S. Brigida  , Arul Amutha Elizabeth
, Arul Amutha Elizabeth , G. Soujania
, G. Soujania and R. Poornima
and R. Poornima
Department of Pharmacology, Sree Balaji Medical College and Hospital, CLC works road, Chrompet, Chennai – 600044, Tamil Nadu, India.
Corresponding Author E-mail: 1506dr.brigida@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2256
Abstract
Introduction: Superficial dermatophytosis is a common public health problem in India, due to its tropical climate with heat and humidity. Today, the triazoles, mainly Itraconazole and the allylamines, chiefly Terbinafine, are the main ammunitions against dermatophytes. This study is undertaken to compare the safety and efficacy of both the drugs. Materials and Methods: This study was conducted to find the efficacy of Oral Terbinafine and Oral Itraconazole in Tinea Corporis/Tinea Cruris infection. The primary efficacy parameter was change in composite score (pruritus, erythema, pigmentations) from baseline to end of the treatment period. And to compare the safety of Oral Terbinafine and Oral Itraconazole by comparing the following parameters, Liver enzymes - SGOT/SGPT before and after treatment with the study drugs. Drug Dosage: Group 1: Drug –Tab. Terbinafine: Dose 500 mg per day once daily at bedtime for 2 weeks. Group 2: Drug –Tab. Itraconazole: Dose 200 mg per day, once daily at bedtime for 2 weeks. Results: The study participants show significant reduction in itching at the second follow up (after 2 weeks of drug completion) in both groups. Pruritis was reduced in 92% subjects in group 1 and 97.5% subjects in group 2. There was 87% reduction in erythema in group 1 and 93% reduction in group 2. Pigmentations were seen in 2% subjects in both groups indicating relapse of infection. Conclusion: The significant outcome of the study was that oral Itraconazole 200mg/day for 14 days(2 weeks) can be the better antifungal.
Keywords
Itraconazole; Superficial dermatophytosis; Terbinafine; Tinea infections
Download this article as:| Copy the following to cite this article: Brigida S, Elizabeth A. A, Soujania G, Poornima R. A Comparative Study of Safety and Efficacy of Oral Terbinafine and Itraconazole in Patients of Tinea Corporis/ Tinea Cruris Infection, a Randomized Open Label Parallel Group Study . Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Brigida S, Elizabeth A. A, Soujania G, Poornima R. A Comparative Study of Safety and Efficacy of Oral Terbinafine and Itraconazole in Patients of Tinea Corporis/ Tinea Cruris Infection, a Randomized Open Label Parallel Group Study . Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/2UoYPAx |
Introduction
Superficial dermatophytosis affecting hair,skin and nail are common public health problem in India, because of the tropical climate with heat and humidity. Cutaneous dermatomycoses caused by filamentous keratinophilic fungi termed as dermatophytes and were classified majorly into three genera: Microsporum, Trichophyton and Epidermophyton. Of which,approximately thirty species of dermatophytes have been identified as pathogens for humans 1-2.
World Health Organization estimates that about twenty five percent of the world population were affected by dermatophytes. Among them, thirty to seventy percent of adults are asymptomatic carriers and that the incidence increases with age. The overall risk of acquiring dermatophytosis in one’s lifetime is estimated between 10 and 20 % the epidemiology and distribution of dermatophytosis are influenced by many factors including climate, race, sex, population migration, social practices and beliefs, host factors and agent factors 3.
Unfortunately, in this era, cure of dermatophytosis, which was once very easy and uncomplicated for dermatologists, has become a nightmare. This is due to relapses following irregular antifungal therapy, poor patient awareness, over the counter(OTC) drugs, misuse of oral antifungal, combination topical medicines with steroids and use of alternative treatment modalities. Hence, there is clinical resistance among patients with dermatophytosis.
Since it is the need of the hour to find out the therapeutic efficacy of the two most widely used oral antifungal drugs Itraconazole and Terbinafine. This study is undertaken to compare the safety and efficacy of both the drugs.
