Sachin Chaudhary1*, Abdel-Nasser El-Shorbagi1 , Ramesh Kumar Gupta2
, Ramesh Kumar Gupta2 and Amit Kumar3
and Amit Kumar3
1Department of Medicinal Chemistry, College of Pharmacy, University of Sharjah, Sharjah, United Arab Emirates, 27272
2Department of Pharmacology, Hygia Institute of Pharmaceutical Education and Research Lucknow, Uttar Pradesh, India, 226020
3School of Pharmaceutical Sciences, IIMT University, Meerut, Uttar Pradesh, India, 250001
Corresponding Author E-mail: schaudhary@sharjah.ac.ae
DOI : https://dx.doi.org/10.13005/bpj/2214
Abstract
The Covid-19 pandemic since 2019 has imparted a massive influence on the human life around the world, irrespective of all the precautionary measures followed worldwide it is strongly suggested that only the effective and safer vaccine can control this vicious pandemic. Nevertheless, the vaccine development strategies for Covid-19 was initiated firstly in china after the outbreak of Covid-19 and then globally after it was declared as pandemic by World Health Organization. Currently, numerous platforms have been designed for developing the most efficacious and safe vaccines designed by different technologies including protein subunit, viral vector, RNA, DNA, inactivated, and live attenuated approach. Here, this review will illustrates the detailed information on above mentioned Covid-19 vaccines development technologies, protocols and their clinical trial phase status. Additionally, this review also includes the details of vaccines failed to progress further.
Keywords
Clinical trial; Covid-19; Inactivated; Live attenuated; SARS-CoV-2; Vaccine
Download this article as:| Copy the following to cite this article: Chaudhary S, El-Shorbagi A. N, Gupta R. K, Kumar A. The Recent Updates on Approaches and Clinical Trials Status of Covid-19 Vaccines Developed Globally. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Chaudhary S, El-Shorbagi A. N, Gupta R. K, Kumar A. The Recent Updates on Approaches and Clinical Trials Status of Covid-19 Vaccines Developed Globally. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/3i7qu1l |
Introduction
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is a positive-strand RNA virus associated toβ-coronaviruses and are classified into four genera namely alpha-corona virus, beta-corona virus, gamma-corona virus and delta-corona virus of which alpha and beta-corona virus strains infect the mammals, gamma and delta-coronavirus infect birds and may also mammals.1-4 Since, 2019 after the outbreak of Covid-19 in China, this novel virus resulted in severe acute respiratory syndrome and induced nearly 38,20,290 confirmed deaths around the world as of 14June 2021.5-8This Covid-19 pandemic has proliferated everywhere social, psychological and economic disaster. The comprehensive projects whose implementation is a denoting challenge like lockdowns, reverse transcription polymerase chain reaction (RT-PCR) testing, isolation of infected patients, social distancing, mask wearing, sanitizing is mandatory to disrupt further escalation of infection.9-14 The non-stop spreading of Covid-19 virus united the global pharmaceutical companies, research industries, educational institutions to initiate and start the development of vaccines and therapeutics at the earliest since vaccines are the only effective mode to control the Covid-19 pandemic.15-18 Currently, numerous vaccines have granted approval for their emergency use internationally. However, admittance of the vaccine is very slow and it is for the wellbeing of human race that everyone gets vaccinated sooner.19-25The longer it takes to vaccinate the humans, more the Covid-19 virus will continue to spread and higher will be the risk of emerging new perilous variants of this virus resulting into greatest economic crises in the world.26-35
Vaccine Strategies
The researchers and scientists around the globe have made great efforts towards the evolution of the varieties of vaccines againstSARS-CoV-2 virus to fend off the pandemic (Figure 1). In the current scenario, until second week of May 2021 globally, 184 vaccines are in pre-clinical phase, 32 vaccines are in phase-I, 35 vaccines are in phase-II and 25 vaccines are in phase-III. As of now, regulatory authorities in different countries have approved 14 vaccines and 4vaccines have been drifted to phase-IV clinical trials.36-38 The enormous spectrum of vaccines approaches have been prospected like protein subunit, viral vector, RNA, DNA, inactivated, live attenuated (Table 1-6). However, considerable factors should be taken into account prior to comprehensive evolution of a vaccine of which the vaccine safety, efficacy, side effects are fundamental.39,40,52
 |
Figure 1: The current clinical trial phase status of vaccines globally. |
Table 1: The recent global framework of Covid-19 protein subunit vaccines, their merits and demerits.
| S.No | Vaccine Name | Clinical Phase | Country | Merits | Demerits |
| 1 | NVX-CoV2373 | III | USA | · Safe as viral protein is unable to induce infection. | · Impart inadequate cellular immunity. |
| 2 | ZF2001 | III | China | · Poor immunogenicity without adjuvants and require booster dose. | |
| 3 | VAT00002 | III | France | ||
| 4 | Finlay-FR-1 | III | Cuba | ||
| 5 | EpiVacCorona | III | Russia | ||
| 6 | Abdala | III | Cuba | ||
| 7 | SCB-2019 | II/III | Australia | ||
| 8 | UB-612 | II/III | USA | ||
| 9 | Plant based VLP | II/III | Canada | ||
| 10 | RBDBV | II | China | ||
| 11 | MVC-CoV1901 | II | Taiwan | ||
| 12 | KBP-201 | I/II | USA | ||
| 13 | BECOV2A | I/II | India | ||
| 14 | GBP510 | I/II | South Korea | ||
| 15 | COVAX-19 | I | Australia | ||
| 16 | Razi Cov-Pars | I | Iran | ||
| 17 | SpFN | I | USA |
Table 2: The recent global framework of Covid-19viral vector vaccines, their merits and demerits.
