Dharmender Sharma1 and Gurinder Kaur Sangha2
and Gurinder Kaur Sangha2
1Department of Zoology, Akal University, Talwandi Sabo (Former PhD Scholar, PAU, Ludhiana) -151302, India.
2Department of Zoology, Punjab Agricultural University, Ludhiana-141004, India.
Corresponding Author E-mail: dsk686@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2228
Abstract
Traditional therapeutic procedures using antioxidant-rich fruits and vegetables have been in vogue for the development of evidence-based biomarkers for assessing reproductive health. Present investigation was designed to study the antioxidative potential of broccoli sprouts aqueous extract (BE), against ovarian toxicity in female rats induced by triazophos (TZ). In the experimental setup, six groups of rats were formed; Control (group 1), BE (group 2), TZ (group 3), and also BE+TZ groups such as BE1 (group 4), BE2 (group 5) and BE3 (group 6) groups. Body weight was weekly recorded of all the rats, while vaginal smear was observed daily during 30 days experiment. After sacrifice, oxidative stress (OS) biomarkers levels viz; catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), glutathione peroxidase (GPx), glutathione-S-transferase (GST) and lipid peroxidation (LPO) were determined along with histopathological and apoptotic observation. Results revealed differentially modified changes in OS biomarkers as CAT, SOD, GR, GPx, and GST, while LPO levels were significantly improved with broccoli supplementation compared to TZ group rats. Plasma progesterone and estradiol levels were also restored along with improved ovarian histoarchitecture among all BE+TZ treated rats. Reduced apoptotic granulosa cells with reduced atresia and normal ovarian surface epithelium height were also observed with BE treatment. BE exerts multi-mechanistic protective effects against TZ induced ovarian toxicity which is attributable to its antioxidant and protective actions.
Keywords
Apoptosis; Broccoli extract; Endogenous antioxidant; LPO; Ovary; Triazophos
Download this article as:| Copy the following to cite this article: Sharma D, Sangha G. K. Ameliorative Potential of Aqueous Extract of Broccoli Sprouts Against Triazophos Induced Ovarian Toxicity in Wistar Rats. Biomed Pharmacol J 2021;14(3). |
| Copy the following to cite this URL: Sharma D, Sangha G. K. Ameliorative Potential of Aqueous Extract of Broccoli Sprouts Against Triazophos Induced Ovarian Toxicity in Wistar Rats. Biomed Pharmacol J 2021;14(3). Available from: https://bit.ly/2UfxAIx |
Introduction
Organophosphorus (OP) pesticides are the most widely used insecticides globally for pest control and their exposure is a major threat to health care and general safety from toxicity1,2. OPs induces neurological disorders, and also act as endocrine disrupters (EDC), which have the potential to alter the normal functioning and development of the female reproductive system3,4,5. Organophosphorus pesticides exposure cause elevated generation of reactive oxygen species (ROS) and ultimately induces toxicity conditions in terms of oxidative stress (OS) in animals6,7. Endogenous antioxidants act as stress biomarkers and play an important role in female reproductive activities, as there abnormal activity levels have been found to influence female subfertility or infertility1,2. Further, supplementation of dietary antioxidants has been indicated as a possible therapeutic strategy for treating reproductive disfuntions and infertility related disorders by controlling ROS production and oxidative stress8.
Triazophos (TZ), is a broad spectrum OP insecticide and humans are exposed to TZ by number of food products including vegetables, drinking water, etc.9,10 and recent literature states that due to its high stability, TZ accumulates in aquatic and other soil ecosystems, as a potential hazard 11,12. TZ has been reported to induce the ovarian and reproductive toxicity2, and also its in utero and lactational exposure has been evidenced to induce the reproductive system abnormalities in offsprings of Rattus norvegicus13.
Natural plant products are rich in antioxidants and are traditionally in practice since long to strengthen the natural immunity. Further ameliorative studies with antioxidant-rich cruciferous vegetables, which contains pharmacologically active substances such as polyphenols, glucosinolates, vitamin C and flavonoids, has protective potential and stimulates defense mechanisms14,15,16,17. There are number of reports suggesting protective effects of natural antioxidants against different classes of pesticides and other environmental contaminants on reproductive stress biomarkers and strengthening potential of fertility parameters14,18,19,20,21, but toxicokinetic assessment of oxidative stress induction in ovaries by OPs with laboratory animals, and reversal by aqueous broccoli extract are absent or limited. Therefore, the aim of this study was to assess the changes in ovarian stress biomarkers level, and to elucidate the ameliorative potential of broccoli sprouts extract against the TZ induced toxicity.
Materials and Methods
All the chemicals were purchased from SRL Pvt. Ltd, Sigma-Aldrich, SD Fine-Chem Ltd, or were either of high analytical grades. ELISA Kits for estimation of Estradiol and Progesterone hormones were purchased from Labor Diagnostika Nord, GmbH & Co. KG. Quantification Kit for apoptosis and necrosis was purchased from Biotium. Ashirwad Industries, Mohali, provided standard rat feed, while Triazophos as Truzo 40 EC was purchased from Meghmani Organics Limited, Chharodi, India.
Broccoli sample
Seeds of Brassica oleracea var. italica, were procured, grown and sprouts were harvested gently on 5 days and were further processed to a dry powdered form of broccoli extract (BE) as per Sharma and Sangha,22 protocol. Quantification of glucosinolates (GSLs), from the powder, was done by Moller et al.23, vitamin C by Adom et al.24, total polyphenols by Ainsworth and Gillespie25, and total flavonoids by Chang et al.26 with slight modifications. Total glucosinolates (GSLs) content was estimated for 200 mg of dried sample and GSLs were considered as a base concentration alongwith other antioxidants in the extract [Table 1]. For experimentation, three different doses of 10, 20 and 30 µmol w.r.t. GSLs were made from dry BE powder in distilled water for subsequent use in present investigation.