Materials and Methods
Study design
This is an open label, randomized, parallel study comparing Oral terbinafine and oral Itraconazole for efficacy and safety in patients with Tinea corporis / Tineacruris infections.
Eligibility criteria
Inclusion criteria
Patients with proven dermatophyte infection of skin – confirmed clinically and by scraping with KOH mount.
Patients without co morbidities and without concurrent medication which could potentially interact with Terbinafine and Itraconazole.
Exclusion criteria
Patients with dermatophyte infections elsewhere i.e. nail (Tinea unguium)/hair (Tinea capitis) / foot (Tinea pedis).
Patients who had been on oral treatment for dermatophytosis within last 1 month and applied topical treatment within the last 2 weeks.
Patients taking drugs which could potentially interact with Terbinafine and Itraconazole.
Patients who are having comorbid conditions such as chronic/ active liver disease/low renal function/ congestive heart failure/Diabetes Mellitus/Hypertension, which are contra indications, and which alters the time frame for treatment with Terbinafine/ Itraconazole.
Women who are lactating the baby and Pregnant.
Patients with underlying keratinization disorders / atopy/hypercortisolism which could complicate dermatophytosis.
Patients taking medications such as statins, astemizole, quinidine, terfenadine, ergot alkaloids anticoagulants were excluded.
Patients taking immunomodulatory, anti-retroviral, anti-depressants and anti-epileptics were not included in the study, unless they had been off the treatment for at least one month.
Withdrawal criteria
Adverse event which warrants withdrawal of subject.
Undue difficulty in obtaining blood sample.
Non-compliance with procedures.
It is in the best interest of the participant that he be withdrawn.
Withdrawal of consent for participation from the study by the subject.
Failure of the study drug to produce the desired effect.
Study procedure
This was started after obtaining approval from the (IEC) Institutional Ethics Committee of Sree Balaji Medical College and Hospital (Ref No:002/SBMC/IHEC/2016/205,dated 17.03.2016) Voluntary written informed consent was taken from all the study participants after explaining the clinical study protocol in detail about the risk and benefit. They were given adequate time to decide for their participation. The informed consent form was available in English as well as local language (Tamil).The Drug Therapy was given free of cost to the patients, till the end of treatment period and they were instructed to bring the empty blister pack, to check for compliance. They were given assurance that any withdrawal from the study would not affect their future treatment in the same hospital. The participants (study subjects) were selected based on the exclusion and inclusion criteria and were randomized with the help of a statistical software SPSS version 20 and allotted a treatment group. Each group had 50 patients. Baseline laboratory investigations were done before the onset of the study and participants received either one of the study drugs for a period of 2 weeks (14 days). The baseline features like demographic data, general, systemic examination were done. Contact numbers of the investigators and emergency physicians were provided to all the study participants for any queries during the study period and for reporting of any adverse events.
There were four scheduled visits during the study, baseline visit, after 1st week, 2nd week (end of treatment visit) then 4th week (follow up for KOH scraping).All cases of dermatophytosis of the skin, diagnosed clinically were recorded along with age, sex and duration of disease. Chronic and non-chronic cases were decided according to disease duration.
The patients who suffered from the disease for more than 24weeks (6 months), with remissions and exacerbations, with or without history of treatment, were taken as chronic cases.If the patient had been applying topical medication, it was stopped for at least 2 weeks and any antifungal systemic treatment was stopped at least 1 month prior to enrolling in the study.
Drug Dosage:Group 1: Drug –Tab. Terbinafine: Dose 500 mg per day once daily at bedtime for 2 weeks. Group 2: Drug – Tab. Itraconazole: Dose 200 mg per day, once daily at bedtime for 2 weeks.
Laboratory investigations
The following Laboratory investigations were done during screening i.e. baseline visit (“0” weeks) and at the end of study i.e. 2 weeks.Blood Biochemistry: Complete blood count,Random blood glucose (only at baseline visit),Repeated blood glucose for diabetics on follow up visits,HbA1C for Diabetics (only at baseline visit),Serum urea and creatinine,Liver function test, Serum glutamic oxaloacetic transaminase (SGOT),Serum glutamic pyruvic transaminase (SGPT).