| S.No | Vaccine name | Clinical Phase | Country | Merits | Demerits |
| 1 | AZD1222 | IV | UK | · Fast to develop. | · Manufacturing process cost is high. |
| 2 | Ad5-nCoV | IV | China | · Invigorate humoral and cellular immunity. | · Recombinant virus may cause illness in patients with compromised immunity. |
| 3 | Sputnik | IV | Russia | · Need low temperature storage. | |
| 4 | Ad26.COV2.S | III | USA | ||
| 5 | GRAd-COV2 | II/III | Italy | ||
| 6 | Viral vector nasal | II | China | ||
| 7 | LIBR-100 | II | Israel | ||
| 8 | Covishield | II/III | India | ||
| 9 | hAD5-Covid-19 | I | USA | ||
| 10 | VXA-CoV2-1 | I | USA |
Table 3: The recent global framework of Covid-19RNA vaccines, their merits and demerits.
| S.No | Vaccine Name | Clinical phase | Country | Merits | Demerits |
| 1 | mRNA-1273 | IV | USA | · Triggers both humoral and cellular immunity. | · Limited immune response. |
| 2 | BNT162b1 | IV | UK | · Vaccine is delivered into cytosol of host cell preventing the risk of integration. | · Stability issues. |
| 3 | CVnCoV | III | Germany | · Needs advanced formulations. | |
| 4 | LUNAR-COV19 | II | USA | · Fast to develop and manufacture. | · Require cool temperature for storage. |
| 5 | ARCov | II | China | ||
| 6 | mRNA-1273.351 | I | USA |
Table 4: The recent global framework of Covid-19DNA vaccines, their merits and demerits.
| S.No | Vaccine Name | Clinical phase | Country | Merits | Demerits |
| 1 | ZyCoV-D | III | India | · Triggers B and T cells. | · Require advanced delivery devices. |
| 2 | AG0303-COVID19 | II/III | Japan | · Long self-life. | · Insertion of foreign DNA may integrate with recipient’s DNA causing mutagenesis. |
| 3 | INO-4800 | II/III | USA | · No risk of illness. | · Induce poor immune response. |
| 4 | GX-19 | I/II | South Korea | · Stable even at high temperature. | |
| 5 | Covid-eVax | I/II | Italy |
Table 5: The recent global framework of Covid-19inactivated vaccines, their merits and demerits.
| S.No | Vaccine Name | Clinical phase | Country | Merits | Demerits |
| 1 | CoronaVac | IV | China | · Fast to develop. | · Booster dose needed. |
| 2 | BBIBP-CorV | IV | China | · Stable and safe. | · Use of adjuvants may cause inflammatory disorders. |
| 3 | Inactivated vero cells | III | China | · Pre-existing technology track record. | · Require plenty of live virus and sophisticated proficiency for large-scale production. |
| 4 | Covaxin | III | India | ||
| 5 | Inactivated vero cells | III | China | ||
| 6 | QazCovid | III | Kazakhstan | ||
| 7 | COVIran Barekat | I | Iran | ||
| 8 | FAKHRAVAC (MIVAC) | I | Iran | ||
| 9 | MV-014-212 | I | USA |
Table 6: The recent global framework of Covid-19live attenuated vaccines, their merits and demerits.
| S.No | Vaccine name | Clinical phase | Country | Merits | Demerits |
| 1 | Codagenix | I | India | · Triggers body defense by inducing pattern recognition receptor system (PRRS) comprising B, CD4 and CD8 cells | · Risk of creating recombinants of viral strain after vaccination |
| 2 | MV-014-212 | I | USA | · Designed for ‘cold adaptive’ viral strains, reassortants and reverse genetics | · Risky to patients with compromised immunity |
| · Single dose without adjuvants is sufficient to trigger immunity |
Vaccine Development Approach
Protein Subunit Vaccine
The protein vector vaccine (Figure 2) is also termed as protein subunit vaccine (acellular vaccine), including antigens fragments of pathogen with substantial immunogenicity capable of revitalizing the immune response in the host resulting in minimizing the risk of adverse effects. However, the protein subunit vaccine exhibits low immunogenicity and requires auxiliary support of an adjuvant to potentiate the vaccine-induced immune responses.It is of various types containing viral or bacterial pathogen; polysaccharide vaccines contain chains of sugar molecules found in the cell wall of some bacterial strains. The viral surface protein vaccine is developed by recombinant DNA technology; bacterial protein vaccine is developed through the purification of whole pathogen preparations. In case of polysaccharide vaccine, growing bacteria in industrial bioreactor produce the polysaccharide, before splitting them open and harvesting the polysaccharide from their cell walls. For conjugate vaccines, the polysaccharide attached to a protein is prepared by growing bacteria in a different bioreactor, later proteins are harvested and chemically bonded to polysaccharide and rest of the vaccine components are added. The protein subunit vaccine for SARS-CoV-2 is viral surface protein, it can be grouped into recombinant spike-protein based vaccine (SPBV), receptor-binding domain based vaccine (RBDBV) and virus like particle based vaccine (VLPBV) and could be expressed in different insect cells, mammalian cells, yeast and plants. So far, S-protein of SARS-CoV-2 is the most suitable antigen to develop antibodies against the virus. The protein subunit vaccines already in use nowadays include hepatitis B, pneumococcal and meningococcal vaccine.41
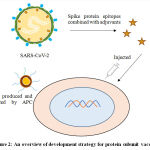 |
Figure 2: An overview of development strategy for protein subunit vaccine. |
Covid-19 Protein Subunit Vaccines
Novavax (United States of America)
The Novax-CoV2373 vaccine designed by using a nanoparticle technology to develop antigen from the spike protein of SARS-CoV-2. The clinical trial phase-II of this vaccine was started in August 2020 in South Africa and phase-III trials were initiated in United Kingdom in the month of September 2020 and in United States of America in December 2020. The results of phase-III clinical trial accomplished in United Kingdom demonstrated an efficacy rate of 89.3%.