Table 1: Broccoli sprouts extract with glucosinolates and other components for dose formation.
| Components | Units/ g of dry BE powder | µ mol | 30 µ mol of GSLs | 20 µ mol of GSLs | 10 µ mol of GSLs |
| Vitamin C | 16.57 ± 1.45 µ mol | 16.57 | 4.569 | 3.046 | 1.523 |
| Total polyphenols | 23.82 ± 2.33 mg gallic acid equivalents | 0.14 | 0.039 | 0.026 | 0.013 |
| Total flavonoids | 0.045 ± 0.003 mg quercetin equivalents | 1.51*10-4 | 4.16*10-5 | 2.78*10-5 | 1.39*10-5 |
| Total glucosinolates | 108.80 ± 3.13 µ mol | 108.8 | 30 | 20 | 10 |
Values expressed as Mean ± SE. (GSLs: glucosinolates)
Animals and Experimental design
Female albino rats aging 9-12 weeks were obtained from the Department of Livestock Production and Management, GADVASU, Ludhiana. Two rats were housed in each polypropylene cages using bedding of paddy husk in the laboratory, where the optimized humidity of 55±5%, temperature around 25±2°C and a 12 to 12 hours light-dark cycle of photoperiod were maintained. Feed and water were provided to housed animals during experimentation and guidelines of CPCSEA, India were used for animal handling, while experiments were duly approved by the “Institutional Animal Ethics Committee (IAEC)”, GADVASU, Ludhiana (date: 06.08.2012 and letter no. 3901-35).
After ten days acclimatization, female rats were segregated into six groups with eight rats in each group as Group I Control rats; Group II as BE rats received 10 µmol of BE; Group III as TZ rats; Group IV as BE1 rats received TZ alongwith 10 µmol of BE; Group V as BE2 rats received TZ and 20 µmol of BE and Group VI as BE3 rats received TZ and 30 µmol of BE. TZ was given as 1/10th of LD50 i.e. 8.2 mg/ kg b.w. in olive oil, while control and BE rats were provided with equal volume of olive oil. Rats with BE supplementation and TZ treatment were collectively referred as Br+TZ rats. During 30 days oral intubation experiment, body weights were recorded weekly, while vaginal smear was observed daily to check the cyclicity of all the rats.
Organs weight and Body weight
After experimentation, female rats were mildly anesthetized using chloroform and their blood sample was collected from heart directly in heparinized vials for its further processing. After that blood was centrifuged for 15 minutes at 2300 r.p.m. and the supernatant as plasma was used for hormonal analysis. Subsequently, reproductive organs were excised, and weighed after clearing off the adhering tissue.
Biochemical and Histological studies
After dissection, whole ovary samples from each rat were homogenized in 0.1 M PBS (pH 7.4), centrifuged and the supernatant was used for the biochemical parameters which were assayed by standard methods. Total proteins was estimated by Lowry et all27; CAT (catalase) by Aebi28; SOD (Superoxide Dismutase) by Marklund and Marklund29; GST (glutathione-S-transferase) by Habig et al.30; GR (glutathione reductase) by Carlberg and Mannervik31; GPx (glutathione peroxidase) by Hafeman et al.32; LPO (Lipid peroxidation) by Stocks and Dormandy33. Progesterone and Estradiol levels of all rats were assessed by ELISA Kits.
Ovary samples from four rats were used to get granulosa cells smears for analysis of the apoptosis and necrosis study as per the standardized protocol by Sharma et al.2 with the help of fluorescent dyes reagents Annexin V (AV) and Ethidium Homodimer (EH) from Quantification Kit. Similarly, ethidium bromide and acridine orange were used in 0.1 M PBS and cells after one minute incubation, were washed with PBS and then smeared on a slide for observation under microscope. Photography was done by fluorescence Nikon ECLIPSE 80i microscope. Ovary tissues from four rats of each group were processed for 24 hours by placing in alcoholic Bouin’s fixative. Using graded series of alcohols ovarian tissues were dehydrated and then cleared using benzene. Subsequently embedded in the paraffin wax having melting point around 58-60°C. Routine laboratory microtome was used to get the 5µm thick sections and further tissue sections were stained by routine procedures with hematoxylin and eosin, and slides were studied and analyzed under OLYMPUS CH20i microscope for photographs.
Statistical Analysis
All values are represented as mean ± standard error of the mean (SEM). Statistical evaluation of collected information was done by one-way ANOVA on a computer by using CPCS1 to check statistical significance at P < 0.05.
Results
Non-significant changes in the final body weight and net body weight gain was observed in all the experimental group rats, but significant change in estrous cycle was observed along with decreased growth rate in TZ treated rats as compared to control rats at P<0.05 [Table 2]; [Table 3]. The number of estrous cycles was reduced significantly in TZ treated animals, and shorter estrus phase with prolonged diestrus was observed compared to control rats at P < 0.05. Estrus, diestrus, and proestrus were significantly improved in BE2 and BE3 rats, while diestrus index was decreased significantly in Br+TZ group rats at a dose-dependent manner of broccoli extract to TZ treated rats at P < 0.05 [Table 2]. There was no significant change observed in weight of ovary, oviduct, and vagina, while weight of uterus was slightly reduced in all Br+TZ group rats [Table 3].