Adverse Event Reporting
All the adverse events observed/complained by the study participants were reported in the case report form along with the other information about the severity such as mild, moderate or severe and any relation to the study medication. Any drastic change in the lab parameters from the initial screening values are also considered as adverse event and documented in the case report form.
Study endpoints
Primary outcome
The primary efficacy parameter was reduction in severity of lesion that is change in composite score (erythema, pruritus,vesicle desquamation) from baseline to end of the post treatment period is taken as the primary end point.
Composite Score
Clinical assessment was based on the proportion of patients with symptoms and signs of tinea lesions namely pruritus(itching) erythema (inflammation) vesicle & desquamation (pigmentation).
For each sign and symptom, it was rated as clinical score 0 to 3: 0: absent; 1: mild. 2: moderate; or 3: severe, for the above three target symptoms.
Effectiveness of the treatment was also assessed by the global clinical evaluation criteria; the clinical findings are rated as
Healed (absence of signs and symptoms),
Markedly improved (>50% clinical improvement),
Considerable residual lesions (< 50% clinical improvement),
No change,
Worse
The other efficacy parameter was negative KOH microscopic examination (considered clinical cure) at the end of post treatment period.
Secondary outcome
To compare the safety profile of Oral Terbinafine and Oral Itraconazole by comparing the following parameters, Liver enzymes – SGOT/SGPT before and after treatment with the study drugs.
Safety Endpoints
Adverse events
Occurrence of any adverse events including any significant lab parameter changes from baseline to the study medications will be recorded. The details of intensity, causal relationship to study drug with proper clinical examination will be documented.
Statistical Analysis
Data was entered into Microsoft excel data sheet and was analyzed using SPSS 20 version software. In the form of Frequencies and proportions categorial data were represented.
Descriptive statistics
Qualitative data significance was tested by using Chi square test.
Mean and standard deviation represent continuous data.
Inferential statistics
Independent t test was used to identify the mean difference between two quantitative variables and qualitative variables as test of significance, respectively.
Graphical representation of data
MS Excel and MS word was used to obtain various types of graphs such as bar diagram. Following were the observations
Results
Comparison of Basic Demographic Statistics
Table 1: Age distribution comparison between two groups (Original)
| Age of Individuals | Group | |||
| Terbinafine | Itraconazole | |||
| Count | % | Count | % | |
| <20 years | 4 | 8.0% | 11 | 22.0% |
| 21 to 30 years | 11 | 22.0% | 24 | 48.0% |
| 31 to 40 years | 15 | 30.0% | 12 | 24.0% |
| 41 to 50 years | 10 | 20.0% | 3 | 6.0% |
| >50 years | 10 | 20.0% | 0 | 0.0% |
Mean of subjects in Terbinafine group was 39.2 ± 13.2 and in Itraconazole group was 27.3 ± 8.3 years.
Majority of subjects in Group 1 were in the age group 31 to 40 years (30%), whereas majority of subjects in Group 2 were in the age group 21 to 30 years.
Table 2: Gender distribution of subjects (Original)
| Gender distribution | Group | |||
| Terbinafine | Itraconazole | |||
| Count | % | Count | % | |
| Female | 24 | 48.0% | 17 | 34.0% |
| Male | 26 | 52.0% | 33 | 66.0% |
Majority of subjects in both the groups were males – 52% in group 1 and 66% in group 2 respectively. Females numbered 48% in group 1 and 34% in group 2.
There was no significant difference in gender distribution between two groups.
Comparison of Baseline Composite Score Between Two Groups
Patients were followed at 1st week, 2nd week and 4th week (after the end of treatment). Clinicalscore and clinical response rates were done for clinical assessment.
The primary outcome of the study is as follows.