Institute of Microbiology, Chinese Academy of Sciences and Anhui Zhifei Longcom Biopharmaceutical (China)
Anhui Zhifei Longcom Biopharmaceutical Company limited and the Chinese Academy of Sciences developed ZF2001 vaccine jointly. The vaccine has completed its phase-I and II clinical trial studies in October 2020 and is currently in final phase of clinical studies in Uzbekistan, Pakistan, and Indonesia. This vaccine has been approved for emergency use in Uzbekistan on 1 March 2021.
Sanofi Pasteur/GlaxoSmithKline (France)
Sanofi following the same approach used for Flublok (vaccine for influenza virus) develops VAT00002 vaccine forSARS-CoV-2. The phase-I/II clinical studies reports of December 2020 demonstrated that the old population is not responding firmly to this vaccine. The phase-II clinical studies of a different formulation of this vaccine were started in February 2021 and phase-IV is expected to be initiated in June 2021 if the results are promising.
Instituto Finlay De Vacunas (Cuba)
Finlay-FR-1 also known as Soberaba 01 is a protein subunit vaccine. In the month of January 2021, the institute had an agreement with Pasteur Institute of Iran for testing the vaccine for phase-III clinical studies.
Vector Vaccine (Russia)
The EpiVacCorona vaccine is designed in Russian Biological Research Center, Russia. It is under phase-III clinical trials; however, the president of Russia had permitted regulatory approval to this vaccine in October 2020. It is the second SARS-CoV-2 vaccines approved by Russian government.
The Center for Genetic Engineering and Biotechnology of Cuba (Cuba)
The phase-II clinical trials of Abdala vaccine have been initiated in February 2021 and are expected to start phase-III clinical studies on further 40,000 volunteers this year.
Clover Biopharmaceuticals (Australia)
The Clover Biopharmaceuticals (SCB-2019) is a protein based SARS-CoV-2, s-trimer vaccine designed by Clover. The company will start phase-II/III clinical studies in December 2020.
Covaxx (United States of America)
The United Biomedical (UB-612), Vaxxinity (formerly called as COVAXX) is a protein subunit vaccine planning to start phase-II/III clinical trials from February 2021 in Brazil.
Medicago (Canada)
It is one of the unique plant based virus like particle vaccine funded by Philip Morris. The species of tobacco plant has been used to design the vaccine, the viral genomes are transported into tobacco leaves and then plant cells design proteins that imitate those found on virus. Medicago has started phase-II/III clinical trials of this vaccine.
West China Hospital of Sichuan University (China)
It is protein subunit vaccine encoding receptor binding domain based vaccine (RBDBV) in a gene.
Medigen (Taiwan)
The Medigen Covid-19 is a protein subunit vaccine. The company has started phase-II clinical trials in January 2021 expecting to involve 3700 individuals ageing between 20 years and old.
Kentucky Bioprocessing (United States of America)
The Kentuchy Bioprocessing (KB-201) vaccine is similar to Medicago (plant based vaccine), designed by using genetically engineered Nicotiana benthamiana for making viral proteins. This company had utilized the same process earlier to design ZMapp drug for treatment of Ebola virus. The clinical phase-I/II trials for this vaccine have been registered in July 2020.
Biological E Limited (India)
The Biological E (BECOV2A) is a protein subunit vaccine, the clinical phase-I/II trials have been launched in November 2020 in India.
SK Bioscience Company Limited (South Korea)
The SK Bioscience company (GBP510) is a protein subunit vaccine; the company has started phase-I/II clinical trial studies recently in February 2021 in exceeding 200 healthy individuals.
Vaxine (Australia)
The South Australian based biotechnology company Vaxine, has designed COVAX-19 a protein subunit vaccine, containing SARS-CoV-2 proteins with adjuvants to stimulate immunogenic response in human body. The Phase-II clinical studies are expected to be initiated in 2021.
Razi Vaccine and Serum Research Institute (Iran)
Razi Cov-Pars is a protein subunit vaccine containing coronavirus like spike proteins. It is administered into the body in three dose, two injections and one nasal spray. It is first injectable inhaled SARS-CoV-2 vaccine.
Walter Reed Army Institute of Research (United States of America)
The Walter Reed Army Institute of Research at United States of America has designed a protein subunit vaccine (SpFN). The company has started phase-I clinical trialsof this vaccine candidate, Spike Ferritin Nanoparticle (SpFN). It is a nanoparticle vaccine against SARS-CoV-2 that deploys spike proteins with a liposomal formulation (ALFQ) adjuvant.
Covid-19 Viral Vector vaccine
The viral vector based vaccine (Figure 3) is different from conventional vaccines, as this type of vaccine does not actually contain antigen, but use body’s own cells to produce them. In virus vector vaccine are the genome of one virus is used to deliver the antigen of other virus, thus by infecting cells and instructing them to make antigens, which can trigger an immune response. This approach employs live (replicating but often attenuated) or non-replicating vectors like adenovirus, measles and many more. The replicating vector vaccines also produce new viral particles in the cells they infect, which then later infect new cells that will make the vaccine antigen. However, non-replicating vector vaccines are unable to make new viral particles; they only produce the vaccine antigen. Currently, the Covid-19 viral vector vaccines under developmentuse non-replicating viral vectors. Once injected into the body, these vaccine viruses begin infecting our cells and inserting their genetic material including the antigen gene into the cell nuclei. Human cells manufacture the antigen as if it was one of their own proteins and this is presented on their surface alongside many other proteins. When the immune cells detect the foreign antigen, they mount an immune response against it. This type of vaccine trigger the antibody producing B cells and cytotoxic T cells, which ultimately leads to the elimination of the viral infected cells. The viral vector vaccine currently in use is recombinant vesicular stomatitis virus-Zaire Ebola virus ( rVSV-ZEBOV) vaccine against Ebola virus.42-44
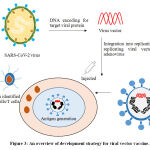 |
Figure 3: An overview of development strategy for viral vector vaccine.
