Manuscript accepted on :14-06-2021
Published online on: 01-07-2021
Plagiarism Check: Yes
Reviewed by: Dr. Rishan
Second Review by: Dr. Deepak Bhattacharya
Rupachandra S1* , Mario Prateek Selvam1
, Mario Prateek Selvam1 , Nisha Muthukumaran1
, Nisha Muthukumaran1 , Sangamithra Senthilkumar1
, Sangamithra Senthilkumar1 , Sandhiya Vaidhyalingam1
, Sandhiya Vaidhyalingam1 , Dharshene K1
, Dharshene K1 and Kasthuri Natarajan2
and Kasthuri Natarajan2
1Department of Biotechnology, School of Bioengineering, SRM Institute of Science and Technology, Kattankulathur, 603 203.
2Department of Translational Medicine and Research, SRM Medical College, Kattankulathur Campus, SRM Institute of Science and Technology. Kattankulathur,603 203.
Corresponding author Email: rupachas@srmist.edu.in
DOI : https://dx.doi.org/10.13005/bpj/2202
Abstract
Plant peptides have gained attention in the medicinal field due to their high anti-microbial and anti-cancer properties. Various plant sources are being used to extract proteins and peptides to be used as a cure for a variety of diseases. The latest studies show that plant peptides are effective in the treatment of cancer due to their ability to preferentially bind to the receptors or membranes of cancer cells leading to tumour growth inhibition, cytotoxicity, decreased proliferation, and apoptosis. The peptides get internalized into the cells causing cancer cell agglutination and aggregation. In addition to acting as therapeutic agents, plant peptides are also used for the targeting of drugs specific to cancer cells. In this study, bioactive peptides were isolated from the seeds of Momordica dioica and leaves of Solanum trilobatum. They were screened and identified using HPLC and MALDI-TOF techniques. In this study, apoptosis was analyzed by the Hoechst 33342 staining method which detected the presence of condensed pycnotic nuclei in apoptotic COLO320DM and COLO205 colon cancer cellsat the maximum concentrations of 150 and 175 µg/mL of plant peptides.Further DCFH-DA staining indicated the intracellular ROS production in the treated COLO320DM and COLO205 colon cancer cells. Thus, the isolated bioactive plant peptides can be formulated towards the development of effective anticancer drugs for the treatment of colon cancer in humans.
Keywords
Anticancer; Apoptosis; Bioactive Peptides; Colon Cancer; Plant Peptides
Download this article as:| Copy the following to cite this article: Rupachandra S, Selvam M. P, Muthukumaran N, Senthilkumar S, Vaidhyalingam S, Dharshene K, Natarajan K. In Vitro Assessment of Cytotoxic Activity of Bioactive Peptides from Momordica Dioica and Solanum Trilobatum Against Human Colon Cancer Cells. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Rupachandra S, Selvam M. P, Muthukumaran N, Senthilkumar S, Vaidhyalingam S, Dharshene K, Natarajan K. In Vitro Assessment of Cytotoxic Activity of Bioactive Peptides from Momordica Dioica and Solanum Trilobatum Against Human Colon Cancer Cells. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3dPRGzR |
Introduction
Cancer is defined as an uncontrolled division of cells and the cell’s ability to invade other tissues, causing vascularization, generation of the tumour mass and metastasis. [1,2] Chemotherapy is one of the significant treatments for cancer, that functions by administering agents that are cytotoxic to the cancer cells. The most important drawback with the conventional chemotherapy methods is the incapability of directly transporting the required quantity of the drug to the tumour cells without any damage to the healthy cells. Lately, the balance of the structural rigidity along with the flexibility has made the small peptides as effective drugs for targeting cancer cells with high affinity and specificity[3].Peptides represent a unique class of pharmaceutical compounds with distinctive therapeutic and biochemical characteristics. [4,5] Peptides have been used as potential drugs that are capable of inhibitingin tracellular molecules such as receptor tyrosine kinases. Peptides can be used directly as drugs (e.g., as angiogenesis inhibitors), as agents that target tumours carrying radionuclides, and cytotoxic drugs (targeted radiation therapy and chemotherapy), vaccines, and hormones. [6]
Solanum trilobatum is a predominant medicinal plant that is rich in iron, crude fibre, calcium, carbohydrates, minerals, fat, and phosphorus in the leaves. It is considered as a cure in the reduction of blood glucose level, asthma, several kinds of leprosy, and also in the arrest of blood vomiting. It also contains antifungal, antibacterial, antioxidant, and antimitotic properties. [7,8]
Momordica dioica is a plant with high medicinal values belonging to the genus Momordica and family Cucurbitaceae. M.dioica possesses anti-hyperglycemic and renal protective activities. The fruits of this plant have diuretic, laxative, hepatoprotective, anti-venomous, antihypertensive, anti-inflammatory, anti-asthmatic, antipyretic, antidiabetic, and antidepressant properties. [9,10] The aim of this study is to examine the cytotoxic properties of bioactive peptides from the seeds of Momordica dioica and leaves of Solanum trilobatum.
