G Roehrborn, Benign Prostatic Hyperplasia: An Overview; Rev Urol. 2005; 7(Suppl 9): S3–S14.
- Bid HemantKumar, Konwar Rituraj, Singh Vishwajeet; Benign prostatic hyperplasia: Is it a growing public health concern for India?; Indian Journal of Medical Sciences, Vol. 62, No. 9, September, 2008, pp. 375-376
CrossRef - L Denis 1, M S Morton, K Griffiths; Diet and its preventive role in prostatic disease; Eur Urol 1999;35(5-6):377-87.
CrossRef - Neelima Dhingra and Deepak Bhagwat; Benign prostatic hyperplasia: An overview of existing treatment; Indian J Pharmacol. 2011 Feb; 43(1): 6–12.
CrossRef - Konstantinos Dimitropoulos and Stavros Gravas; Solifenacin/tamsulosin fixed-dose combination therapy to treat lower urinary tract symptoms in patients with benign prostatic hyperplasia; Drug Des Devel Ther. 2015; 9: 1707–1716.
CrossRef - P Rigatti, M Brausi, R M Scarpa, D Porru, H Schumacher & C A Rizzi; A comparison of the efficacy and tolerability of tamsulosin and finasteride in patients with lower urinary tract symptoms suggestive of benign prostatic hyperplasia; Prostate Cancer and Prostatic Diseases volume 6, pages315–323(2003)
CrossRef - Leonard S Marks; 5α-Reductase: History and Clinical Importance; Rev Urol. 2004; 6(Suppl 9): S11–S21.
- Alvin Lee, Han Jie Lee, and Keong Tatt Foo; Can men with prostates sized 80 mL or larger be managed conservatively?; Investig Clin Urol. 2017 Sep; 58(5): 359–364.
CrossRef - Herbert Lepor; Medical Treatment of Benign Prostatic Hyperplasia; Rev Urol. 2005; 7(Suppl 4): S42–S48.
CrossRef - David Wofsy, Jan L. Hillson, and Betty Diamond; Comparison of Alternative Primary Outcome Measures for Use in Lupus Nephritis Clinical Trials; Arthritis Rheum. 2013 Jun; 65(6): 1586–1591.
CrossRef - F M Debruyne 1, A Jardin, D Colloi, L Resel, W P Witjes, M C Delauche-Cavallier, C McCarthy, C Geffriaud-Ricouard; Sustained-release alfuzosin, finasteride and the combination of both in the treatment of benign prostatic hyperplasia. European ALFIN Study Group; Eur Urol 1998 Sep;34(3):169-175
CrossRef - Hack-Lyoung Kim, Jae-Bin Seo, Woo-Young Chung, Sang-Hyun Kim, Myung-A Kim, and Joo-Hee Zo; The incidence and predictors of overall adverse effects caused by low dose amiodarone in real-world clinical practice; Korean J Intern Med. 2014 Sep; 29(5): 588–596.
CrossRef - Marie T. Brown, and Jennifer K. Bussell; Medication Adherence: WHO Cares?; Mayo Clin Proc. 2011 Apr; 86(4): 304–314.
CrossRef







