Giorgio Attinà1 , Raffaele Tepedino2 and Antonio Ruggiero1*
, Raffaele Tepedino2 and Antonio Ruggiero1*
1Pediatric Oncology Unit, Fondazione Policlinico Universitario A.Gemelli IRCCS, Universita’ Cattolica Sacro Cuore, Rome, Italy
2Medicine and Surgey Faculty. Università La Sapienza, Rome, Italy
Corresponding Author E-mail: antonio.ruggiero@unicatt.it
DOI : https://dx.doi.org/10.13005/bpj/2273
Abstract
Tumor lysis syndrome (TLS) can be a life-threatening complication that occurs following the onset of chemotherapy treatment, most commonly in association with high-grade lymphoproliferative pathologies such as acute lymphoblastic leukemia and Burkitt lymphoma. The massive cell lysis caused by cytotoxic therapy leads to the rapid release in the blood of intracelullary products and the onset of severe metabolic and electrolytic complications (hyperkalemia, hyperphosphatemia, hypocalcemia and hyperuricemia) upto the acute renal failure. This article describes the incidence and pathophysiological basis of TLS, focusing on the new therapeutic strategies implemented over the last few years, especially with regard to the treatment of hyperuricemia. In particular, it highlights the characteristics of a recent drug, Rasburicase, as a safe and effective alternative, compared to traditional allopurinol therapy, for prophylaxis and treatment of children with hyperuricemia induced by chemotherapy.
Keywords
Allopurinol; Hyperuricemia; Rasburicase; Tumor Lysis Syndrome
Download this article as:| Copy the following to cite this article: Attinà G, Tepedino R, Ruggiero A. Acute Tumor Lysis Syndrome: A Metabolic Emergency in Cancer Patients. Biomed Pharmacol J 2021;14(5). |
| Copy the following to cite this URL: Attinà G, Tepedino R, Ruggiero A. Acute Tumor Lysis Syndrome: A Metabolic Emergency in Cancer Patients. Biomed Pharmacol J 2021;14(5). Available from: https://bit.ly/3DkQS07 |
Introduction
Acute Tumor Lysis Syndrome (TLS) is a metabolic and electrolytic disorder due to a rapid increase in the blood of intracellular products, originating from massive lysis of cancer cells following the beginning of cytotoxic therapy. The release of intracellular products determines hyperkalemia, hyperphosphatemia, hypocalcemia and hyperuricemia with metabolic consequences on the normal homeostatic mechanisms, up to the acute renal failure 1. Therefore, TLS is a potentially lethal complication of chemotherapy, which requires proper prophylaxis and adequate treatment.
Epidemiology
TLS is commonly associated with high-grade lymphoprolifeative diseases particularly frequent in children, such as acute lymphoblastic leukemia and Burkitt lymphoma 1. It can be also a complication of chronic lymphatic leukemia, acute myeloid leukemia, multiple myeloma, and isolated plasmacytoma.
Some cases are reported in the course of other haematological disorders, such as non-Hodgkin lymphomas, Hodgkin lymphomas, chronic myeloid leukemia (in the phase of blastic crisis) and myeloproliferative disorders. TLS has been rarely observed in solid tumors characterized by a high degree of proliferation and high response to cytotoxic therapy, such as testicular cancer, breast cancer, and pulmonary microcytoma.
Physiopathology
Hyperkalemia
Hyperkalemia is the result of massive potassium release into the extracellular space because of cellular lysis. It can occur from 6 to 72 hours from the beginning of chemotherapy 2.
Some concomitant conditions such as acute renal failure, chronic renal disease, acidosis, or excessive iatrogenic administration of potassium during the induction phase of chemotherapy may contribute to exacerbating such electrolyte imbalance.
Clinical symptoms commonly include nausea, vomiting, diarrhea, and anorexia. In presence of severe hyperkalemia (>7 mmol/l) neuromuscular signs and symptoms may also appear, such as cramps, weakness, paresthesias, paralysis, and cardiovascular alterations, such as arrhythmias (tachycardia or ventricular fibrillation), electrocardiographic alterations (loosening of the QRS complex and pointed T-wave), syncope, and sudden death 3-7.
Hyperphosphatemia
Hyperphosphatemia can occur between 24 and 48 hours from the start of chemotherapy and can cause nausea, vomiting, diarrhea, lethargy, and seizures 2.