Click here to view figure |
AstraZeneca/University of Oxford, United Kingdom
The University of Oxford United Kingdom develops Astra Zeneca Covid-19(AZD1222) vaccine; the efficacy of this vaccine has been estimate to be up to 90%. The government of United Kingdom, Argentina, India, Mexico, Brazil and European Medicines Agency (EMA) has certified and acknowledged its use for vaccination.The phase-III clinical trial of this vaccine was executed in exceeding ten thousand citizens (children/old) ofUnited Kingdom.The clinical trial phase-IV have been instituted in February 2021 in association with Ministry of the Interior and Health, Denmark.The government of Brazil, India, and United States has certified this vaccine.
CanSino Biologics Inc (China)
Thevaccine (Convidecia), developed by Cansino Biologics in collaboration with Academy of Military Medical Sciences China. The approach used to develop this vaccine is the same utilized for designing Ebola virus vaccine. The health ministry of Kingdom of Saudi Arabia (KSA) on 9 August 2020 authorized to start the phase-III clinical trial studies of this vaccine in their region.
Gamaleya Research Institute (Russia)
The SputnikV or Gam-Covid-Vac is a viral vector vaccine. The phase-III clinical trial studies of this vaccine was conducted in more than forty thousand individuals in Russia, Latin America and United Arab Emirates, the outcome revealed the vaccine efficacy scale of 91.6%. The government of India is planning to certify this vaccine for emergency use in their region.
Janssen/ Johnson & Johnson (United States of America)
The Janssen or Johnson & Johnson Covid-19 vaccine is a viral vector vaccine, the company Johnson & Johnson initially designed the vaccine for Ebola virus using recombinant Adenovirus serotype 26 (Ad26) and has now developed one more vaccine for SARS-CoV-2 virus. The clinical phase-III trial studies were initiated in September 2020 over 60,000 Latin Americans. The vaccine was found to have an efficacy rate of 66%.
ReiThera (Italy)
The ReiThera (GRAd-COV2) is a viral vector vaccine based on Adenovirus designed by ReiThera, an Italian company. The results of phase-I clinical trials in July 2020 expressed promising results in participants. The phase-II/III clinical studies have been launched recently.
Beijing Wantai Biological Pharmacy (China)
It is viral vector nasal spray vaccine using SARS-CoV-2 spike proteins. The phase-II clinical trials have been started in November 2020 involving 720 volunteers of Jiangsu province in China.
Israel Institute for Biological Research (Israel)
Iibr-100 is a viral vector vaccine and has started phase-I clinical studies in 1000 individuals, expected to reach phase-III clinical trials in 2021.
Serum Institute of India (India)
Covishield is a viral vector vaccine designed and developed by Serum Institute of India. It is recombinant, replication-deficient chimpanzee adenovirus vector encoding the SARS-CoV-2 Spike (S) glycoprotein. It is produced in genetically modified human embryonic kidney (HEK) 293 cells. The Indian government has authorized the use of this vaccine in the country.
ImmunityBio and Nantkwest (United States of America)
The ImmunityBio (hAd5) a viral vector vaccine, the company has started phase-I clinical trials in California population ageing up to 55 years. It is an adenovirus (Ad5) vaccine designed to deliver both spike protein and nucleocapsid DNA to trigger both cellular and antibody immunity.
Vaxart (United States of America)
The Vaxart (VXA) is a viral vector vaccine designed for oral administration using an adenovirus (Ad5) for delivering SARS-CoV-2 viral content into the body to drive immunity.
Covid-19 RNA Vaccine
The augmentation of mRNA synthesis, alteration, and delivery automation technique, provoked the research on mRNA in the consecutive 20 years. The mRNA is an arising, non-contagious, and a non-integrating scaffoldhaving minimal risk of mutagenesis. Nevertheless, mRNA vaccines (Figure 4) have the competency to establish vaccine discovery and development globally, and promote an acceleratedfeedback to appearingvirulentpandemics. The mRNA vaccine contains spike proteins of SARS-CoV-2 in a lipid polysaccharide nano shell. The ultimate target is to permit entering ofthese nano-particles containing mRNA into the body; later the cellular components of human body identify and translate the mRNA to initiate generation of spike proteins. Finally, the spike proteinsact as an antigen to promote the adaptive immune process in humans. Currently, the non-replicating conventional mRNA and the virus derived self-replicating RNA vaccines are being developed universally. The conventional mRNA vaccine contains the coding sequenceof the antigen of interest flanked by regulatory regions. The uppermostsuperiority of conventional mRNA vaccine approach are their untroublesome designing techniques and proportionately modest size of the RNA molecule. However, conventional mRNA in vivohas limited stability and efficacy because of disposition of cells to limit duration of expression. Additionally, optimization ofRNA structural elements and formulation technique can escalate antigen expression and stability. The self-amplifying mRNA vaccines are commonly based on theengineered RNA genome of single stranded RNA virus, such as alphavirus, flavivirus, and picornavirus. In overall scenario, mRNA mimics the replication process ofsinglestranded RNA viruses aiming to inflate the endurance and significance of expression process, in conjunction with subsequent immunogenicity of the encoded antigen. The outstanding self-amplified mRNA molecules have been derived from alphavirus genomes, like thoseof the Sindbis virus (SINV), Semliki Forest virus (SFV), and Venezuelan equine encephalitisvirus (VEEV). The development of RNA-based vaccines can also be achieved by employing negative-sense single-stranded RNA viruses, such as rhabdovirus andmeasles virus.45,46.