Table 2: Effect of BE and TZ treatment on estrous cycle.
| Groups | No. of Cycles | Duration in days | Diestrus index | |||
| Estrus | Metestrus | Diestrus | Proestrus | |||
| Control | 6.01 ± 0.23 | 7.09 ± 0.21 | 5.77 ± 0.13 | 11.04 ± 0.67 | 5.67 ± 0.31 | 37.89 ± 1.21 |
| BE | 6.41 ± 0.35 | 7.23 ± 0.17 | 5.78 ± 0.11 | 10.87 ± 0.77 | 5.73 ± 0.23 | 36.67 ± 1.43 |
| TZ | 4.17 ± 0.11* | 5.21 ± 0.21* | 4.72 ± 0.31 | 16.13 ± 0.77* | 16.89 ± 0.67* | 54.67 ± 1.63* |
| BE1 | 5.67 ± 0.21^ | 6.15 ± 0.23 | 5.47 ± 0.11 | 13.67 ± 0.67 | 13.67 ± 0.47* | 46.78 ± 1.89*^ |
| BE2 | 5.89 ± 0.24^ | 6.67 ± 0.19^ | 5.56 ± 0.23 | 13.23 ± 0.21^ | 13.33 ± 0.23*^ | 44.97 ± 1.67*^ |
| BE3 | 6.21 ± 0.11^ | 6.89 ± 0.35^ | 5.67 ± 0.43 | 12.50 ± 0.69^ | 12.50 ± 0.69*^ | 41.78 ± 1.53^ |
Values expressed as Mean ± SE (n=8) (*Significant difference as compared to control; ^Significant difference as compared to TZ at P ≤ 0.05).
Table 3: Effect of BE and TZ treatment on body weight (g) and reproductive organs weight (g/100g b.w.).
| Parameters | Control | BE | TZ | BE1 | BE2 | BE3 |
| Initial b.w. (g) | 161.75 ± 4.19 | 162.50 ± 5.76 | 163.52 ± 4.57 | 160.75 ± 4.84 | 164.50 ± 3.87 | 164.75 ± 5.03 |
| Final b.w. (g) | 195.50 ± 4.15 | 196.75 ± 5.31 | 190.45 ± 3.43 | 187.72 ± 3.67 | 195.75 ± 3.21 | 193.50 ± 4.11 |
| Growth rate (g/week/100g b.w./rat) | 3.01 ± 0.15 | 3.03 ± 0.27 | 2.38 ± 0.35 | 2.43 ± 0.30 | 2.63 ± 0.14 | 2.47 ± 0.29 |
| Ovary | 0.017±0.001 | 0.016±0.001 | 0.018±0.001 | 0.016±0.001 | 0.017±0.000 | 0.016±0.001 |
| Oviduct | 0.007±0.000 | 0.007±0.001 | 0.005±0.000 | 0.006±0.000 | 0.006±0.000 | 0.006±0.000 |
| Uterus | 0.129±0.021 | 0.127±0.017 | 0.128±0.014 | 0.115±0.003 | 0.104±0.002 | 0.109±0.014 |
| Vagina | 0.064±0.005 | 0.071±0.003 | 0.068±0.005 | 0.065±0.005 | 0.065±0.004 | 0.066±0.005 |
Values expressed as Mean ± SE (n=8) (*Significant difference as compared to control; ^Significant difference as compared to TZ at P ≤ 0.05)
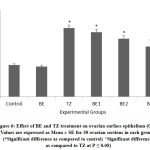
Altered activity levels of various antioxidative parameters were observed in all TZ and Br+TZ treated group rats [Table 4]. Proteins levels in the ovary were non-significant in all group rats. CAT activity was significantly increased with TZ treatment and was restored slightly in all Br+TZ group rats at P<0.05 [Table 4]. SOD activity levels were significantly decreased in TZ group rats and were restored significantly in all Br+TZ treated rats. Significantly increased GST activity levels were reported in TZ group rats, which were also improved significantly with broccoli extract supplementation in BE2 and BE3 group rats at P<0.05. Glutathione reductase activity was comparable, while GPx activity was significantly high and improved in BE3 group rats, as compared to control and TZ group rats at P<0.05 [Table 4].
Table 4: Effect of TZ and BE treatment on ovarian enzyme activity.
| Parameter | Control | BE | TZ | BE1 | BE2 | BE3 |
| Protein | 4.33±0.41 | 4.47±0.32 | 4.86±0.56 | 4.51±0.23 | 4.57±0.31 | 4.71±0.29 |
| CAT | 6.71±1.03 | 5.63±0.65 | 8.92±0.31* | 6.83±0.72 | 7.64±0.54 | 8.06±0.45 |
| SOD | 2.53±0.15 | 2.61±0.15 | 1.53±0.51* | 3.88±0.42*^ | 4.73±0.62*^ | 4.75±0.42 *^ |
| GST | 0.035±0.002 | 0.036±0.002 | 0.061±0.005* | 0.075±0.003* | 0.044±0.011^ | 0.039±0.006^ |
| GPx | 0.43±0.11 | 0.43±0.08 | 0.44±0.01 | 0.52±0.08 | 0.54±0.03 | 0.78±0.07 *^ |
| GR | 0.005±0.001 | 0.006±0.001 | 0.007±0.001 | 0.006±0.001 | 0.007±0.001 | 0.008±0.001 |
Units: Proteins (mg/100 mg tissue), CAT (µmole of H2O2 decomposed/min/mg protein), SOD (U/mg protein), GST (µmoles of GSH-CDNB conjugate formed/ min/mg protein), GR (µmoles of NADPH oxidized/ min/mg protein), GPx (U/mg protein).
Values expressed as Mean ± SE (n=8) (*Significant difference as compared to control; ^Significant difference as compared to TZ at P ≤ 0.05)
TZ caused significant increase in the ovarian MDA levels as a result of lipid peroxidation and was restored and improved significantly in all Br+TZ treated rats at P<0.05 [Figure 1]. Plasma estradiol levels were significantly increased and progesterone levels were significantly reduced in TZ group rats compared to control group rats at P<0.05. Further estradiol and progesterone levels were restored significantly in BE2 and BE3 experimental group rats as compared to TZ treated rats (P<0.05) [Figure 2]; [Figure 3].