The evidence that cancer cells may contain up to 4 times more organic and inorganic phosphorus than non-malignant cells 8 favours its onset. In addition, acute renal insufficiency, particularly when associated with uric acid precipitation or other complications induced by chemotherapy, may contribute to a significant exacerbation of this imbalance 9,10.
Hypocalcemia
Hypocalcemia is generally due to precipitation of calcium phosphate crystals into the renal tubule because of hyperphosphatemia 8.
When the calcium-phosphorus product exceeds a value of 70, the risk of deposition of calcium phosphate at the level of the kidney and other organs becomes particularly high 8,11,12, resulting in metastatic calcifications. In particular, at the renal level it is possible to find clinical conditions ranging from the nephrocalcinosis to the nephrolithiasis up to the acute obstructive uropathy 3,4.
Hypocalcemia can lead to several complications: 1) musclular: cramps, spasms, paresthesias, and tetanus; 2) cardiovascular: ventricular arrhythmias, cardiac arrest, and hypotension; 3) neurological: confusion, delirium, hallucinations, and convulsions.
In addition, severe hypocalcemia can also lead to severe clinical manifestations, such as heart failure, coma and, rarely, death 3-5, 13-15.
Hyperuricemia
Hyperuricemia is the result of the rapid catabolism of intracellular nucleic acids released following the lysis of cancer cells and occurs, in general, between 48 and 72 hours from the beginning of therapy 2,16,17.
In TLS, hyperuricemia is severe, as the tumor cells have increased replicative activity and, therefore, a greater amount of nucleic acids.
Uric acid has a pKa of 5.4-5.7 and is slightly soluble in water. Its clearance occurs at the renal level and normally a quantity of 500 mg/day is excreted. At normal concentration and physiological pH, more than 99% of uric acid is present in ionised form 13. In the presence of acidic pH and a high concentration, however, uric acid precipitates at the tubular level in the form of crystals, resulting in obstruction, development of acute obstructive uropathy and renal dysfunction 3.4.
Obstructive uropathy is responsible for several clinical manifestations, such as hematuria, flank pain, hypertension, hyperazotemia, acidosis, edema, oliguria, anuria, lethargy, and drowsiness 4,5.
Uremia
Uremia is a clinical syndrome that follows the acute renal failure caused by metabolic and electrolytic alterations of the TLS. It is a multifactorial process that recognizes different causes, such as the formation and deposition of uric acid crystals into the renal tubules, the deposition of calcium phosphate crystals and/or xanthine crystals, the tumor infiltration of the kidney, ureteral obstruction, the use of nephrotoxic drugs, and acute sepsis 8,18-20.
The clinical manifestations are related to the renal failure and include nausea, vomiting, oliguria, anuria, edema, hypertension, congestive heart failure, metabolic disorders, exacerbation of hyperphosphatemia and hyperkalemia, severe hematuria and acidosis, up to the impairment of the state of consciousness with confusion, drowsiness, lethargy, convulsions, and coma 3,4,5.
Risk Factors
TLS risk factors belong to two categories: a) pre-existing risk factors such as reduced urinary flow, pre-existing hyperuricemia, dehydration, low urinary pH, and kidney failure (8,19); b) tumour-related risk factors, including tumour volume, proliferative rate, kidney tumor infiltration, chemotherapy sensitivity, and LDH level 1,21.
Prevention and Treatment
Preventive strategies
The implementation of strategies to prevent and/or reduce the severity of clinical manifestations of the TLS plays a crucial role. Patients at high risk of developing TLS or suffering from TLS, during the first 48-72 hours of chemotherapy, should be subjected to close monitoring of the following laboratory parameters: the assessment, every 4-6 hours, of serum electrolyte levels, of uric acid, creatinine and urea nitrogen, could allow a rapid correction of electrolyte disorders, reducing the risk of fatal complications. It would also be appropriate to correct hypovolemia if present, avoid the administration of nephrotoxic drugs and agents able to block the tubular reabsorption of uric acid. These measures are accompanied by other important prophylactic and therapeutic measures described below.
Hyperhydration
Hydration is recommended for all patients at risk of TLS : the decrease of serum concentration of uric acid, phosphate and potassium and the increase of urinary flow, with reduced concentration of solutes in the renal tubules, decrease the formation and precipitation of the crystals of urate and calcium phosphate 22-26.
Hydration should start immediately following the patient’s admittance to the ward and continued up to 2-3 days after chemotherapy. It is important that the fluids reserved for hydration do not contain potassium, calcium and phosphate 3,4,27.
