Endang Mutiawati1,2* , K.R.T. Lucas Meliala3, Ginus Partadiredja4, Dhirgo Adji5
, K.R.T. Lucas Meliala3, Ginus Partadiredja4, Dhirgo Adji5 and Raden Wasito5
and Raden Wasito5 
1Department of Neurology, School of Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia
2Department of Neurology, Dr. Zainoel Abidin Hospital, Banda Aceh, Indonesia
3Department of Neurology, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia
4Department of Physiology, Faculty of Medicine, Gadjah Mada University, Yogyakarta, Indonesia
5Department of Pathology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia
Corresponding Author E-mail: endangmutiawati@unsyiah.ac.id
DOI : https://dx.doi.org/10.13005/bpj/2205
Abstract
The objective of this study wasto assess the effect of methylcobalaminonmechanical allodynia and the voltage-gated sodium channels (VGSCs) expression of injured nerves in spinal nerve ligation-induced neuropathic pain model in animals.Three different doses of methylcobalamin were administrated intramuscularly into neuropathic pain rat model, twice a week for 14 weeks. The effect of methylcobalamin on neuropathic pain was assessed using mechanical allodynia (using the von Frey filaments) while its effect on VGSC expression was assessed using immunohistochemistry. ANOVA and independent t-test were employed to compared the effect of methylcobalamin on mechanical allodynia between groups.The size of von Frey filament that induced the first onset of mechanical allodynia was smaller in control group compared to 50µg methylcobalamin group (p=0.013) and methylcobalamin 100µg group (p=0.019). There is a dose–response relationship between methylcobalamin dose and the average duration of mechanical allodynia (43.8, 38.2, 30.6 and 29.6 days for control, 50µg, 100µg, and 150µg methylcobalamin group, respectively) with a significant different observed between control and 150µg methylcobalamin group only (p=0.027). Nerve tissues from all animals within control group expressed VGSC while all nerve tissues from both 100µg, and 150µg methylcobalamin, had no VGCS expression. In conclusion, methylcobalamin is potentially shorten the duration of mechanical allodynia and increase pain threshold in neuropathic pain animal model. These effects might associate with reduction of VGSC expression on the injured neurons.
Keywords
Neuropathic pain; Methylcobalamin; Pathology; Sodium ion channel
Download this article as:| Copy the following to cite this article: Mutiawati E, Meliala K. R. T. L, Partadiredja G, Adji D, Wasito R. Effect of Methylcobalamin on Voltage-Gated Sodium Channels (Vgscs) Expression in Neuropathic Painanimal Model. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Mutiawati E, Meliala K. R. T. L, Partadiredja G, Adji D, Wasito R. Effect of Methylcobalamin on Voltage-Gated Sodium Channels (Vgscs) Expression in Neuropathic Painanimal Model. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/36iGAza |
Introduction
Neuropathic pain is defined as a sensation arises from the results of a lesion or disease of the peripheral or central somatosensory nervous system; some of the examples are postherpetic neuralgia, painful polyneuropathy, trigeminal neuralgia, and post-stroke pain.1Burning pain, painful sensitivity to touch, and pain attacks are the common complains reported and these significantly reduce the quality of life of the patients and impose economic burdens on individuals and society.2, 3 The worldwide prevalence of chronic neuropathic pain ranging between 6.9% and 10%.4
The pathophysiology neuropathic pain is complex and not completely understood.5 One of pathophysiology mechanisms is alteration of ion channels within the affected neurons, leading to altered electrical excitability of sensory neurons.5, 6 Voltage-gated sodium channels (VGSC), one of the ion channels, allow rapid influx of sodium, causes depolarization of action potentials in excitable cells.7VGSCs are integral membrane glycoproteins on neurons and alteration of this ion channel such as overexpressed on neurons is critical for development of pain sensation of neuropathic pain.6, 8, 9
Regardless of its origin (peripheral or central), the pharmacotherapy alternatives of neuropathic pain are similar. Tricyclic antidepressants, selective serotonin-norepinephrine reuptake inhibitors, and anticonvulsants are the first choice of drugs for neuropathic pain, while opioids can only be used when other drugs have not been effective or in need of a more rapid onset of pain relief.10 Methylcobalamin, an activated form of vitamin B12, exerts neuronal protection by promoting regeneration of injured nervesand reduces glutamate-induced neurotoxicity.11Recent experimental and clinical studies suggested that methylcobalamin also has potential analgesic effects on neuropathic pain by improving nerve conduction, promoting regeneration of injured nerves, and preventing spontaneous discharges of injured sensory neurons.11, 12Despite of rigorous studies on the efficacy of methylcobalamin as the treatment for pain, there is still no study investigating the effect of methylcobalamin on VGSC expression in neuropathic pain. This study sought to assess the effect of methylcobalamin on VGSC expression on nerves of neuropathic pain animal model.