Materials and Methodologies
Plant material and reagents
Roswell Park Memorial Institute medium1640 (RPMI-1640), 50X Penicillin and Streptomycin Antibiotic, and Fetal bovine serum (FBS) were acquired from Hi-Media. Ethidium Bromide, Acridine Orange, and DCFDA were purchased from Santa Cruz. Hoechst 33342 was acquired from Thermofisher Scientific. COLO 205 and COLO 320 DM cell lines were procured from NCCS, Pune, India, and were maintained at a temperature of 37°C and 5% CO2 atmosphere. The cell lines were cultured in RPMI-1640 medium consisting of 1% antibiotic (penicillin and streptomycin cocktail) and 5% Fetal Bovine Serum.
Preparation of Peptide extracts
The seeds of Momordica dioica were washed with distilled water, shade dried and powdered. About, 30 g of the seed powder was extracted with 300 mL of Sodium Phosphate buffer and was kept in a rotary shaker at 100 rpm, overnight. The extract was filtered and subjected to 80% Ammonium Sulphate Precipitation method to precipitate the peptides from the buffer extract. Further, the extracted peptides were dialysed using a membrane with a cut-off MW 10kDa to isolate peptides with a molecular weight less than 10KDa. [11]The leaves of Solanum trilobatum were shade dried and powdered. 8g of the leaf powder was extracted with dichloromethane and methanol (1:1). The extract was filtered using a muslin cloth and partitioned with an equal volume of dichloromethane and water. The organic layer was discarded and the aqueous layer of methanol – water was concentrated using a rotary evaporator. [12]The extracted peptides from both the plant sources were lyophilized and stored at -20 0C for further use.
Identification of Peptides in the plant extracts
The lyophilized peptide samples of selected plant sources were fractionated by High-Performance Liquid Chromatography (HPLC) on a C18 column (4.6*250mm*5µm, 300˚A)with a linear gradient of 0-60% acetonitrile in 0.1% trifluoroacetic acid at a flow rate of 1 mL/min. The absorbance was read at 280 nm for the peptides extracted from M. dioica and S. trilobatum. [13]The mass spectrum of the extracted peptides of M. dioica and S. trilobatum were obtained by MALDI-TOF analysis.
Hoechst staining
The apoptosis of cancer cells depicts nuclear condensation which can be visualized by staining with Hoechst 33342 stain. The COLO 320 DM cells (10^5 cells/mL) were cultured in a 6-well plate and treated with the peptides isolated from M.dioica. The cells were stained with Hoechst 33342 dye for 15 mins at room temperature and were visualized using a fluorescence microscope. [14]
Acridine Orange/Ethidium Bromide Staining
COLO 205 and COLO 320 DM cells were grown at the density of 1×106 on the coverslips in 6-well plates and incubated for 48 h. COLO 205 colon cancer cells were treated with 175 μg/mL of peptides extracted from S.trilobatum for 24 h and 48h. COLO 320DM cells were treated with 150 μg/mL of peptides extracted from M.dioica for 24 h and 48 h. A fixative 4% paraformaldehyde was used to fix the COLO 205 cells with an incubation of 30 min and permeabilized with 0.1% Triton X-100 for 15 min. The cells were stained with 2μg/mL of AO/EB for 10 min and the dead cells were washed with PBS. Cells were mounted and photographed using fluorescence microscopy. [15]
DCFDA staining
Oxidative stress was analysed by evaluation of ROS using DCFDA staining in COLO 205 colon cancer cells for 24 h and 48 h. COLO 205 colon cancer cells were treated with 175μg/mL of peptides extracted from S. trilobatum for 24 h and 48 h and were photographed in fluorescence microscopy. [16]
Results and Discussion
Structural Analysis of Peptide Extracts
The peptides extracted from M. dioica[Fig.1] and S. trilobatum[Fig.2]were analyzed using a C-18 HPLC column (4.6*250mm*5µm, 300˚A) at the absorbance of 280 nm.HPLC analysis of the peptides extracted from Momordica dioicaat 280nmdepicted the presence of a single peak at the retention time of 13.099 min. This is found to be in concordance with the results obtained by the HPLC analysis of antioxidant peptides isolated from α-Lactalbumin and β-Lactoglobulin in Hernández-Ledesma et al. 2005. [17] The highest peak for the peptide extracts from Solanum trilobatum was observed at the retention time of 33.230 min by eluting in the C-18 HPLC column at the absorbance of 280 nm.