Patients should receive hyperhydration of at least 3 l/m2/day (200 ml/kg/day in children up to a weigh of 10 kg) and maintain a urinary flow of 100 ml/m2/hour (3 ml/kg/hour in children up to a weight of 10 kg), with a specific urine weight at ≤1,010. In patients without acute obstructive uropathy and/or hypovolemia, diuretics may also be required (mannitol with a dose of 0,5mg/kg or furosemide with a dose of 0,5-1 mg/kg)(27-30).
Alkalinization of urine
Alkalinization of urine has been recommended for prevention and/or treatment by TLS, based on the evidence that alkaline urine facilitates the excretion of uric acid released during TLS. However, nowdays the use of this practice is controversial for two reasons:
Alkaline urinary pH promotes precipitation of calcium phosphate at the renal level and other organs, increasing the risk of renal insufficiency;
Alkalinization also significantly reduces the solubility of xanthine, facilitating the formation and precipitation of xanthine crystals, with consequent increased risk of kidney failure.
Hyperkalemia
Based on the serum potassium level, 2 therapeutic approaches are suggested:
asymptomatic and moderate hyperkalemia (≥ 6 mmol/l): avoid the administration of potassium, both intravenously and orally; monitor the patient through the execution of ECG control. The treatment includes the administration of sodium sulfonate polystyrene (1g/kg with sorbitol 50%) for oral administration or endorectally. However, not having an immediate effect, should not be used in patients with severe arrhythmia;
symptomatic and/or severe hyperkalemia (> 7 mmol/l): administer calcium gluconate (100-200 mg/kg iv) and/or regular insulin (0,1 U/kg iv) + dextrose 25% (2 ml/kg iv) 25,31.
Calcium gluconate allows stabilization of the heart cell membrane, preventing arrhythmias and, due to its short half-life, should be repeated at the need. Insulin, on the other hand, promotes the transition of potassium from the extra-to the intracellular space and is administered together with dextrose to prevent a hypoglycemic status 5,32.
Sodium bicarbonate might also be used as, in addition to having a mechanism of action similar to insulin, corrects metabolic acidosis. In patients with kidney damage should be administered cautiously for the risk of inappropriate volume expansion. More aggressive treatments, such as dialysis, are performed in patients with acute renal failure 22,33.
Hyperphosphatemia
Hyperphosphatemia is defined by phosphorus values of ≥2,1 mmol/l in children and ≥ 1,45 mmol/l in adults.
In presence of moderate hyperphosphatemia it is important not to infuse phosphate iv and the treatment of choice provides for the administration of phosphorus chelating agents, such as aluminum hydroxide, which can be taken through nasogastric tube feeding at a dose of 15 ml every 6 hours (50-150 mg/kg/24 h). If phosphorus chelating agents do not control hyperphosphatemia, it can be necessary to utilize more aggressive treatments, such as peritoneal dialysis, hemodialysis or continuous veno-venous haemofiltration. Some authors have pointed out that phosphorus clearance is significantly better as a result of haemodialysis (9.8 mg/min) than continuous venous haematofiltration (1 mg/min) and peritoneal dialysis (0.3 mg/min) 4,34-40.
Hypocalcemia
Hypocalcemia is a metabolic disorder related to hyperphosphatemia causing the tissue precipitation of calcium phosphate. Hypocalcemia is defined as a total serum calcium concentration < 8.8 mg/dL (< 2.20 mmol/L) in the presence of normal plasma protein concentrations or as a serum ionized calcium concentration < 4.7 mg/dL (< 1.17 mmol/L).
No treatment is recommended for asymptomatic hypocalcemia. Following the appearance of the first symptoms, however, the patient must be treated with calcium gluconate, with a dose of 50-100 mg/kg iv. This treatment, however, could increase the risk of calcium phosphate deposition, resulting in acute obstructive uropathy (4).
Hyperuricemia
Hyperuricemia (defined for uric acid values ≥480 μmol/l) is an important risk factor for the formation and precipitation of urate crystals in the renal tubules. Therefore, it is fundamental that adequate hydration is performed. Serum levels of uric acid can also be reduced by inhibiting its formation, obtained by allopurinol, or by increasing its renal clearance by treatment with Rasburicase.
Allopurinol is an analogue of xanthine which prevents the formation of uric acid through the inhibition of xanthine oxidase (Fig.1) 41-43.