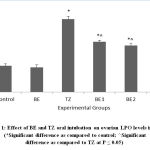 |
Figure 1: Effect of BE and TZ oral intubation on ovarian LPO levels in rats. (*Significant difference as compared to control; ^Significant difference as compared to TZ at P ≤ 0.05). |
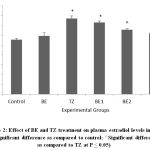 |
Figure 2: Effect of BE and TZ treatment on plasma estradiol levels in rats. (*Significant difference as compared to control; ^Significant difference as compared to TZ at P ≤ 0.05). |
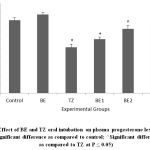 |
Figure 3: Effect of BE and TZ oral intubation on plasma progesterone levels in rats. (*Significant difference as compared to control; ^Significant difference as compared to TZ at P ≤ 0.05). |
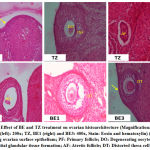
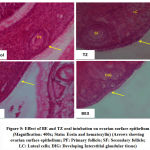
All phases of the follicular development were observed in the ovarian tissue sections [Figure 4]; [Table 5]. Increased follicular atresia was observed in TZ and BE1 group rats as compared to control rats. The follicular diameter of secondary and tertiary follicle was abnormal and reduced in TZ treated rats and all Br+TZ treated group rats [Table 5]. Ovarian interstitial glands were also observed in TZ, BE1, and BE2 group rats, while degenerating oocytes were observed in BE1 group rats and degenerating oocytes were numerous in TZ group rats [Figure 4]; [Table 5]. Control rats ovarian surface epithelium (OSE) was well organized with cuboidal cells single layer, while OSE height was increased TZ treated rats, and was also restored significantly in BE3 group rats compared to TZ group rats at P<0.05 [Figure 5]; [Figure 6]. Granulosa cells smear of ovarian tissues showed only a few apoptotic cells in control rats [Table 6] and gave green fluorescence with annexin V and also fewer necrotic cells, which gave red fluorescence with ethidium homodimer [Figure 6] were observed. Significant increase in the number of apoptotic and necrotic cells was observed in TZ group rats ovarian granulosa cells, while reduced apoptotic and necrotic cells were noticed in all Br+TZ experimental group rats [Table 6]. Also, more live cells in all Br+TZ group rats were observed, compared to TZ treated rats at P<0.05 [Table 6]; [Figure 7].
Table 5: Histopathological changes in ovary of TZ and BE treated rats.
| S. No. | Feature | Control | BE | TZ | BE1 | BE2 | BE3 |
| 1 | Follicular atresia | + | + | +++ | +++ | ++ | ++ |
| 2 | Degenerating oocyte | – | – | +++ | ++ | ++ | + |
| 3 | Apoptotic and necrotic granulosa cells | + | + | +++ | +++ | ++ | ++ |
| 4 | Increased height of ovarian surface epithelium | – | – | +++ | ++ | + | + |
| 5 | Ovarian interstitial glands development | – | – | ++ | + | + | – |
| 6 | Follicular diameter of Secondary and Tertiary follicle | N | N | ↓ed, +++ | ↓ed,++ | ↓ed, ++ | ↓ed, + |
– nil; + minimal (<10%); ++ mild (<25%); +++ moderate (<40%); N: normal; ↓ed: Reduced.
Table 6: Apoptosis and Necrosis observation in ovarian granulosa cells of TZ and BE treated rats
| Treatment | Control | BE | TZ | BE1 | BE2 | BE3 |
| Apoptotic Cells | 12.50 ± 1.14 | 12.37 ± 1.47 | 28.43 ± 3.23* | 27.71 ± 2.37* | 24.66 ± 2.95* | 23.13 ± 2.48* |
| Necrotic Cells | 25.50 ± 3.04 | 24.67 ± 3.54 | 67.50 ± 8.50* | 54.22 ± 5.47* | 50.57 ± 4.41*^ | 46.75 ± 4.71*^ |
| Apoptotic and Necrotic Cells | 6.50 ± 2.29 | 7.27 ± 1.27 | 20.70 ± 3.41* | 18.07 ± 1.54* | 16.67 ± 1.57* | 16.13 ± 1.05* |
All values are expressed as Mean ± SE from 4 rats in each group from smeared granulosa cells (200µl sample). (*Significant difference as compared to control; ^Significant difference as compared to TZ at P ≤ 0.05)
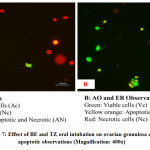 |
Figure 7: Effect of BE and TZ oral intubation on ovarian granulosa cells apoptotic observations (Magnification: 400x). |
Discussion
OPs are the most widely used pesticide of choice in agricultural practices due to their effectiveness and relatively low persistence34, and many of these tested pesticides have been proved more toxic to females of a species35. Apart from allergenic sensitization, pesticides toxicity can cause cancer, number of genetic disorders along with number of birth defects, sterility and miscarriage related issues can also develop in response to OS1,6,36,37. Broccoli and other cruciferous vegetables, contains pharmacologically active dietary antioxidants such as vitamin C, polyphenol-rich compounds, glucosinolates as the precursor of sulforaphane (SFN) and flavonoids, has protective potential and stimulates defence mechanisms16,38,39,40. The glucosinolates have been shown to provide protection both in in vitro and in vivo system from oxidative stress through scavenging ROS and have capability to minimize the risk of stress and diseased conditions by inducing endogenous antioxidant defenses41,42,43,44. Non-significant changes in body and organs weight are in agreement with literature, where pesticides toxicity related outcomes were not been found as potential threat to pose significant effects on body and organs weight of exposed animals45,46. The altered estrous cyclicity with prolonged diestrus phase and reduced estrus are correlated with secretion of estrogen and reduced secretion of gonadotropins, thus causing hormonal imbalance2. Similar observations for the number of reduced estrous cycles, and significant increase in the duration of diestrus and diestrus index were also reported in rats treated with organophosphate pesticides47,48 further consolidate our findings and their reversal in all Br+TZ rats can be corroborated to the antioxidative potential of broccoli sprouts. Similarly, daily intake of antioxidant-rich vegetables, fruits, legumes, and other plant products having high levels of antioxidants, has been confirmed to have positive effects on improving fertility potential by reducing the adverse effects of OS49,50 also consolidate our present findings.