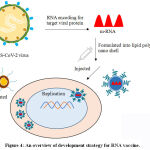 |
Figure 4: An overview of development strategy for RNA vaccine. |
Moderna
Moderna vaccine (mRNA-1273) developed in Cambridge, Massachusetts and financed by National Institute of Allergy and Infectious Diseases (NIAID), is mRNA vaccine to be reserved in ultra-cold deep freezer. The phase-III clinical trial studies of this vaccine performed in 30,000 health citizens of United States endorsed 94% of vaccine efficacy. The clinical trial phase-IV have been instituted in February 2021 in association with Ministry of the Interior and Health, Denmark.
Pfizer/BioNTech (Germany)
It is RNA based vaccine (BNT162b1), approved firstly by government of United Kingdom. The clinical trial-II/III was started in 27 July 2020 on 30,000 individuals of United States, Argentina, Brazil and Germany, additionally in October 2020 Pfizer and BioNTech initiated clinical phase-III studies in South Africa and data revealed the vaccine efficacy of 95%.
CureVac (Germany)
The clinical phase-III studies of CVnCoVhave been started in December 2020 involving 36,500 participants. The company CureVac in association with Elon Musk (owner of Tesla) is working to design “mRNA-factories” that could be delivered globally for developing billions of vaccine doses.
Arcturus/Duke-Nus (United States of America/Singapore)
The Arcturus (ARCT-021) is a RNA vaccine and phase-II clinical trials of this vaccine have been initiated in Singapore and United States of America.
Academy of Military Medical Sciences/Suzhou Abogen Biosciences/Walvax Biotechnology (China)
The Walvax (ARCoV) is a RNA vaccine developed in China. The vaccine is under clinical trials phase-II studies.
Moderna/National Institute of Allergies and Infectious Diseases (United States of America)
It is a RNA vaccine (mRNA-1273.351), this vaccines is designed to target SARS-CoV-2 B.1.351 variant identified first in South Africa.
Covid-19 DNA Vaccine
The utmostsubversiveexecutionofvaccine design and development is DNA vaccine (Figure 5) that drives adaptive immune response in humans. DNA vaccines are comparativelymanageable to develop and are moderately stable. DNA vaccines contain antigen-encoding genes inserted into a bacterial plasmid. Customarily, these bacterial plasmids contain mammalian expression promoters and the gene that encodes the spike protein, which is expressed in the individual after vaccination. The DNA vaccine stimulates humoral and cell mediated immunity response in the individual. The DNA plasmids in these vaccine containing the antigen is injected into the muscles, but the vital challenge is permitting the plasmid to cross the cellular membrane and reaching to cell nucleus to be translated into protein. Hence, DNA vaccination requires sophisticated delivery modules like electroporation and bio-injection.47,48
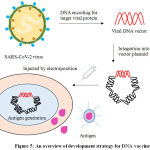 |
Figure 5: An overview of development strategy for DNA vaccine. |
Zydus Cadila (India)
The Cadila Healthcare (ZyCoV-D) vaccine is a DNA vaccine, the phase-III clinical trials of this vaccine is under initiation involving 300,000 candidates.
Osaka University/AnGes (Japan)
The company AnGes has designed(AG0303-Covid-19) vaccine in collaboration with Osaka University and Takara Bio. The phase-I trials have been started in June 2020 and phase-II/III was initiated in December last year.
Inovio Pharmaceuticals (United States of America)
The Inovio (INO-4800) is DNA vaccine, the clinical trials-II/III of this vaccine has started in population of United States, China and South Korea.
Genexine Consortium (South Korea)
The Genexine (GX-19)vaccine developed in joint collaboration with Genexine, Binex, GenNBio, International Vaccine Institute, Korea, Advanced Institute of Science and Technology and Pohang University of Science and Technology. The vaccine is heading towards phase-I/II clinical trials on 190 healthy individuals ageing between 18-50 years.
Takis and Rottapharm Biotech (Italy)
The Covid-eVax is DNA vaccine, the clinical phase-I/II trials have been started in February 2021. The vaccine must be administered by an intramuscular injection following electro-gene-transfer (EGT) procedure.
Covid-19 Live Attenuated vaccine
It is among the predominant traditional technique of developing a vaccine using whole virus to trigger an immunogenic response in an individual. Live attenuated vaccines (Figure 6) use an exhausted configuration of the virus, which can still flourish and reduplicate, but does not cause sickness. Attenuation can be executed by conforming the virus to unpromising status (like, growth at declined temperature, growth in non-human cells) or by rational modification of the virus (like, by codon de-optimization or by deleting genes that are responsible for counteracting innate immune recognition).Live attenuated vaccines are sometimes considered incompatible in people with immune compromised integral body system like HIV patients, pregnant females. Since, even a weakened virus may trigger the infection in these kinds of individuals. Additionally, rarely the live attenuated vaccines can revert to a more pathogenic form, triggering disease in vaccinated individuals or people in contact. This has been observed in oral polio vaccine containing the live attenuated polio virus, the immune system responds as it would to any other cellular invader, mobilizing a range of defense mechanism against it, including killer T cells (which identify and destroy infected cells), helper T cells (which support antibody production) and antibody-producing B cells (which target pathogens in the body). This immune response continues until the virus is cleared from the body, meaning there is plenty of time for development of memory cells against the virus. Because of this, live attenuated vaccines can trigger an immune response that is almost as good as being exposed to the virus, but without falling sick.49,50
 |
Figure 6: An overview of development strategy for live attenuated virusvaccine. |
Serum Institute of India (India)
The company has designed the second vaccine (Codagenix) forSARS-CoV-2 infection,it is live attenuated vaccine and has started phase-I clinical trials in January 2021.