Therefore, the formation of new uric acid decreases and reduces the risk of obstructive uropathy 41,31.
It’s been available in oral form since 1966 and in intravenous form in the United States since 1999. The recommemded dose level is 200-400 mg/m2/day in adult patients and 200 mg/m2/day in children, divided in 1-3 doses, up to a maximum dose of 600 mg/day.
This type of treatment has important limitations:
slow start of action; the reduction of serum uric acid levels in patients in allopurinol therapy occurs after 2-3 days;
while preventing the new formation of uric acid, it does not reduce the levels of uric acid pre-existing at the beginning of therapy;
the dose should be reduced in patients with renal damage since oxypurinol remains active for 18-30 hours and being eliminated by the renal route may have a substantially increased half-life in patients with renal insufficiency 5,22;
leads to an increase in the precursors of uric acid, xanthine and hypoxanthin, favouring the development of urinary crystals and acute obstructive uropathy (42, 44-46);
increases the medullary toxicity of cyclophosphamide and reduces the degradation of 6-mercaptopurine and azathioprine. Therefore, during the concomitant treatment with allopurinol, it is recommended to reduce the dose of these drugs, in particular 6-mercaptopurine, by 50-70%. Allopurinol also interferes with the pharmacokinetics of other drugs, such as dicumarol, cyclosporine, and thiazide diuretics;
3% of patients develop hypersensitivity reactions to this drug, and in some patients a severe allergic reaction has also been described, such as Steven-Johnson syndrome.
An alternative therapeutic strategy to allopurinol involves the use of agents that, instead of inhibiting the formation of uric acid, facilitate kidney clearance, through conversion to more soluble products mediated by the urate oxidase.
In 1975 it was introduced in Europe as a prophylactic treatment of TLS, the oxidase urate not recombinant, an enzyme isolated from Aspergillus flavus and proved effective in reducing serum levels of uric acid 47-53.
Its use has, however, been limited by the high risk of anaphylaxis that the drug involved, although the introduction of glycolpolyethylene has reduced the incidence 50, 54.
For this reason, a form of recombinant oxidase urate has recently been developed, Rasburicase. It is an enzyme produced from a genetically modified strain of Saccharomyces cerevisiae, the use of which was approved in Europe in 2001.
Rasburicase catalyzes enzymatic oxidation of uric acid to allantoin, a product which, at the urinary level, is 5-10 times more soluble than uric acid (Fig. 1)55,56. Being a recombinant enzyme, it has a higher degree of purity, therefore higher specificity (> 50% compared to the native enzyme) and greater tolerability.
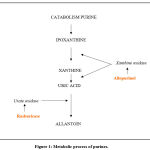 |
Figure 1: Metabolic process of purines. |
Being a recombinant enzyme, it has a higher degree of purity, therefore higher specificity (> 50% compared to the native enzyme) and greater tolerability.
Rasburicase should be administered immediately before and during the starting of chemotherapy. The recommended dose is 0.2 mg/kg/day. It is administered once a day by intravenous infusion of 30 minutes in 50 ml of 0.9% sodium chloride solution. The duration of treatment can vary between 5 and 7 days. However, recent studies have shown that low doses (0.05-0.20 mg/kg) and/or short-term treatments (1-3 days) could have the same efficacy of the dose and the original schedule, allowing, a better cost-benefit ratio through reduced costs and exposure to Rasburicase 57-62.
Rasburicase has a half-life of 19 hours and a clearance of about 3.5 ml/ kg/h. Its metabolism occurs by peptide hydrolysis, so liver failure does not influence the pharmacokinetics and in patients with renal insufficiency it is not necessary to reduce the dosage (57,58).
In addition, in a randomized phase III study, the efficacy of Rasburicase (0.20 mg/kg/day administered iv ) vs allopurinol (300 mg/m2/day for os in 3 doses) was compared in 52 pediatric patients with leukemia or lymphoma. The two-hypouricemizing agents were administered randomly during chemotherapy. It has been observed that, compared to allopurinol, Rasburicase has a greater effectiveness in the treatment of hyperuricemia (p<0,0001) and has a faster mechanism of action. In fact, after 4 hours from the first dose, plasmatic uric acid levels were reduced by 86% in patients treated with Rasburicase and by 12% in those treated with allopurinol (p<0,0001)(31). Sample size was too small to be able to determine a difference in the incidence of kidney failure. In the allopurinol-treated group, a 12-year-old patient with adavanced Burkitt lymphoma needed hemodialysis during the study period, while in the group treated with Rasburicase it has been documented that a 15-year-old patient with renal baseline insufficiency improved the renal conditions during the therapy, without the need for dialysis or haematofiltration. For a better evaluation of the relationship between renal function and treatment, were also analyzed levels of creatinine in hyperuricemic patients during the first 96 hours of therapy.