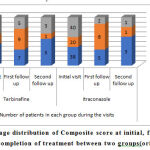 |
Figure 1: Percentage distribution of Composite score at initial, first Follow up and after completion of treatment between two groups(original). |
Table 3: Composite scores in GROUP 1 and GROUP 2(original)
| Group 1 n=50 | Group 2 n=50 | |||||||
| At baseline | At 1st week | At 2nd week | At 4th week | At baseline | At 1st week | At 2nd week | At 4th week | |
| Mean ± SD | 6.42 ± 1.55 | 5.90 ± 1.79 | 5.18 ± 1.85 | 1.28 ± 2.29 | 6.43±1.50 | 5.31 ± 1.92 | 3.21 ± 2.20 | 0.79 ± 1.76 |
| ZVALUE | 4.10 | 5.08 | 6.03 | 5.16 | 5.51 | 5.58 | ||
| PVALUE | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | ||
There was reduction in itching significantly at the second follow up (after 2 weeks of drug completion) in both the groups. Pruritis was reduced in 92% subjects in group 1 and 97.5% subjects in group 2. There was 87% reduction in inflammation (erythema) in group 1 and 93% reduction in group 2.
Pigmentations were seen in 2% subjects in both groups indicating relapse of infection.
The secondary outcome of the study is as follows.
Post-treatment LFT
Table 4: Post treatment LFT comparison between two groups (Original)
| Post treatment LFT | Number and percentage of patients in each Group | |||
| Terbinafine | Itraconazole | |||
| Normal | 47 | 94.0% | 49 | 98.0% |
| Abnormal | 3 | 6.0% | 1 | 2.0% |
In Group 1, 6% had an abnormal LFT.
In Group 2, 2% developed abnormal LFT after treatment.
Both results showed a transient raise in liver enzymes which returned to normal within 4 weeks of completion of treatment. This was not clinically significant.
Post treatment LFT was assessed in all patients and it was found that 3 patients in Group 1/ Terbinafine group and 1 patient in Group 2/ Itraconazole group had mildly elevated liver enzymes. These patients did not have history of liver disease/ did not take any medication that was hepatotoxic. The elevated levels returned to normal within 4 weeks of completion of treatment. Hence it was not significant.
Both terbinafine 1 and Itraconazole have propensity to cause hepatotoxicity but it is very rare [4]. These patients did not have any clinical symptoms or features of liver injury; therefore, the rise in enzymes was mild and transient.
Adverse events
Table 5: Adverse events comparison between two groups (Original).
| Adverse Events | Number and percentage of patients in each Group | |||
| Terbinafine | Itraconazole | |||
| Headache | 14 | 28.0% | 13 | 26.0% |
| GI Symptoms | 14 | 28.0% | 7 | 14.0% |
| None | 22 | 44.0% | 30 | 60.0% |
In Group 1, 28% developed headache, 28% developed GI symptoms and 44% had no adverse effects.
In Group 2, 26% developed headache, 14% developed GI symptoms and 60% had no adverse effect. This was not clinically significant. There was no discontinuation of treatment due to adverse effects. There was no significant difference in adverse event between two groups.
Discussion
Several RCTs support the efficacy of systemic antifungal drugs.There are not so many studies on comparison between Terbinafine and Itraconazole pulse therapy. Terbinafine has shown 90% cure rate at a dose of 250 mg/day when administered for 2 weeks. Off late many clinical failure and relapses were noticed due to terbinafine resistance 5-7.
Majid et al8 in their12 weeks study, 43 out of 100 tinea infected patients show relapse after a 2 week 250 mg oral terbinafine standard treatment. They found out that the incomplete mycological cure leads to relapse. One among the primary mechanisms of antifungal drug resistance is decrease in effective drug concentration, for terbinafine this standard dose of 250 mg extensively getting accumulated in skin and adipose tissue. Fungal resistance is aggravated due to increased and inappropriate use, over the counter sale, inappropriate prescription and hence the current standard terbinafine therapy with 250 mg/day dose is insufficient in current scenario. Although clear cut solution for this can’t be derived,appropriate prescription with correct dosing that is avoiding low dose antifungals can significantly contribute to this issue 9.