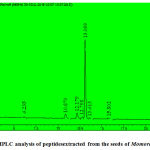 |
Figure 1: HPLC analysis of peptidesextracted from the seeds of Momordica dioica. |
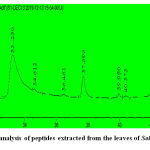 |
Figure 2: HPLC analysis of peptides extracted from the leaves of Solanum trilobatum. |
The molecular weight of the peptides extracted from M. dioica[Fig.3] and S. trilobatumwere examined using MALDI-TOF analysis [Fig.4]. The crude sample of M.dioica had the peptide with the highest molecular weight of 855.085Da and the peptides from S.trilobatum has the highest molecular weight of 1446.911Da. The results are homologous to the results obtained in Hashempour et al., 2013 by the characterization of cyclic peptides from Viola ignobilis. [18]
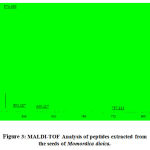 |
Figure 3: MALDI-TOF Analysis of peptides extracted from the seeds of Momordica dioica. |
 |
Figure 4: MALDI-TOF analysis of peptides extracted from the leaves of Solanum trilobatum. |
Bioactive compounds isolated from plants possess anti-microbial, anti-inflammatory, anti-tumour and anti-pyretic properties. [19] The present study focuses on the isolation, characterization and determination of the anti-cancer properties of the peptides extracted from the two selected plant sources, M. dioica and S. trilobatum against human colon cancer cells.
Cell Viability Analysis of COLO 320DM and COLO 205 cells
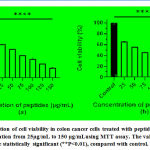
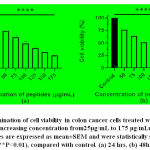
The reduction in the number of viable cancer cells was observed using a phase contrast microscope [Fig.5 and Fig.6]after being treated with the peptides isolated from M.dioica and S.trilobatum for 48 hours, which indicates the potential anti-cancer activity of these peptides. The COLO 320DM cells exhibited increased cytotoxicity of 81% when treated with the maximum concentration of 150 µg/mL of the peptides extracted from M. dioica after 48 h of incubation [Fig.7]. The COLO 205 cells treated with the maximum concentration of 175 µg/mL of the peptides extracted from S. trilobatum after 48 h showed increased cell cytotoxicity of 75% [Fig.8]. [20,21]
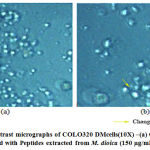 |
Figure 5: Phase contrast micrographs of COLO320 DMcells(10X) –(a) Control (untreated) (b) Treated with Peptides extracted from M. dioica (150 µg/mL) – 48 hr. |
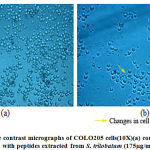 |
Figure 6: Phase contrast micrographs of COLO205 cells(10X)(a) control (untreated) (b) Treated with peptides extracted from S. trilobatum (175µg/mL) – 48 hr. |
The anti-cancer properties of the novel peptides extracted from M. dioica and S. trilobatum were studied against human colon cancer cell lines, COLO 320DM and COLO 205 cells. Our findings demonstrate that the peptides extracted from M.dioica have potent cytotoxic activity against COLO 320DM cells and the peptide extracts from S.trilobatum exhibit cytotoxic activity against COLO 205 cells. Similar results were obtained in Li et al., 2013 by treating the human colorectal cancer cell lines with the crude peptide extracts from Ipomoea batatas. [22]
Examination of cell death and oxidative stress in human colon cancer cells
Nuclear changes are characteristic to apoptosis and is a major factor that distinguishes apoptosis from necrosis. Hoechst 33342 staining revealed nuclear changes such as nuclear fragmentation and chromatin condensation which are associated with apoptosis in COLO 320DM cells treated with peptides extracted from M. dioica at the concentration of 150 µg/mL as seen in [Fig.9]. [23,24]Characteristic features of apoptosis include nuclear changes such as chromatin condensation and nuclear fragmentation. [25] This was studied in COLO 320DM indicating changes in the morphology of the nucleus of the cells treated with peptides isolated from M. dioica, by Hoechst 33342 staining method that are characteristic to apoptosis such as fragmented and condensed apoptotic bodies. Similar results were observed in a study of the mechanism of apoptotic induction by gecko peptide mixtures in treated human hepatocellular carcinoma cells. [26]