Patients treated with Rasburicase showed at baseline a creatinine value of 144% compared to the average defined by age/sex and following the first 4 days decreased to 102%; in the group treated with allopurinol, however, the values have worsened from 132% to 147%. Therefore, Rasburicase when compared to allopurinol allows not only a faster and more efficient control of uric acid levels, but also an important improvement of renal function. For this reason, alkalinization of urine is currently being discussed in patients undergoing this type of treatment, especially in relation to the complications it entails 63.
Finally, we recall that treatment with Rasburicase can cause fever, nausea, vomiting; diarrhea, and headache. Allergic side effects were also described, mainly rashes, bronchospasm ( <1%) and severe hypersensitivity reactions, including anaphylaxis ( <1%). In addition, in patients with glucose-6-phosphate dehydrogenase deficiency and with non-hereditary hemolytic anemia this drug is contraindicated, leading to an increased risk of hemolytic anemia or metahemoglobinemia 57,64-71.
Uremia and acute renal failure
The treatment of renal insufficiency associated with acute TLS requires a careful monitoring of fluids introduced, the control of hypertension, the correction of electrolytic alterations and the reduction of the dosage of drugs normally excreted by the renal route.
Sometimes, the response to the medical therapy is poor and it is necessary to adopt aggressive treatments, such as hemodialysis, peritoneal dialysis or continuous veno-venous haemofiltration. For uric acid clearance, the best results were reported by haemodialysis patients (70-145 ml/min) compared to those who performed peritoneal dialysis (15 ml/min) and continuous veno- venous haemafiltration (6,2 ml/min) (4).
Conclusions
TLS is a serious metabolic and electrolytic disorder that occurs during chemotherapy, resulting from the lysis of cancer cells. It is characterized by the presence of hyperkalemia, hyperphosphatemia, hypocalcemia, hyperuricemia and can lead to acute renal failure. Therefore, the identification of patients at risk, the development of preventive strategies, the careful monitoring and the adequate correction of clinical and laboratory parameters play a crucial role, allowing to reducing the risk of fatal complications. In particular, hyperuricemia should be carefully treated adopting appropriate hydration and medical therapies such as allopurinol or rasburicase favouring the uric acid clearance and reducing the incidence of nephropathy.
Conflict Of Interest
The authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Funding Source
The authors received no specific funding for this work.
References
- Hande KR, Garrow GC. Acute tumor lysis syndrome in patients with high-grade non-Hodgkin’s lymphoma. Am J Med 1993; 94:133–139.
CrossRef - Flombaum CD. Metabolic emergencies in the cancer patient. Semin Oncol 2000;27:322–334.
- Adreoli SP, Clark JH, McGuire WA, Bergstein Purine excretion during tumor lysis in children with acute lymphocytic leukemia receiving allopurinol: relationship to acute renal failure. J Pediatr 1986; 109: 292–298.
CrossRef - Jones DP, Mahmoud H, Chesney RW. Tumor lysis syndrome: pathogenesis and management. Pediatr Nephrol 1995; 9; 206–212.
CrossRef - Jeha S. Tumor lysis syndrome. Semin Hematol 2001; 38: 4–8.
CrossRef - Ruggiero A, Maurizi P, Larocca LM, Arlotta A, Riccardi R. Childhood CD4+/CD56+ hematodermic neoplasm: case report and review of the literature. Haematologica 2006;91(12 Suppl):ECR48.
CrossRef - Cefalo MG, Ruggiero A, Maurizi P, Attinà G, Arlotta A, Riccardi R. Pharmacological management of chemotherapy-induced nausea and vomiting in children with cancer. J Chemother 2009;21(6):605-10.
CrossRef - Frei EI, Bentzel CJ, Rieselbach R, Block JB. Renal complications of neoplastic disease. J Chron Dis 1963;16: 757–776.