Similarly, for Cutaneous fungal infections, studies using Itraconazole 100 mg/day demonstrate that 2-week treatment courses generally produce clinical and mycological cure/marked improvement in 80% of patients with dermatophyte infections. complete healing (clinical cure and negative mycology) may be observed in approximately fifty to eighty percent of patients. Pulse that is short(7 days or 14 days) higher itraconazole dosages (200 or 400mg/day for a week) also produce same significant cure rates, but a more rapid response is seen and are also beneficial in dermatophytosis10.
Summary
This study has aimed to compare the efficacy of two widely used systemic antifungals- terbinafine and Itraconazole in tinea corporis and tinea cruris patients.
100 patients with clinically and mycological (KOH scraping) proven dermatophytosis of skin were randomly allocated into 2 groups –
Group 1 was treated with Oral Terbinafine 500 mg once daily for 2 weeks and Group 2 was treated with Oral Itraconazole 200mg once daily for 2 weeks.
Patients were regularly followed up and improvement was assessed using composite score. Patient compliance was noted, safety profile was investigated based on adverse events, pre- and post-treatment LFT.
The significant outcome of the study was that oral Itraconazole 200mg/day for 2 weeks was found to be the better antifungal. Drug-drug interactions with Itraconazole are witnessed in one patient. Careful patient selection, patient education and frequent monitoring can address this issue. Safety profile was similar in both groups with no major adverse events.
Conclusion
Overall, oral Itraconazole 200mg/day for 2 weeks proved to be a better agent with excellent and significantly better cure rates than oral Terbinafine 500mg/day for 2 weeks. With Itraconazole, the contra-indications, drug interactions must be kept in mind to prevent loss of efficacy/ potentially hazardous interactions. Both drugs had good safety profile and few minor adverse events.
Acknowledgement
Authors wish to thank the patients who gave their consent for participation and their adherence to their prescription and this prestigious institution for carrying out this study.
Conflicts of Interest
None
Funding source
None
References
- Havlickova B, Czaika VA, Friedrich M. Epidemiological trends in skin mycoses worldwide. Mycoses. 2008;51: 2-15.
CrossRef - Kannan P, Janaki C, Selvi GS. Prevalence of dermatophytes and other fungal agents isolated from clinical samples. Indian Journal of Medical Microbiology. 2006;24: 212.
CrossRef - Madhavi S, Rama Rao MV, Jyothsna K. Mycological study of Dermatophytosis in rural population. Ann Biol Res. 2011;2: 88-93.
- Del Rosso JQ. Oral Antifungals: What you should know about drug interactions. Podiatry Today. 2004;116: 61-5.
- Hemangi Ramesh Walke, Anil A Gaikwad, Shrikant S Palekar. Clinico-mycological profile of dermatophytosis in patients attending dermatology OPD in tertiary care hospital, India. Int.J.Curr. Microbiol and App. Sci 2014 ;3: 432- 440.
- Sahoo AK, Mahajan R. Management of tinea corporis, tinea cruris, and tinea pedis: A comprehensive review. Indian dermatology online journal. 2016; 7:77.
CrossRef - J,Fritsch P, PicotoW,et al., Short-term Itraconazole versus Terbinafine in the treatment of superficial dermatophytoses of the glaborous skin (tinea corporis or cruris).Eur J Dermatol 1997;7:353-7.
- Majid I, Sheikh G, Kanth F, Hakak R. Relapse after oral terbinafine therapy in dermatophytosis: A clinicaland mycological study. Indian J Dermatol2016; 61:529-33.
- Braun-Falco O, Plewig G, Wolff HH, Burgdorf WH. Fungal diseases. Dermatology, 2nd ed. Berlin, Germany: Springer-Verlag publishers; 2000.p.313-58.
- Gupta AK, Cooper EA. Update in antifungal therapy of dermatophytosis. Mycopathologia. 2008; 166:353-67.
CrossRef