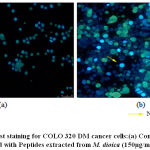 |
Figure 9: Hoechst staining for COLO 320 DM cancer cells:(a) Control (untreated) (b) Treated with Peptides extracted from M. dioica (150µg/mL) – 48 hr. |
Further, Acridine Orange / Ethidium Bromide staining was performed to study the morphological changes that lead to cell death in COLO 320DM cells treated with peptides extracted from M.dioica at the maximum concentration of 150 µg/mL and COLO 205 cells treated with peptides extracted from S.trilobatum at the maximum concentration of 175 µg/mL [Fig.10 and Fig.11]. [27] Further Acridine Orange / Ethidium Bromide fluorescent staining technique was performed on COLO 320DM cells treated with peptides isolated from M. dioica and COLO 205 cells treated with peptides from S. trilobatum to study the changes that occur in cellular morphology during apoptosis. [28] The untreated control cells appeared green in colour while the cells treated with the peptides extracted from M. dioica and S trilobatum appeared orange and red in colour. The cells treated with the maximum concentration of 150 µg/mL peptides extracted from M.dioica and S.trilobatum , showed shrunken nuclei and fragmented apoptotic bodies as opposed to the untreated control cells with large and normal nucleus. A comparative study was performed by Umayaparvathi et al., 2014, studying the anticancer effect and antioxidant activity of bioactive peptides extracted from the enzyme hydrolysates of Saccostrea cucullata and by Ebrahim etal., 2014, evaluating the anti-cancer activity of cobra venom polypeptide Cytotoxin-II against MCF-7 cell lines. [29,30]This study supports the apoptotic property of the extracted novel peptides against human colon cancer cell lines. These findings clearly suggest the anti-tumour properties of the peptide extracts from M.dioica and S.trilobatum. The novel isolated peptides are proved to be potential drug for the treatment of human colon cancer.
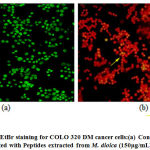 |
Figure 10: AO/EtBr staining for COLO 320 DM cancer cells:(a) Control (untreated) (b) Treated with Peptides extracted from M. dioica (150µg/mL) – 48 hr. |
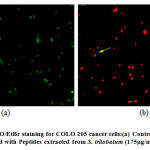 |
Figure 11: AO/EtBr staining for COLO 205 cancer cells:(a) Control (untreated) (b) Treated with Peptides extracted from S. trilobatum (175µg/mL) – 48 hr. |
The oxidative stress in COLO 205 cells treated with peptide extracts from S. trilobatum at the concentration of 150 µg/mL and the changes in the morphology of the nucleus was studied using the 2’,7’-dichlorofluorescein diacetate (DCFDA), an oxidation sensitive dye. [31,32] Fluorescent microscope images of the cells treated with the peptides isolated from S. trilobatum showed an increase in ROS levels when compared to the untreated control cells [Fig.12]. An analogous work was carried out by treating the human hepatocarcinoma cells with peptides isolated from maize with the generation of reactive oxygen species. [33]
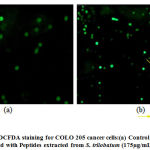 |
Figure 12: DCFDA staining for COLO 205 cancer cells:(a) Control (untreated) (b) Treated with Peptides extracted from S. trilobatum (175µg/mL) – 48 hr. |
Discussion
The peptides obtained from M.dioicaand S.trilobatum were identified with a C-18 HPLC column. Similar work was performed by Pritchard et al., 2010, where the bioactive peptides in Cheddar cheese were extracted and identified using a C-18 RP-HPLC column[34]. Similar work was carried out by Neves et al., 2017, in which the enzyme hydrolysates of Salmo salar were analysed in a RP-HPLC column[35]. MALDI-TOF analysis was performed to obtain the molecular mass of the peptide extracts from M.dioicaand S.trilobatum which were concordant with the results of Ahmed et al., 2015, in which the molecular mass of the antioxidant peptides from goat milk were also studied in a similar manner[36].