CrossRef - Vachvanichsanong P, Maipang M, Dissaneewate P, Wongchanchailert M, Laosombat V. Severe hyperphosphatemia following acute tumor lysis syndrome. Med Pediatr Oncol 1995; 2:, 63–66.
CrossRef - Ruggiero A, Ferrara P, Attinà G, Rizzo D, Riccardi R. Renal toxicity and chemotherapy in children with cancer. Br J Clin Pharmacol 2017;83(12):2605-2614.
CrossRef - Wechsler DS, Kastan MB, Fivush BA. Resolution of nephrocalcinosis associated with tumor lysis syndrome. Pediatr Hematol Oncol 1994; 11: 115–118
CrossRef - Zusman J, Brown DM, Nesbit ME. Hyperphosphatemia, hyperphosphaturia and hypocalcemia in acute lymphoblastic leukemia. NEJM 1973;289:1335–1340.
CrossRef - Ruggiero A, Cefalo MG, Coccia P, Mastrangelo S, Maurizi P, Riccardi R. The role of diet on the clinical pharmacology of oral antineoplastic agents. Eur J Clin Pharmacol 2012;68(2):115-122.
CrossRef - Attinà G, Ruggiero A, Maurizi P, Arlotta A, Chiaretti A, Riccardi R. Transdermal buprenorphine in children with cancer-related pain. Pediatr Blood Cancer 2009;52(1):125-7.
CrossRef - Triarico S, Rinninella E, Cintoni M, Capozza MA, Mastrangelo S, Mele MC, Ruggiero A. Impact of malnutrition on survival and infections among pediatric patients with cancer: a retrospective study. Eur Rev Med Pharmacol Sci 2019;23(3):1165-1175.
- Seegmiller JE, Laster L, Howell RR. Biochemistry of uric acid and its relationship to gout. NEJM 1963;268:712–716.
CrossRef - Van den Berghe G. Purine and pyrimidine metabolism between millennia: what has been accomplished, what has to be done? Adv Experiment Med Biol 2000;486:1–4.
CrossRef - Klinenberg JR, Goldfinger S, Seegmiller JE. The effectiveness of the xanthine oxidase inhibitor allopurinol in the treatment of gout. Ann Int Med 1965;62:638.
CrossRef - Tsokos GC, Balow JE, Spiegel RJ, Magrath IT. Renal and metabolic complications of undifferentiated and lymphoblastic lymphomas. Medicine 1981;60:218–229.
CrossRef - Bishop MR, Coccia PF. Tumor lysis syndrome. In: Clinical Oncology (ed. by Abeloff MD, Niederhuber JE, Armitage JO, Lichter AS), Churchill Livingstone, New York, 2000:750–754.
- Howard SC, Jones Jones, DP DP, Pui CH. The tumor lysis syndrome. NEJM 2011;364:1844–1854.
CrossRef - Lorigan PC, Woodings PL, Morgenstern GR, Scarffe JH. Tumour lysis syndrome, case report and review of the literature. Ann Oncol 1996;7:631–636.
CrossRef - Sondheimer JH, Migdal SD. Toxic nephropathies. Crit Care Clin 1987;3:883–907.
CrossRef - Rinninella E, Ruggiero A, Maurizi P, Triarico S, Cintoni M, Mele MC. Clinical tools to assess nutritional risk and malnutrition in hospitalized children and adolescents. Eur Rev Med Pharmacol Sci 2017;21(11):2690-2701.
- Sallan S. Management of acute tumor lysis syndrome. Semin Oncol 2001;28(suppl 5):9–12.
CrossRef - Razis E, Arlin Z, Ahmed T, et al. Incidence and treatment of tumour lysis syndrome in patients with acute leukaemia. Acta Haematol 1994;91:171–174.
CrossRef - Silverman P, Distelhorst CW. Metabolic emergencies in clinical oncology. Semin Oncol 1989;16:504–515.
- Ruggiero A, Triarico S, Trombatore G, Battista A, Dell’acqua F, Rizzari C, Riccardi R. Incidence, clinical features and management of hypersensitivity reactions to chemotherapeutic drugs in children with cancer. Eur J Clin Pharmacol 2013;69(10):1739-46.
CrossRef - Falsini B, Chiaretti A, Barone G, et al. Topical nerve growth factor as a visual rescue strategy in pediatric optic gliomas: a pilot study including electrophysiology. Neurorehabil Neural Repair 2011; 25: 512-520.