Broccoli extract rich in glucosinolates such as sulforaphane and other antioxidants as vitamin C has an extraordinary ability to induce expression of several indirect enzymes via the KEAP1, Nrf2, ARE pathway, which play important role in toxicokinetics of xenobiotic substances51,52. Further, broccoli extract upregulates various Nrf2/ARE genes and forms phase II detoxification enzyme systems by causing their expression, and have protective affinity against the OS by maintaining redox homeostasis and activity of free radical scavengers53 and thus, can be exploited for strengthening immune defenses against pesticide induced toxicity. The activities of superoxide dismutase, catalase, and glutathione peroxidase constitute first line antioxidant defense system, which plays a major role in the total defense mechanisms in biological systems54. CAT activity was increased with TZ treatment and was restored slightly with BE supplementation may be assumed to block the free radical load55, while SOD activity increased significantly in all Br+TZ group rats, which may be due to SOD gene upregulation by antioxidants of broccoli sprouts, and supposed to counter the toxic effects of TZ. Similarly, phoxim and methomyl mixture has also been evidenced to induce reproductive toxicity through reduced SOD activity56 and intake of antioxidants rich white grape juice has been confirmed to improve metabolic status in women by increasing SOD levels, and has been attributed to reducing the risk of number of diseases57 also consolidate present findings. Further, the GST activity levels were altered, along with GPx levels and these were restored in all Br+TZ treated group rats. Studies have demonstrated that oleic acid as natural antioxidant contributes to the cellular antioxidant defenses and was observed beneficial against mitochondrial OS through cellular glutathione peroxidase activation and enhances clearance of ROS,58 which further strengthens our findings. Significant increase in ovarian MDA levels by TZ was also reversed significantly in all Br+TZ treated rats. Increased MDA levels following OPs exposure, has been linked to the increased production of ROS in ovaries and can induce OS,59,60 and TZ induced OS can be reversed with the application of glucosinolates rich broccoli extract. Further, the protective role of natural antioxidants have been evidenced in ovarian toxicity61 also supports our findings.
Most of the OPs act as a potential endocrine disrupter (EDC) and in severity can induce the developmental and other defects of female reproductive system2,3,62. Restored estradiol and progesterone levels in BE2 and BE3 group rats were reported compared to TZ rats. Similarly, the protective antioxidant properties of Pistacia lentiscus oil (PLO), on chlorpyrifos induced changes in the reproductive hormone levels of female rats, are in agreement with the present investigation, where progesterone levels were restored significantly20. TZ induced toxicity decreases progesterone levels in the plasma, probably by direct damage to the granulosa cells structure and function, while the formation of interstitial glands has induced the over-expression of genes responsible of elevated levels of estrogens. It has been observed that broccoli supplementation has the potential in ameliorating the TZ induced oxidative damage and thus consolidates its antioxidative properties against pesticides toxicity.
Physiologically, ROS levels fluctuates in different phases of ovarian cycle such as after preovulatory gonadotropin surge and during steroidogenesis, but are neutralized by regulatory endogenous or exogenous antioxidant molecules63,64 to maintain homeostasis, as it is a pre-requirement for assuring the regulated reproductive functions in a healthy organism36,65. OS characterized by the changes in antioxidants status or ROS scavenging enzymes, is detrimental as it can damage macromolecules such as nucleic acids, lipids, and proteins, which can threaten the integrity of cell membranes and cytosolic organelles1. Ovarian histoarchitecture observation showed restored features of different stages of follicles including follicular diameter, reduced follicular atresia and degenerating oocytes in all Br+TZ treated group rats. Recent findings has shown that subchronic exposure of OPs has the potential to accumulate and damage ovarian integrity and histoarchitecture2,66. Similarly increased ROS levels can bind with DNA genetic material and increase preapoptotic signals which subsequently induces externalization of phosphatidylserine (PS) along with elevated LPO levels in live cells in response to toxicants exposure63,67. These act as death signals and activates caspase cascade, which leads to cell death or apoptosis in granulosa cells68. Fluorescent-labeled annexin V used to visualize the externalization of PS on the granulosa cells2, and present investigation revealed reduced apoptotic and necrotic cells in all Br+TZ rats and further it has been attributed to the protective potential of BE. Similar observations with antioxidants Vitamins C and E supplementation has been reported, where decreased oxidative stress‐mediated granulosa cells apoptosis was observed due to its efficiency to diminish glyphosate‐induced oxidative stress and thereby, preventing associated fertility disorders14. These corroborate with our present findings and free radical-scavenging activity of BE protects the tissues from oxidative damage by restoring the levels of MDA, SOD, GPx, CAT, etc. which confirms its strong antioxidizing potential against TZ toxicity. TZ is thought to be estrogenic and is responsible for increased ovarian surface epithelium (OSE)2. OSE expresses estrogen receptors and also act as the site susceptible for ovarian cancer development47. Further, increased levels of estradiol and increased height of OSE in TZ were restored by the broccoli extract supplementation, which might have role in the down-regulation of bcl-2 gene expression and thus can play a protective role in ovarian cancer development in OSE cells, because OSE has been found associated with the development of number of ovarian cancers and its cystic derivatives69 and thus confirms antioxidative properties of broccoli sprouts.