Meissa Vaccines (United States of America)
The Meissa Vaccine (MV-014-212) is a live attenuated vaccine designed for intranasal mode of administration;it is an adjuvant free single dose vaccine.The clinical trials phase-I of this vaccine has been started in March 2021.
Covid-19Inactivated Vaccine
Vaccines based on killed microorganisms (inactivated vaccines) belong to a very traditional technological platform that has led to numerous vaccines. The inactivated vaccine (Figure 7) also contains virus or a part of it but their genetic material has been inactivated by chemical or physical methods. The vaccines produced using this method are more stable than live attenuated vaccines and can be given to people with compromised immune response. Even though the genetic material of the pathogen has been inactivated but still it contains many proteins to which the immune system can respond, inactivated vaccines only stimulate antibody-mediated response in the body. Hence, is mainly related to the short duration of immune memory, which demands inoculation of higher amounts of vaccine, booster doses or the association of the inactivated microorganism with an adjuvant to stimulate the immune system.1-5,51
 |
Figure 7: An overview of development strategy for inactivated virusvaccine. |
Sinovac (China)
CoronaVac is an inactivated vaccine developed by Sinovac, performed phase-III clinical trial studies in population of Brazil, Indonesia and Turkey. The government of China has authorized the emergency limited use of this vaccine in July 2020. The phase-IVclinical trial of this vaccine has started in recently in February 2021.
Beijing Institute of Biological Products (China)
Beijing Institute an integral organization of Sinopharm in teamwork with the Chinese Center of Disease Control and Prevention develops BBIBP-CorV. Recently, the phase-IVclinical trial of this vaccine has started.
Wuhan Institute of Biological Products (China)
It is an inactivated vaccine, the phase-IIIclinical trial studies of this vaccine showed 86% of efficacy. InJuly 2020, Wuhan Institute of Biological Products (China) in collaboration with Sinopharm initiated phase-III clinical trials in individuals of United Arab Emirates. In September 2021, United Arab Emirates turned into the first country around the globe to approve this vaccine for emergency use.
Bharat Biotech International Limited (India)
It is an inactivated vaccine (Covaxin) developed in alliance with National Institute of Virology (NIV) Pune, Indian Council of Medical Research (ICMR) New Delhi, and Bharat Biotech (Hyderabad). The clinical trial phase-III has been started across 25 centers in India and Indian government has authorized its use in vaccination programme across the country.
Chinese Academy of Medical Sciences (China)
It is an inactivated vaccine developed by Institute of Medical Biology at Chinese Academy of Medical Sciences, China. In the month of July 2020, clinical trial phase-II was initiated; later 29,000 volunteers participated in phase-III clinical studies. This institute has already developed polio and hand-foot-and-mouth vaccine.
Research Institute for Biological Safety Problems (Kazakhstan)
QazCovid vaccine is developed in Kazakhstan, the clinical phase-II studies were established in September 2020 and reported that vaccine produces maximal immune response in the participants. Recently the clinical phase-III trials have been started and vaccine is expected to be approved by March 2021.
Shenzhen Kangtai Biological Products/Beijing Minhai Biotechnology Company (China)
It is an inactivated vaccine developed by Shenzhen Kangtai Biological Products in partnership with AstraZeneca.
Shifa Pharmed Industrial Company (Iran)
COVIran Barekat vaccine is developed in Iran and clinical phase-I trails have been started in Tehran involving 56 volunteers.
Organization of Defensive Innovation and Research (Iran)
FAKHRAVAC (MIVAC) is an inactivated vaccine. It will be tested at two different dose strengths, each injected as part of a two-dose schedule two and three weeks apart. The clinical phase-I trials of this vaccine has been started in March 2021.
Vaccines Failed to Progress
V590 and V591 (Merck, United States of America)
In the Month of January 2021, Merck reported to stop developing SARS-CoV-2 vaccine candidates V590 and V591 (replicating viral vector vaccines). The company stated to accentuate its Covid-19 research methodologies and production competencies on advancing two other vaccines MK-4482 and MK-7110. The replicating viral vector vaccine candidates V590 and V591 in their clinical trial phase-I were evaluated to be well tolerated, but the immunogenic feedbacks were observed to be subordinate as compared to natural infection and other Covid-19 vaccines.35,36
COVAC1 (Imperial College London, United Kingdom)
COVAC1 is RNA vaccine candidate developed by using synthetic strands of SARS-CoV-2 genetic material (RNA) is a self-amplifying ribonucleic acid (saRNA) vaccine candidate. The basic background concept of this vaccine is similar to mRNA vaccine. However, saRNA are expected to make multiple duplications quicker resulting to produce more proteins.36,37
Covid-19-101 (Institut Pasteur, France)
The Institut Pasteur has developed a replicating viral vector vaccine candidate. The results of clinical trial phase-I obtained in August 2020, revealed that vaccine candidate was well tolerated, but the immune response developed was minor in people recovered from Covid-19 infection and other vaccines authorized globally.36
V451 (The University of Queensland, Australia)
The University of Queensland developed a protein subunit vaccine candidate (V451) for treating Covid-19 infection. The phase-I clinical studies represented that it obtained a powerful response against the virus by generating antibodies towards protein fragment (gp41) and has a good safety index. However, the Australian government decided to stop progress of vaccine to clinical trial phase-II/III because it showed HIV positive results in people involved in clinical phase-I.36
Conclusion
The voluminous research needs to be done and a lot is still to be learned about Covid-19 in particular, in addition to protective immunity induced by vaccines and miscellaneous vaccines is required for infants, children, pregnant ladies, immuno-deficient people in particular. Some reports expressed that innate immune response is also essential in inducing protection against Covid-19 in addition to adaptive immunity. The influential research targets the mechanism of genetic drivers of infection vaccine-induced humoral and cellular immunity to SARS-CoV-2, determining and understanding the precise targets of humoral and cellular immunity at epitope level, indicating the B-cell/T-cell receptors evoked by Covid-19 infection or post vaccination to verify the long term protective immune response in individuals.