Materials and Methods
Study setting
A study to assess the effects of methylcobalamin on expression of VGSCs on injured neurons was conducted in nerve ligation-induced neuropathic pain rats. The methylcobalamin was administrated in three different doses, intramuscularly, twice a week for 14 weeks. Apart from assessing the expression of VGSC by immunohistochemistry (IHC), the effect of methylcobalamin in reducing neuropathic pain was also evaluated by assessing the neuropathic pain behavior (mechanical allodynia) in animals.
Animals and neuropathic pain induction
Twenty male Sprague-Dawley rats, 2 months old, weighing 150-250 g, were used. The animals went through an adaptation process for a week prior the study under laboratory conditions(temperature 23±1oC, 60% of humidity, and 12h light-dark cycle),and were fed ad libitum as explained previously.13
To induce the neuropathic pain, segmental spinal nerve ligation (SNL) technique14 was adopted. The ligation was conducted on lumbar 5 (L5) nerve.Briefly, the animals were anesthetized and the left hind paw was shaved and sterilized. A 5cm longitudinal incision midway of the left tight was performed. A 0-6 silk thread was inserted to the nerve distal and the nerve was ligated tightly as described previously.15 The open wound was washed with penicillin-streptomycin solution and closed by thoroughly stitching the muscles using 4-0 chromic cat-gut thread and 0-3 cotton thread for the skin.
Administration of methylcobalamin
The animalswere randomly divided into 4 groups of 5; one control group and three methylcobalamin groups (M50, M100, M150). Control groupwas given 0.9% sodium chlorideintramuscularly, while M50, M100 and M150 groups were received methylcobalamin 50µg, 100µg, and 150µg, respectively, intramuscularly, twice a week for 14 weeks.
Mechanical allodynia assessment
To assess the effect of methylcobalamin on neuropathic pain, mechanical allodyniawas evaluated in animals. Mechanical allodynia was assessed using the von Frey filaments (BiosebLab, France), by pressing an actuator filament slowly against the hind paw until it buckles, with a frequency of 1/sand each intensity was repeated for 10 times. Mechanical allodynia was defined when 5 out of 10 of specific size von Frey stimuli caused the rats to withdraw the paw. Mechanical allodynia was assessed on the 1st, 3rd, 5th, 7th day, and every weekend afterwards as suggested previously.16 Three indicators were used: (a) onset time, the length of the time from L5 ligation to time of the first onset of mechanical allodynia appeared; (b)von Frey filament size, the size of von Frey filament which induced mechanical allodynia in the first onset; and (c) duration of mechanical allodynia, the length of the time between the first time when neuropathic pain appeared and when it resolved.
Evaluation of VGSC expression
After 14 weeks of treatment, all animalswere deeply anesthetized and then sacrificed via cervical dislocation per protocol.17 The surgical wound was re-opened and the ligated nerve was extracted together with nearby tissues, and kept in preservative solution (saturated picric acid, formalin (37-40%), and glacial acetate acid with proportion of 15:5:1). The nerve tissues were then dehydrated, processed and cut at the required thickness followed standard operation procedure for histological preparation technique 18. These nerve tissues where then stained with IHC to assess VGSC expression. IHC staining was conducted using primary anti-pan NaV antibody(Alomone Labs, Jerusalem, Israel). The staining was visualized by using horseradish peroxidase (HRP) – chromogen 3,3’-diaminobenzidine (DAB) detection IHC kit (ab64259, Abcam). All procedures were performed based on the manufacturer’s instructions.
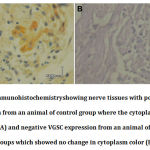
The expression of VGSC was divided into two: active and inactive VGSC. Active VGSC indicating that the VGSC was open and therefore NaV antibody could enter to neuron cells and bind with epitope, which located intracellular loop between domains III and IV domain of VGSC (i.e.cytoplasm colored as brownish after secondary training). Inactive VGSC indicating that the GSC was closed and therefore the NaV antibody could not enter the neurons (i.e.cytoplasm does not turn brownish secondary training). Interpretation of VGSC expression was conducted by two pathologists.
Statistical analysis
To compare the effect of methylcobalamin on mechanical allodynia between groups ANOVA and independent t-test were employed. The expression of VGSC from IHC wasanalyzed descriptively. Significance was assessed at α=0.05 and analyses were conducted using SPSS version 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
Mechanical allodynia
Onset of mechanical allodynia
The first observed mechanical allodynia was in day 3 for control and M50, and day 5 for M100and M150. On day 14, mechanical allodynia was observed in all animals within all groups. The mean onset time of mechanical allodynia was 5.4±1.6 days for control group, 4.6±2.1 days for M50, 9.0±4.6 days for M100, and 8.6±4.9days for M150. Statistical analysis indicated the mean onset time had no different among groups.