In this study the cytotoxic properties of the peptides extracted from M.dioicaand S.trilobatum were evaluated. In Li et al., 2013, human colorectal cancer cell lines were treated with the crude peptide extracts ofIpomoea batatas. The cell proliferation was observed to decrease by 49%with increase in the peptide dosage at 40 μmol/L[37]. Another study by Kumar et al., 2019, examined the in vitro anticancer properties of collagen peptides isolated from Aluterusmonoceros[38]. Hoechst 33342and Acridine Orange/ Ethidium Bromide (AO/EtBr) are fluorescent staining techniques that binds to the DNA of the cells and thus aids in studying the morphological changes that occur in the nucleus. In the present study, the COLO 320DM cells treated with peptide extracts from M.dioica were stained with Hoechst 33342 stain and modifications in the nuclear morphology such as nuclear fragmentation, characteristic to apoptotic cells were observed. These results are homologous with the studies of Jinet al., 2016, in which human hepatocellular carcinoma cells (HepG2) were treated with different concentrations of gecko peptide mixture which depicted chromosomal condensation in HepG2 cells[39].
AO/EtBr staining technique was further used to study the cellular changes that are distinctive to apoptotic cell death in Colo 320DM cells treated with peptide extracts fromM.dioicaand the Colo 205 cells treated with peptide extracts from S.trilobatum. This staining technique can be used to visualize the morphological changes that occur in the cell membrane of apoptotic cells. It can also be used to effectively determine the stages of apoptosis in the treated cells[40]. Similar results were obtained with the studies of Taniya et al., 2020 where amaranth seed protein hydrolysates were observed to induce apoptosis in breast cancer cell lines[41].
2′,7′ –dichlorofluorescin diacetate (DCFDA) is another fluorescent staining method that helps to identify the levels of reactive oxygen species (ROS) in live cells. High concentration of ROS is often indicative of apoptosis in cells that is caspase dependent and involves mitochondrial disintegration. The Colo 205 cells treated with peptides extracted from S.trilobatum were observed to exhibit increasing levels of ROS in a dose dependent manner. Similar results were also obtained by Brodskáet al., 2011,where the leukaemia cell line(CML-T1) was treated with various anti-cancer drugs[42].
Conclusion
The peptides extracted from Momordica dioica and Solanum trilobatum were found to inhibit cell proliferation and cause cell death in COLO 320DM cells and COLO 205 cells respectively. Cell death was examined by fluorescent staining techniques such as Hoescht, DAPI and AO/EtBr. The results indicated chromosomal condensation and nuclear fragmentation in the treated human colon cancer cell lines which is characteristic of apoptosispathway. ROS generation was detected in COLO 205 cells treated with peptides extracted from Solanum trilobatum by DCFDA staining technique, which indicates the onset of apoptosis of the COLO 205 cells through the production of ROS.
The results obtained from this study suggest that the peptides extracted from Momordica dioica and Solanum trilobatum can be used as potential novel drugs against human colon cancer.
Acknowledgement
We would like to express our deepest gratitude to the Department of Biotechnology, School of Bioengineering, Faculty of Engineering & Technology, SRM Institute of Science and Technology, Kattankulathur 603203, Tamilnadu, India for helping with the laboratory facilities and also thank SRM-DBT for providing the facility to use the fluorescent microscopy.
Conflict of Interests
Authors declare that there is no conflict of interest.
References
- Moore AE, Sabachewsky L, Toolan H. Culture characteristics of four permanent lines of human cancer cells. Cancer research. 1955; 15:598-602.
- Labianca R, Nordlinger B, Beretta GD, Mosconi S, Mandalà M, Cervantes A, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of oncology. 2013; 24:64-72.
CrossRef - Capello A, Krenning EP, Bernard BF, Breeman WA, Erion JL, de Jong M. Anticancer activity of targeted proapoptotic peptides. J Nucl Med. 2006;47:122-129.
- Germain H, Chevalier E, Matton DP (2006). Plant bioactive peptides: an expanding class of signaling molecules. 2006; 84:1-19.
CrossRef - Guzmán-Rodríguez JJ, Ochoa-Zarzosa A, López-Gómez R, López-Meza JE. Plant antimicrobial peptides as potential anticancer agents. Bio Med Res Int. 2015.
CrossRef - Lee ACL, Harris JL, Khanna KK, Hong JH. A comprehensive review on current advances in peptide drug development and design. Int J Mol Sci. 2019; 20:2383.