SARS-CoV-2 has become a sizzling research field since the day it was announced as pandemic by World Health Organization. The researchers around the universe are collaborating for designing and developing innovative vaccines with pharmaceutical companies, medical agencies and educational institutions. The vaccine development protocols are very tedious involving several trials, including pre-clinical phase and clinical development. But, since during this Covid-19 pandemic the sufficient data is available so skipping few stages has been recommended to stimulate vaccine development technology with a quick regulatory review, approval, manufacturing and quality control.
Hence, ultimate fact is that, if Covid-19 remains dangerous to a single individual, it will remain dangerous to every human being. The strong collaborations and understanding must be developments through partnerships between pharmaceutical industries, government health regulatory organizations and international institutions for universal benefits of every individual in this world.
Acknowledgment
The work on this review was supported by University of Sharjah.
Conflict of interest
The authors declare that there is no conflict of interest in this review.
References
- Poland GA, Ovsyannikova IG, Kennedy RB. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet., 2020; 396(10262): 1595-1606.
CrossRef - Kaur SP, Gupta V. COVID-19 Vaccine: A comprehensive status report. Virus Res., 2020; 198114.
CrossRef - Amanat F, Krammer F. SARS-CoV-2 Vaccines: Status report. Immunity., 2020; 52(4): 583-589.
CrossRef - Ou X, Liu Y, Lei X, Li P, Mi D, Ren L, Li G, Chen T, Hu J, Xiang Z, Mu Z, Chen X, Chen J, Hu K, Jin Qi, Wang J, Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Commun.,2020; 11(1620): 1-12.
CrossRef - Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Mol. Immunol., 2020; 17: 613-620.
CrossRef - Erensoy S. SARS-CoV-2 and microbiological diagnostic dynamics in COVID-19 pandemic. Mikrobiyol Bul., 2020; 54(3): 497-509.
CrossRef - Coyle PK, Gocke A, Vignos M, Newsome SD. Vaccine considerations for multiple sclerosis in the COVID-19 era. Adv. Ther., 2021; June 1: 1-39.
CrossRef - Long QX, Tang XJ, Shi QL, Li Q, Deng HJ, Yuan J, Hu J, Xu W, Zhang Y, Lv FJ, Su K, Zhang F, Gong J, Wu B, Liu XM, Li JJ, Qiu JF, Chem J, Huang AL. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Med., 2020; 26:1200-1204.
CrossRef - Soleimanpour S, Yaghoubi A. COVID-19 vaccine: where are we now and where should we go? Expert Rev. Vaccines., 2021; 20(1): 23-44.
CrossRef - Lin F, Ichim TE, Pingle S, Jones LD, Kesari S, Ashili S. Mesenchymal stem cells as living anti-inflammatory therapy for COVID-19 related acute respiratory distress syndrome. World J. Stem Cells., 2020; 12(10): 1067-1079.
CrossRef - Channappanavar R, Fett C, Zhao J, Meyerholz DK, Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol., 2014; 88:11034-11044.
CrossRef - Lipsitch M, Grad YH, Sette A, Crotty S. Cross-reactive memory T cells and herd immunity to SARS-CoV-2. Nat. Rev. Immunol., 2020; 20(11): 709-713.
CrossRef - Tan M, Liu Y, Zhou R, Deng X, Li F, Liang K, Shi Y . Immunopathological characteristics of coronavirus disease 2019 cases in Guangzhou, China. Immunol., 2020; 160: 261-280.
CrossRef - Lucas C, Wong P, Klein J et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature, 2020; 584: 463-469.
CrossRef - Nigrovik PA. COVID-19 cytokine storm: what is in a name? Ann. Rheum. Dis., 2021; 80: 1-5.
CrossRef - Tian X, Li C, Huang A, Xia S, Lu S, Shi Z, Lu L, Jiang S, Yang Z, Wu Y, Ying T. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. MicrobesInfect., 2020; 9: 382-385.
CrossRef - Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virol. Sin., 2020; 35:266-271.
CrossRef - Arvin AM, Fink K, Schmid MA, Cathcart A, Spreafico R, Havenar-Daughton C, Lanzavecchia A, Corti D, Virgin HW. A perspective on potential antibody-dependent enhancement of SARS-CoV-2. Nature, 2020; 584: 353-363.
CrossRef - Guo YR, Cao QD, Hong ZS, Yuan-Yang T, Chen SD, Jin HJ, Tan KS, Wang DY, Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak- An update on the status. Mil. Med. Res., 2020, 7(11): 1-10.
CrossRef - Cui J, Li F, Shi ZL. Origin and evaluation of pathogenic coronoaviruses. Nat. Rev. Microbiol., 2019, 17(3): 181-192.
CrossRef - Shang J, Wan Y, Luo C, Geng Q, Auerbach A, Li F. Cell entry mechanisms of SARS-CoV-2. PNAS., 2020, 117: 11727-11734.
CrossRef - Salvatori G, Luberto L, Maffei M, Aurisicchio L, Roscilli G, Palombo F, Marra E. SARS-CoV-2 SPIKE PROTEIN: An optimal immunological target for vaccines. J. Transl Med.,2020, 18(222): 1-3.