Table 1: Effect of methylcobalamin on day of first onset and duration of mechanical allodynia.
| Group | Mean of first day of onset (day) | von Frey filament size | Duration of mechanical allodynia (day) |
| Control | 5.4 ± 1.6 | 14.6 ± 1.9 | 43.8 ± 6.2 |
| M150µg | 4.6 ± 2.1 | 18.0 ± 1.4 | 38.2 ± 17.5 |
| M2100µg | 9.0 ± 4.6 | 17.4 ± 0.8 | 30.6 ± 16.4 |
| M3150µg | 8.6 ± 4.9 | 15.8 ± 1.3 | 29.6 ± 9.1 |
von Frey filament size
Our study showed that the size of von Frey filament which induced the pain on controlled group was smaller compared to the ones used for methylcobalamin groups, with mean 14.6±1.9, 18.0±1.4, 17.4±0.8 and 15.8±1.3 for control, M50, M100, M150, respectively (Table 1). Statistical analysis indicated that there was significant different among the four groups (p=0.007). Significant different only observed between control and M50 group (p=0.013), and between control and M100 group (p=0.019).
Duration of mechanical allodynia
Our data suggested that the duration of mechanical allodynia was different among groups. In control group, mechanical allodynia was observed in all animals till day 42 while mechanical allodynia already resolved in all animals of M150 group in day 42 (Fig.1). On the last day of the examination, day 52, one animal from control and M50 group still exhibited mechanical allodynia.
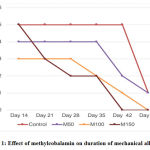 |
Figure 1: Effect of methylcobalamin on duration of mechanical allodynia. |
In average, the longest and the shortest duration of mechanical allodynia was observed in control and M150group, respectively (43.8±6.2 vs. 29.6±9.1 days). There was a dose–response relationship between methylcobalamin dose and duration of mechanical allodynia where the higher the dose, the shorter the duration (Table 1). However, the significant different found between control and M150 group only (p=0.027).
VGSC expression
Using IHC staining, the expression VGSC protein on the effected nerves was classified as positive (cytoplasm colored into brownish indicates VGSCs were open) and negative (cytoplasm unstained indicating VGSC were closed or inactive). Figure 2 presented ICH of VGSC expression from nerve tissue from control group (positive VGSC expression) and M100 group (negative VGSC expression).Our data showed that all nerve tissues from all animals within control group were expressed VGSC and two animals (40%) of M50 group also had positive VGSC expression. All nerve tissues from all animals of both M100 andM150 group, had negative expression of VGCS.
Discussion
SNL procedure is standard technique to induce neuropathic pain,14, 15 adopting this technique ensured the production of neuropathic pain in animal model. Accumulation of VGSC will induce ectopic pacemaker, whose together with sensitization of other receptors (mechanical, thermal, or chemical) will induce neuropathic pain.19 This process requires some time and neuropathic pain does not directly arise after neural injury.20 When we measure the onset time, our data found there was no significant different of onset time of mechanical allodynia between control and treatment groups. However, a previous study found administration of methylcobalamin on dorsal root ganglion significantly delay the early onset of pain.11One of the possible reasons is the onset time of mechanical allodynia (representing neuropathic pain) was not measured every day in the current study. Therefore, although the assessment time was followed the previous study,16 we might failed to measure the exact onset time of mechanical allodynia among groups.
Unlike the onset time of mechanical allodynia, our study found the duration of mechanical allodynia reduced as methylcobalamin dose increased suggesting that methylcobalamin enabled to reduce neuropathic pain. In the same study it was demonstrated alsothat methylcobalamin significantly shorten the duration of mechanical allodynia by lowering spike amplitude of ectopic discharge.11 Further more, the present study also found that the bigger size of von Frey filament was required to induce pain in methylcobalamin groups compare to control group, suggesting that methylcobalamin could increase the pain threshold. Altogether, our data suggest that methylcobalamin could improve the outcome of neuropathic pain. To the best of our knowledge, this was the first study assessing the effect of methylcobalamin on VGSC expression in neuropathic pain animal model. Our study indicated that methylcobalamin reduced the expression of VGSC on injured nerves highlighting a potential mechanism of methylcobalamin in reducing neuropathic pain.VGSC is a critical development of pain sensation in neuropathic pain6, 8, 9making VGSC is one of the main therapeutic target of chronic neuropathic pain.21 Our study highlights that methylcobalamin improves the neuropathic pain through downregulation and/or inactivation of VGSC on the neurons.