CrossRef - Iuvone L, Peruzzi L, Colosimo C, et al. Pretreatment neuropsychological deficits in children with brain tumors. NeuroOncol 2011;13(5):517-524
CrossRef - Goldman SC, Holcenberg JS, Finklestein JZ, et al. A randomized comparison between rasburicase and allopurinol in children with lymphoma or leukemia at high risk for tumor lysis. Blood 2001;97:2998–3003.
CrossRef - Altman A. Acute tumor lysis syndrome. Semin Oncol 2001; 28(suppl 5):3–8.
CrossRef - Sewani HH, Rabatin JT. Acute tumor lysis syndrome in a patient with mixed small cell and non-small cell tumor. Mayo Clinic Proc 2002;77:722–728.
CrossRef - Heney D, Essex-Cater A, Brocklebank JT, Bailey CC, Lewis I.J. Continuous arteriovenous haemofiltration in the treatment of tumour lysis syndrome. Pediatr Nephrol 1990; 4: 245–247.
CrossRef - Timeus F, Crescenzio N, Longoni D, et al. Paroxysmal nocturnal hemoglobinuria clones in children with acquired aplastic anemia: a multicentre study. PLOS One 2014;9(7):e101948
CrossRef - Sakarcan A, Quigley R. Hyperphosphatemia in tumor lysis syndrome: the role of hemodialysis and continuous veno-venous hemofiltration. Pediat Nephrol 1994;8:351–353.
CrossRef - Ferrara P, Marrone G, Emmanuele V, et al. Homotoxicological remedies versus desmopressin versus placebo in the treatment of enuresis: a randomised, double-blind, controlled trial. Pediatr Nephrol 2008;23(2):269-274.
CrossRef - Findakly D, Luther RD 3rd, Wang J. Tumor Lysis Syndrome in Solid Tumors: A Comprehensive Literature Review, New Insights, and Novel Strategies to Improve Outcomes. Cureus 2020;12(5):e8355.
CrossRef - Ruggiero A, Rizzo D, Trombatore G, Maurizi P, Riccardi R. The ability of mannitol to decrease cisplatin-induced nephrotoxicity in children: real or not?. Cancer Chemother Pharmacol 2016;77(1):19-26.
CrossRef - Chiaretti A, Ruggiero A, Barone G, et al. Propofol/alfentanil and propofol/ketamine procedural sedation in children with acute lymphoblastic leukaemia: safety, efficacy and their correlation with pain neuromediator expression. Eur J Cancer Care (Engl) 2010;19(2):212-220.
CrossRef - Krakoff IH, Meyer RL. Prevention of hyperuricemia in leukemia and lymphoma: use of allopurinol, a xanthine oxidase inhibitor. JAMA 1965;193:89–94.
CrossRef - Spector T. Inhibition of urate production by allopurinol. Biochem Pharmacol 1977;26: 355–358.
CrossRef - Skoczyńska M, Chowaniec M, Szymczak A, Langner-Hetmańczuk A, Maciążek-Chyra B, Wiland P. Pathophysiology of hyperuricemia and its clinical significance – a narrative review. Reumatologia 2020;58(5):312-323.
CrossRef - DeConti RC, Calabresi P. Use of allopurinol for prevention and control of hyperuricemia in patients with neoplastic disease. NEJM 1996;274:481–486.
CrossRef - Band PR, Silverberg DS, Henderson JF, Ulan RA, Wensel RH, Banerjee TK, Little AS. Xanthine nephropathy in a patient with lymphosarcoma treated with allopurinol. NEJM 1970;283:354–357.
CrossRef - Landgrebe AR, Nyhan WL, Coleman M. Urinary-tract stones resulting from the excretion of oxypurinol. NEJM 1975;292:626–627.
CrossRef - Patte C, Sakiroglu O, Sommelet D. European experience in the treatment of hyperuricemia. Semin Hematol 2001;38(suppl10):9–12.
CrossRef - Chiaretti A, Ruggiero A, Barbi E, et al. Comparison of propofol versus propofol-ketamine combination in pediatric oncologic procedures performed by non-anesthesiologists. Pediatr Blood Cancer 2011;57(7):1163-1167.
CrossRef - Rizzo D, Scalzone M, Ruggiero A, et al. Temozolomide in the treatment of newly diagnosed diffuse brainstem glioma in children: a broken promise?. J Chemother 2015;27(2):106-110.
CrossRef - Calvo Villas JM. Tumour lysis syndrome. Med Clin (Barc) 2019;152(10):397-404.