Overall health, in females, is determined by the proper physiological functioning of ovaries and reproductive system. Similarly, ROS generated during ovarian physiological metabolism, and endogenous antioxidants maintain the balance between ROS generation and their clearance from living system8. Present study demonstrate that TZ alters the levels of endogenous stress biomarkers including reproductive hormones and also influences estrous cyclicity by causing substantial oxidative damage in the ovarian tissues. BE can ameliorate the detrimental OS biomarkers changes with reduced MDA levels and apoptotic granulosa cells along with improved histoarchitecture. Protective effects of BE is probably due to the antioxidant properties of major compounds of BE, such as glucosinolates, vitamin C, polyphenols, etc., which scavenge and helps in clearance of excessively produced free radicals and prevent the oxidative damage by TZ induced toxicity.
Conclusion
Aqueous extract of broccoli sprouts has strong antioxidant affinity to reverse the TZ toxic effects as BE increases cell viability by reducing ROS generation and suppresses granulosa cells apoptosis, by inhibition of lipid peroxidation and restoring estrogen/progesterone balance. Endogenous antioxidants play an important role in follicular growth, oocyte maturation, and cyclicity, and moreover scientific attention is required to elucidate the underlying molecular mechanisms for better understanding of the possible protective roles of natural antioxidants, which can be used for treating pesticides induced female infertility and associated disorders with in vivo antioxidant supplementation.
Acknowledgment
The design of the study was carried out by Dr D Sharma and Dr GK Sangha. The experiment was done by Dr D Sharma and the statistical analysis was carried out by Dr GK Sangha. The manuscript was written by Dr D Sharma and revised by both authors. All authors approved the final version. Authors are thankful to Head, Department of Zoology, PAU Ludhiana, Punjab (India) for providing financial assistance and infrastructure to carry out the research.
Conflict of interest
None
Funding Source
Not applicable
References
- Agrawal A and Sharma B. Pesticides induced oxidative stress in mammalian systems: Review Article. Int. J. Biol. Med. Res., 2010; 1(3):90-104.
- Sharma D, Sangha G. K and Khera K. S. Triazophos induced oxidative stress and histomorphological changes in ovary of female wistar rats. Pestic. Biochem. Physiol., 2015; 117:9-18.
CrossRef - Bhardwaj J. K, Mittal M, Saraf . and Kumari P. Pesticides induced oxidative stress and female infertility: a review. Toxin Reviews, 2018; DOI:10.1080/15569543.2018.1474926
CrossRef - Stamati P. N, Hans L and Howard C. V. Pesticides as endocrine disrupters: Identification of hazards for female reproductive functions. In: Reproductive Health Environment (Eds: N. Rexia and A. Mantovani). Vol. 2, Springer Verlag, Netherland; 2007; p. 227-248.
- Bretveld R. W, Thomas C. M, Scheepers P. T, Zielhuis G. A and Roeleveld N. Pesticide exposure: the hormonal function of the female reproductive system disrupted? Reprod Biol Endocrinol., 2006; 4:30.
CrossRef - Sharma D and Sangha G. K. Triazophos induced oxidative stress and histomorphological changes in liver and kidney of female albino rats. Pestic. Biochem. Physiol., 2014; 110:71-80.
CrossRef - Sharma D, Sangha G. K and Khera K. S. Triazophos induced oxidative stress in female albino rats. Int. J. Adv. Res., 2014; 2(1): 746-754.
- Wang S, He G, Chen M, Zuo T, Xu W and Liu X. The Role of Antioxidant Enzymes in the Ovaries. Oxidative Medicine Cellular Longevity, 2017; Article ID 4371714 doi.org/10.1155/2017/4371714
CrossRef - Li W, Qiu S and Wu Y. Triazophos residues and dissipation rates in wheat crops and soil. Ecotoxicol Environ Saf., 2008; 69(2): 312-16.
CrossRef - Rani S, Madan V. K and Kathpal T. S. Persistence and dissipation behavior of triazophos in canal water under Indian climatic conditions. Ecotoxicol. Environ. Saf., 2001; 50(1): 82-84.
CrossRef - Juan W, Feng Y, Dai Y, Cui N, Anderson B and Cheng S. Biological mechanisms associated with triazophos (TAP) removal by horizontal subsurface flow constructed wetlands (HSFCW). Science of The Total Environment., 2016; 553: 13-19.
CrossRef - Bajeer M. A, Mallah M. A, Sherazi S. T. H, Bhanger M. I and Nizamani S. M. Investigation of Dissipation, Adsorption, Degradation, and Leaching of Triazophos Pesticide in Various Soils. Polycyclic Aromatic Compounds., 2016; 36: 229-241.
CrossRef - Bhanot R and Sangha G. K. Effect of in utero and lactational exposure of triazophos on reproductive system functions in male offsprings, Rattus norvegicus. Drug Chemical Toxicol., 2018; DOI: 10.1080/01480545.2018.1457048
crossRef - Bhardwaj J. K, Mittal M and Saraf P. Effective attenuation of glyphosate‐induced oxidative stress and granulosa cell apoptosis by vitamins C and E in caprines. Mol. Reprod. Dev., 2019; 86: 42–52.
CrossRef - Sajeesh T, Arunachalam K and Parimelazhagan T. Antioxidant and anti-pyretic studies on Pothos scandens L. Asian Pac. J. Trop. Med., 2011; 4(11):889–899.
CrossRef - Chen Y. J. D, Conte J, Lin Y, Gemelli A, Castro A, Yarlagadda S, et al. Sulforaphane, an extract from broccoli, prevents skin cancer. J. Plast. Dermatol., 2008; 4:165–166.
- Lee J. Y, Hwang W. I and Lim S. T. Antioxidant and anticancer activities of organic extracts from Platycodon grandifl orum A. De Condole roots. J. Ethnopharmacol., 2004; 93: 409-15.