This study had some limitations that need to be discussed. The number of animals was relatively small for each group; therefore, further study with bigger number of samples is warranted. In our study, the expression of VGSC was measured without specify the VGSC family. Further analysis to determine whether methylcobalamin effects specific VGSC family only maybe required. In this study the expression of VGSC was evaluated by IHC staining only making a further study usingmultiple approaches to measure VGSC expression is crucial to elucidate the finding of this study.
Conclusion
This present study suggests that methylcobalamin improve the symptoms of SNL-induced neuropathic pain animal model by shorten the duration of pain and increasing pain threshold. This improvement might be associated with reduction of VGSC expression on affected nerves.
Acknowledgment
We would like to thank to the patients and HT Editorial Service for the assistance during manuscript preparation.
Conflict of Interest
Authors do not have any conflict of interests.
Funding Source
This study received no external funding.
References
- Baron R, Binder A, Wasner G. Neuropathic pain: Diagnosis, pathophysiological mechanisms, and treatment. Lancet Neurol. 2010; 9:807-19.
CrossRef - Tarride JE, Moulin DE, Lynch M, Clark AJ, Stitt L, Gordon A, et al. Impact on health-related quality of life and costs of managing chronic neuropathic pain in academic pain centres: Results from a one-year prospective observational Canadian study. Pain Res Manag. 2015; 20:327-33.
CrossRef - Jensen MP, Chodroff MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007; 68:1178-82.
CrossRef - van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014; 155:654-62.
CrossRef - Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, et al. Neuropathic pain. Nat Rev Dis Primers. 2017; 3:17002.
CrossRef - Devor M. Sodium channels and mechanisms of neuropathic pain. J Pain. 2006; 7:S3-S12.
CrossRef - Cummins TR, Sheets PL, Waxman SG. The role of sodium channels in nociception: Implication for mechanisms of pain. Pain. 2007; 131:243-57.
CrossRef - Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M. Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol. 2010; 9:413-24.
CrossRef - Chahine M, Chatelier A, Babich O, Krupp JJ. Voltage-gated sodium channels in neurological disorders. CNS Neurol Disord Drug Targets. 2008; 7:144-58.
CrossRef - Binder A, Baron R. The pharmacological therapy of chronic neuropathic pain. Dtsch Arztebl Int. 2016; 113:616-26.
CrossRef - Zhang M, Han W, Hu S, Xu H. Methylcobalamin: A potential vitamin of pain killer. Neural Plast. 2013; 2013.
CrossRef - Buesing S, Costa M, Schilling JD, Moeller-Bertram T. Vitamin B12 as a treatment for pain. Pain Physician. 2019; 22:45-52.
CrossRef - Amoateng P, Adjei S, Osei-Safo D, kukuia KKE, Kretchy IA, Sarkodie JA, et al. Analgesic effects of a hydro-ethanolic whole plant extract of Syndrella nodiflora (L.) Gaertn in paclitaxel-induced neuropathic pain in rats. BMC Res Notes. 2017; 10.
CrossRef - Chung JM, Kim HK, Chung K. Segmental spinal nerve ligation model of neuropathic pain. Methods Mol Med. 2004; 99:35-45.
CrossRef - Tsantoulas C, Denk F, Signore M, Nassar M, Futai K, McMahon S. Mice lacking Kcns1 in peripheral neurons show increased basal and neuropathic pain sensitivity. Pain. 2018; 159:1641-51.
CrossRef - Persson A, Wastermark S, Merrick D, Sjölund B. Validity of electrical stimulus magnitude matching in chronic pain. J Rehabil Med. 2009; 41:898-903.
CrossRef - CCAC. CCAC guidelines on: euthanasia of animals used in science. Canadian Council on Animal Care, Ottawa, Canada. 2010; https://www.ccac.ca/Documents/Standards/Guidelines/Euthanasia.pdf.
CrossRef - Anderson G, Bancroft J. Tissue processing and microtomy. Bancroft J, editor. Edinburgh Churchill Livingstone; 2020.
CrossRef - Dunteman ED. Mechanisms and treatment issues for neuropathic pain. Canadian Journal of Anesthesia. 2004; 51:R46-R9.
CrossRef - Nishimoto S, Tanaka H, Okamoto M, Okada K, Murase T, Yoshikawa H. Methylcobalamin promotes the differentiation of Schwann cells andremyelination in lysophosphatidylcholine-induced demyelination of the rat sciatic nerve. Front Cell Neurosci. 2015; 9.
CrossRef - Ma RSY, Kayani K, Whyte-Oshodi D, Whyte-Oshodi A, Nachiappan N, Gnanarajah S, et al. Voltage gated sodium channels as therapeutic targets for chronic pain. J Pain Res. 2019; 12:2709-22.
CrossRef