CrossRef - Wan Y, Shang J, Graham R, Baric RS, Li F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol.,2020; 94(7): 1-9.
CrossRef - Jiang S. Don’t rush to deploy COVID-19 vaccines and drugs without sufficient safety guarantees. Nature, 2020; 579: 321.
CrossRef - Mantovani A, Netea M. Trained Innate Immunity, epigenetics and Covid-19. N. Engl. J. Med., 2020;383:1078-1080.
CrossRef - Khamsi R. If a coronavirus vaccine arrives, can the world make enough? Nature., 2020; 580: 578-580.
CrossRef - Dogra A, Goyal B, Sharma AM. Corona virus: A novel outbreak. Biomed. Pharmacol J., 2020; 13(1): 5-10.
CrossRef - Trivedi V, Biswas K, Fattepur S, Sreeharsha N. Study on genome sequence of novel coronavirus (SARS-CoV-2) strains in different countries. Biomed. Pharmacol J., 2020; 13(4): 2015-2024.
CrossRef - Arif TB. The 501.V2 and B.1.1.7 variants of coronavirus disease 2019 (COVID-19): A new time-bomb in the making? Infect. Control Hosp. Epidemiol., 2021; 1-2.
CrossRef - Cui J, Li F, Shi ZL. Origin and evaluation of pathogenic coronaviruses. Nat. Rev. Microbiol., 2019; 17(3): 181-192.
CrossRef - Srivastava S, Banu S, Singh P, Sowpati DT, Mishra RK. SARS-CoV-2 genomics: An Indian perspective on sequencing viral variants. J. Biosci, 2021, 46(1): 22.
CrossRef - Forni G, Mantovani A, COVID-19 vaccines: where we stand and challenges ahead. Cell Death Differ., 2021, 28; 626-639.
CrossRef - Lurie N, Saville M, Hatchett R, Halton J. Developing Covid-19 vaccines at pandemic speed. NEngl JMed, 2020; 382: 1969-1973.
CrossRef - Draft landscape of COVID-19 candidate vaccines. 2020.
CrossRef - Callaway E. The underdog coronavirus vaccines that the world will need if front runners stumble. Nature, 2020; 585: 332-333.
CrossRef - Gates B. When a COVID-19 vaccine is ready, this group will make sure the whole world can access it. Gates Foundation. 2020.
CrossRef - Ye T, Zhong Z, Garcia-Sastre A, Schotsaert M, De Geest BG. Current Status of COVID-19 (Pre)clinical vaccine development. Angewandte Chemie Int. Ed., 2020; 59: 18885-18897.
CrossRef - Strizova Z, Smetanova J, Bartunkova J, Milota T. Principles and Challenges in anti-COVID-19 vaccine development. Int. Ach. Allergy and Immunol., 2021; 182: 339-349.
CrossRef - Zheng J. SARS-CoV-2: An emerging coronavirus that causes a global threat. Int. J. Biol. Sci., 2020;16(10): 1678-1685.
CrossRef - Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat. Reviews Immunol., 2020; 20(10): 615-632.
CrossRef - Zhang L, Wang W, Wang S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines., 2015; 14(11): 1509-1523.
CrossRef - Tregoning JS, Brown ES, Cheeseman HM, Flight KE, Higham SL, Lemm NM, Pierce BF, Stirling DC, Wang Z, Pollock KM. Vaccine for COVID-19. Cli. Exp. Immunol., 2020; 202: 162-192.
CrossRef - Le TT, Andreadakis Z, Kumar A et al. The COVID-19 vaccine development landscape. Nature, 2020; 19: 306.
CrossRef - Dror AA, Eisenbach N, Talber S, Morozov NG, Mizrachi M, Zigron A, Srouji S, Sela E. Vaccine hesitancy: the next challenge in the fight against COVID-19. Eur. J. Epidemiol., 2020; 35: 775-779.
CrossRef - Woo PC, Huang Y, Lau SK, Yuen K. Coronavirus genomics and bioinformatics analysis. Viruses., 2010; 2(8): 1804-1820.
CrossRef - Moore JP, Klasse PJ. COVID-19 vaccines: “Warp Speed” needs mind melds, notwarped minds. J. Virol., 2020; 94(17): 1-32.
CrossRef - Krammer F. SARS-COV-2 vaccines in development. Nature, 2020; 586: 516-527.
CrossRef - Zhang J, Zeng H, Gu J, Li H, Zheng L, Zou Q. Progress and prospects on vaccine development against SARS-CoV-2. Vaccines, 2020; 8(153): 1-12.
CrossRef - Rosales-Mendoza S, Márquez-Escobar VA, González-Ortega O, Nieto-Gomez R, Arevalo-Villalobos JI. What does plant-based vaccine technology offer to the fight against COVID-19? Vaccines, 2020; 8(183): 1-19.
CrossRef - Haque A, Pant AB. Efforts at COVID-19 vaccine development: Challenges and successes. Vaccines, 2020; 8(739): 1-16.
CrossRef - Abdel-Alim, AAM, El-Shorbagi ANA, Abdel-Moty SG, Abdel-Allah HHM. Synthesis and anti-inflammatory testing of some new compounds incorporating 5-aminosalicylic acid (5-ASA) as potential prodrugs. Arch. Pharm. Res., 2005; 28 (6): 637-647.
CrossRef - Chaudhary S, Kumar S, Tarazi H. Peptide derivatives of 1, 2-dihydro-3-methyl-2-oxoquinoxaline-6-carboxylic acid: Synthesis and evaluation of antimicrobial, antifungal and antiviral potential. Pharm. Chem. J., 2016; 50(5): 331-338.
CrossRef