CrossRef - Riccardi A, Mazzarella G, Cefalo G, et al. Pharmacokinetics of Temozolomide given three times a day in pediatric and adult patients. Cancer Chemother Pharmacol 2003;52:459-464.
CrossRef - Falsini B, Ziccardi L, Lazzareschi I, et al. Longitudinal assessment of childhood optic gliomas: relationship between flicker visual evoked potentials and magnetic resonance imaging findings. J Neurooncol 2008;88:87-96.
CrossRef - Mika D, Ahmad S, Guruvayoorappan C. Tumour lysis syndrome: implications for cancer therapy. Asian Pac J Cancer Prev 2012;13(8):3555-60.
CrossRef - Rozenberg S, Koeger AC, Bourgeois P. Urate-oxydase for gouty arthritis in cardiac transplant recipients. J Rheumatol 1993;20:2171.
- Brogard JM, Coumaros D, Franckhauser J, Stahl A, Stahl J. Enzymatic uricolysis: a study of the effect of a fungal urateoxydase. Rev Eur Etud Clin Biol 1972;17:890–895.
- Alakel N, Middeke JM, Schetelig J, Bornhäuser M. Prevention and treatment of tumor lysis syndrome, and the efficacy and role of rasburicase. Onco Targets Ther 2017;10:597-605.
CrossRef - Elitek (rasburicase) [prescribing information]. New York, New York: Sanofi-Synthelabo Inc.; July 2002.
- Pui CH. Rasburicase: a potent uricolytic agent. Exp Opin Pharmacother 2002;3:433–452.
CrossRef - Benn CL, Dua P, Gurrell R, et al. Physiology of hyperuricemia and urate-lowering treatments. Front Med (Lausanne) 2018;5:160.
CrossRef - Perez-Ruiz F, Dalbeth N, Bardin T. A review of uric acid, crystal deposition disease, and gout. Adv Ther 2015;32:31–41.
CrossRef - Dincer HE, Dincer AP, Levinson DJ. Asymptomatic hyperuricemia: to treat or not to treat. Cleve Clin J Med 2002;69:600–602.
CrossRef - Chen C, Lü J-M, Yao Q. Hyperuricemia-related diseases and xanthine oxidoreductase (XOR) inhibitors : an overview. Med Sci Monit 2016;22:2501–2512.
CrossRef - Herbert LA, Leman J, Peterson JR. Studies of the mechanisms by which phosphate infusion lowers calcium concentration. J Clin Invest 1966; 45:1886-94.
CrossRef - Pui C, Mahmoud HH, Wiley JM, et al. Recombinant urate oxidase for the prophylaxis or treatment of hyperuricemia in patients with leukemia or lymphoma. J Clin Oncol 2001;19:697–704.
CrossRef - Ruggiero A, Rizzo D, Mastrangelo S, Battaglia D, Attinà G, Riccardi R. Interactions between antiepileptic and chemotherapeutic drugs in children with brain tumors: is it time to change treatment?. Pediatr Blood Cancer 2010;54(2):193-198.
CrossRef - Brant JM. Rasburicase: an innovative new treatment for hyperuricemia associated with tumor lysis syndrome. Clin J Oncol Nurs 2002;6:12–16.
CrossRef - Dinnel J, Moore BL, Skiver BM, Bose P. Rasburicase in the management of tumor lysis: an evidence-based review of its place in therapy. Core Evid 2015;10:23-38.
CrossRef - Lazzareschi I, Ruggiero A, Riccardi R, Attinà G, Colosimo C, Lasorella A. Hypersensitivity reactions to carboplatin in children. J Neurooncol 2002; 58:33-37.
CrossRef - Firwana BM, Hasan R, Hasan N, Alahdab F, Alnahhas I, Hasan S, Varon J. Tumor lysis syndrome: a systematic review of case series and case reports. Postgrad Med 2012;124(2):92-101.
CrossRef - Triarico S, Maurizi P, Mastrangelo S, Attinà G, Capozza MA, Ruggiero A. Improving the Brain Delivery of Chemotherapeutic Drugs in Childhood Brain Tumors. Cancers (Basel) 2019;11(6):824.
CrossRef - Wilson FP, Berns JS. Tumor lysis syndrome: new challenges and recent advances. Adv Chronic Kidney Dis 2014;21(1):18-26.
CrossRef