CrossRef - Estakhri M. A, Shokrzadeh M, Jaafari M. R, Karami M and Mohammadi H. R. Organ toxicity attenuation by nanomicelles containing curcuminoids: Comparing the protective effects on tissues oxidative damage induced by diazinon. Iran. J. Basic. Med. Sci., 2019; 22: 17-24.
- Mekircha F, Chebab S, Gabbianelli R and Leghouchi E. The possible ameliorative effect of Olea europaea L. oil against deltamethrin-induced oxidative stress and alterations of serum concentrations of thyroid and reproductive hormones in adult female rats. Ecotoxicol. Environmental Safety., 2018; 161: 374-382.
CrossRef - Chebab S, Mekircha F and Leghouchi E. Potential protective effect of Pistacia lentiscus oil against chlorpyrifos-induced hormonal changes and oxidative damage in ovaries and thyroid of female rats. Biomedicine & Pharmacotherapy., 2017; 96: 1310-1316.
CrossRef - Nna V. U, Usman U. Z, Ofutet E. O and Owu D. U. Quercetin exerts preventive, ameliorative and prophylactic effects on cadmium chloride – induced oxidative stress in the uterus and ovaries of female Wistar rats. Food Chemical Toxicol., 2017; 102:143-155. doi.org/10.1016/j.fct.2017.02.010.
CrossRef - Sharma D and Sangha G. K. Antioxidative effects of aqueous extract of broccoli sprouts against Triazophos induced hepatic and renal toxicity in female Wistar rats. J. Applied Biomedicine., 2018; 16:100–110.
CrossRef - Moller P, Ploger A and Sørensen H. Quantitative analysis of total glucosinolatecontent in concentrated extracts from double low rapeseed by the Pd-glucosinolate complex method. In: Sørensen, H. (Ed.), Advances in theProduction and Utilization of Cruciferous Crop. Martinus Nijhoff JunkPublishers, Dordrecht. 1985; p. 97–110.
- Adom K. K and Liu R. H. Antioxidant activity of grains. J. Agric. Food Chem., 2002; 50(21): 6182-7.
CrossRef - Ainsworth E. A and Gillespie K. M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc., 2007; 2(4): 875-7.
CrossRef - Chang C, Yang M. H, Wen H. M and Chern J. C. Estimation of total flavonoid content in propolis bytwo complementary colorimetric methods. J. Food Drug Analysis., 2002; 10: 178-182.
CrossRef - Lowry O. H, Rosebrough N. J, Farr A. L and Randall A. J. Protein measurement with folin phenol reagent. J. Biol. Chem., 1951; 193: 265-75.
CrossRef - Aebi H. Catalase methods in enzymatic analysis (ed) Bergmeryer HU Vol 3, Academic Press, New York;. 1983; p. 276-86.
- Marklund S and Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Euro. J. Biochem., 1974; 47:469-474.
CrossRef - Habig W. H, Pabst M. J and Jakoby W. B. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem., 1974; 246: 7130-7139.
CrossRef - Carlberg I and Mannervik B. Glutathione reductase. Methods Enzymol., 1985; 113: 484-490.
CrossRef - Hafeman D. G, Sunde R. A and Hoekstra W. G. Effect of dietary selenium erythrocyte and liver glutathione peroxidase in the rat. J. Nutr., 1984; 104: 580-587.
CrossRef - Stocks J and Dormandy T. L. The autoxidation of human red cell lipids induced by hydrogen peroxide. British J. Haem., 1971; 20(1):95-111.
CrossRef - Uchendu C, Ambali S. F and Ayo J. O. The organophosphate, chlorpyrifos, oxidative stress and the role of some antioxidants: A review. African J. Agric. Res., 2012; 7(18):2720-2728.
CrossRef - Mancini F, van Bruggen A. H. C, Jiggins J. L. S, Ambatipudi A. C and Murphy H. Acute pesticide poisoning among female and male cotton growers in India. Int. J. Occup. Environ. Health., 2005; 11: 221–232.
CrossRef - Agarwal A, Aponte-Mellado A, Premkumar B. J, Shaman A and Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod. Biol. Endocrin., 2012; 10: 49.
CrossRef - Sangha G. K, Kaur K, Khera K. S and Singh B. Toxicological Effects of Cypermethrin on Female Albino Rats. Toxicol. Int., 2011; 18(1):5-8.
CrossRef - Koh E, Wimalasiri K. M. S, Chassy A. W and Mitchell A. E. Content of ascorbicacid, quercetin, kaempferol and total phenolics in commercial broccoli. J. Food Compos. Anal., 2009; 22:637–643.
CrossRef - Vallejo F, Tomas-Barberan F. A and Garcia-Viguera C. Potential bioactive compounds in health promotion from broccolicultivars grown in Spain. J. Sci. Food Agric., 2002; 82:1293–1297.
CrossRef - Fahey J. W, Zhang Y and Talalay P. Broccoli sprouts: an exceptionally rich source of inducers of enzymes that protect against chemical carcinogens. Proc. Natl. Acad. Sci. U. S. A., 1997; 94(19): 10367-72.
CrossRef - Traka M and Mithen R. Glucosinolates, isothiocyanates and human health. Phytochem. Rev., 2009; 8(1):269-82.
CrossRef - Jeffery E. H and Araya M. Physiological effects of broccoli consumption. Phytochem. Rev., 2009; 8(1):283-98.
CrossRef - Dinkova-Kostova A. T, Fahey J. W, Wade K. L, Jenkins S. N, Shapiro T. A, Fuchs E. J. et al. Induction of the phase 2 response in mouse and human skin by sulforaphane containing broccoli sprout extracts. Cancer Epidemiol. Biomarkers Prev., 2007; 16: 847–51.
CrossRef - Murashima M, Watanabe S, Zhou X. G, Uehara M and Kurashige A. Phase 1 study of multiple biomarkers for metabolism and oxidative stress after one-week intake of broccoli sprouts. Biofactors., 2004; 22:271–5.
CrossRef - Uzun F. G and Kalender Y. Protective effect of vitamin C and E on malathion induced nephrotoxicity in male rats. Gazi Univ. J. Sci., 2011; 24(2):193-200.
- Tuncmen H and Tuzmen M. N. Biochemical Effects of pesticide contaminated drinking water on lipid peroxidation and free-radical scavenger. Hacettepe J. Biol. Chem., 2007; 35(2):111-16.
- Nishi K and Hundal S. S. Chlorpyrifos induced toxicity in reproductive organs of female Wistar rats, Food Chem. Toxicol., 2013; 62:732–738.
CrossRef - Rao R. P and Kaliwal B. B. Monocrotophos induced dysfunction on estrous cycle and follicular development in mice. Ind. Health., 2002; 40:237–244.
CrossRef - Lian H. Y, Gao Y, Jiao G. Z, Sun M. J, Wu X. F, Wang T. Y et al. Antioxidant supplementation overcomes the deleterious effects of maternal restraint stress-induced oxidative stress on mouse oocytes. Reproduction., 2013; 146:559–568.
CrossRef - Aseervatham G. S, Sivasudha T, Jeyadevi R and Ananth D. A. Environmental factors and unhealthy lifestyle influence oxidative stress in humans—an overview. Environ. Sci. Poll. Res. Int., 2013; 20: 4356–4369.
CrossRef - Shimozono R, Asaoka Y, Yoshizawa Y, Aoki T, Noda H, Yamada M, Kaino M and Mochizuki H. Nrf2 activators attenuate the progression of non-alcoholic steatohepatitis-related fibrosis in a dietary rat model. Mol. Pharmacol., 2013; 84:62–70.
CrossRef - Boddupalli S, Mein J. R, Lakkanna S and James D. R. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: perspectives in maintaining the antioxidant activity of vitamins A, C, and E. Front. Genet., 2012; 3:7. doi:10.3389/fgene.2012. 00007
CrossRef - Guerrero-Beltrán C. E, Calderón-Oliver M, Tapia E, Medina-Campos O. N, Sánchez-González D. J and Martínez-Martínez C. M. Sulforaphane protectsagainst cisplatin-induced nephrotoxicity. Toxicol. Lett., 2010; 192: 278–285.
CrossRef - Ighodaro O. M and Akinloye O. A. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid. Alexandria J. Medicine., 2018; 54: 287-293.
CrossRef - Attia A. M and Nasr H. M. Dimethoate-induced changes in biochemical parameters of experimental rat serum and its neutralization by black seed (Nigella sativa l.) oil. Slovak. J. Animal Sci., 2009; 42(2): 87-94.
- Ming-ming H, Xiu-fang L, Xia G, Jie L and Yan-hua N. Joint action of phoxim and methomyl on female rats reproductive toxicity. Carcinog. Teratog. Mutagen., 2008; 20(6):470-74.
- Zuanazzi C, Maccari P. A, Beninca S. C, Branco C. S, Theodoro H, Vanderlinde R. et al. White grape juice increases high-density lipoprotein cholesterol levels and reduces body mass index and abdominal and waist circumference in women. Nutrition., 2019; 57:109-114.
CrossRef - Duval C, Augé N, Frisach M. F, Casteilla L, Salvayre R and Nègre-Salvayre A. Mitochondrial oxidative stress is modulated by oleic acid via an epidermal growth factor receptor-dependent activation of glutathione peroxidase. Biochem. J., 2002; 367(Pt 3): 889-94.
CrossRef - Güney M, Demirin H, Oral B, Ozgüner M, Bayhan G and Altuntas I. Ovarian toxicity in rats caused by methidathion and ameliorating effect of vitamins E and C. Hum. Exp. Toxicol., 2007; 26(6): 491–498.
CrossRef - Bergamini C. M, Gambetti S, Dondi A and Cervellati C. Oxygen, reactive oxygen species and tissue damage. Curr. Pharm. Des., 2004; 10: 1611–1626.
CrossRef - Roopha P. D and Padmalatha C. Effect of Herbal Preparation on Heavy Metal (Cadmium) Induced Antioxidant System in Female Wistar Rats. J. Medical Toxicol., 2012; 8(2):101–107.
CrossRef - Mansour S. A and Mossa A. H. Oxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zinc. Pestic. Biochem. Physiol., 2010; 96:14–23.
CrossRef - Yener N. A, Sinanoglu O, Ilter E, Celik A, Sezgin G, Midi A et al. Effects of Spirulina on cyclophosphamide-induced ovarian toxicity in rats: biochemical and histomorphometric evaluation of the ovary. Biochem. Res. Int., 2013; Article ID 764262, doi:10.1155/2013/764262.
CrossRef - Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D and Dekel N. Reactive oxygen species are indispensable in ovulation. Proc. Nat. Acad. Sci. USA., 2011; 108(4):1462-67.
CrossRef - Fujii J, Iuchi Y and Okada F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol., 2005; 3: 43.
CrossRef - Tanvir E. M, Afroz R, Chowdhury M. A. Z, Gan S. H, Karim N, Islam M. N. et al. A model of chlorpyrifos distribution and its biochemical effects on the liver and kidneys of rats. Hum. Exp. Toxicol., 2016; 35(9):991–1004.
CrossRef - Devine P. J, Perreault S. D and Luderer U. Roles of reactive oxygen species and antioxidants in ovarian toxicity. Biol. Reprod., 2012; 86(2):1–10.
CrossRef - Hussein M. R. Apoptosis in the ovary: molecular mechanisms. Hum. Reprod. Update., 2005; 11(2):162–178.
CrossRef - Ho S. M. Estrogen, progesterone and epithelial ovarian cancer. Reprod. Biol. Endocrinol., 2003; 1:73. doi:10.1186/1477-7827-1-73.
CrossRef