CrossRef - Latha PS, Kannabiran K. Antimicrobial activity and phytochemicals of Solanum trilobatumAfr J Biotechnol. 2006.
- Doss A, Mubarack HM, Dhanabalan R. Antibacterial activity of tannins from the leaves of Solanum trilobatumIndian J Sci Technol. 2009: 2:41-43.
CrossRef - Jain A, Soni M, Deb L, Jain A, Rout SP, Gupta VB, et al. Antioxidant and hepatoprotective activity of ethanolic and aqueous extracts of Momordica dioica leaves. J Ethnopharmacol. 2008;115:61-66.
CrossRef - Talukdar SN, Hossain MN. Phytochemical, phytotherapeutical and pharmacological study of Momordica dioica. J Evidence-Based Complementary Altern Med. 2014.
CrossRef - Al Akeel R, Mateen A, Syed R, Alyousef AA, Shaik MR. Screening, purification and characterization of anionic antimicrobial proteins from Foeniculum Vulgare. Molecules. 2017; 22:602.
CrossRef - Chen B, Colgrave ML, Dal NL, Rosengren KJ, Gustafson KR, Craik DJ. Isolation and Characterization of Novel Cyclotides from Viola hederaceae solution structure and anti-hiv activity of vhl-1, a leaf-specific expressed cyclotide. J Biol Chem. 2005;280:22395-22405.
CrossRef - Uchoa AF, Souza PAS, Zarate RML, Gomes Filho E, Campos FAP. Isolation and characterization of a reserve protein from the seeds of Opuntia ficus-indica (Cactaceae). Braz J Med Biol Res. 1998;31:757-761.
CrossRef - Pitchai D, Roy A, Ignatius C. In vitro evaluation of anticancer potentials of lupeol isolated from Elephantopus scaber L. on MCF-7 cell line. J Adv Pharm Technol Res. 2014;5:17.
CrossRef - Roy S, Rawat AK, Sammi SR, Devi U, Singh M, Gautam S, et al. Alpha-linolenic acid stabilizes HIF-1 α and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget. 2017;8(41):70049–71.
CrossRef - Wu D, Yotnda P. Production and detection of reactive oxygen species (ROS) in cancers. J Vis Exp. 2011; (57):2–5.
CrossRef - Hernández-Ledesma B, Dávalos A, Bartolomé B, Amigo L. Preparation of antioxidant enzymatic hydrolysates from α-lactalbumin and β-lactoglobulin. Identification of active peptides by HPLC-MS/MS. J Agric Food Chem. 2005;53:588-593.
CrossRef - Hashempour H, Koehbach J, Daly NL, Ghassempour A, Gruber CW. Characterizing circular peptides in mixtures: sequence fragment assembly of cyclotides from a violet plant by MALDI-TOF/TOF mass spectrometry. Amino acids; 2013;44: 581-595.
CrossRef - Pistollato F, Giampieri F, Battino M. The use of plant-derived bioactive compounds to target cancer stem cells and modulate tumor microenvironment. Food Chem Toxicol; 201575:.58-70.
CrossRef - Voss C, Eyol E, Berger MR. Identification of potent anticancer activity in Ximenia americana aqueous extracts used by African traditional medicine. Toxicol Appl Pharmacol. 2006; 211:177-187.
CrossRef - Chan FL, Choi HL, Chen ZY, Chan PS, Huang Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Letters. 2000;160:219-228.
CrossRef - Li PG, Mu TH, Deng L. Anticancer effects of sweet potato protein on human colorectal cancer cells. World J Gastroenterol.2013;19:.3300.
CrossRef - Li JT, Zhang JL, He H, Ma ZL, Nie ZK, Wang ZZ, et al. Apoptosis in human hepatoma HepG2 cells induced by corn peptides and its anti-tumor efficacy in H22 tumor bearing mice. Food Chem Toxicol.2013;51:297-305.
CrossRef - Kim YS, Li XF, Kang KH, Ryu B, Kim SK. Stigmasterol isolated from marine microalgae Navicula incerta induces apoptosis in human hepatoma HepG2 cells. BMB reports.2014;47:433.
CrossRef - Venugopal K, Ahmad H, Manikandan E, Arul KT, Kavitha K., Moodley MK, et al. The impact of anticancer activity upon Beta vulgaris extract mediated biosynthesized silver nanoparticles (ag-NPs) against human breast (MCF-7), lung (A549) and pharynx (Hep-2) cancer cell lines. J Photochem Photobiol. 2017; 173:99-107.
CrossRef - Jin Y, Duan LX, Xu XL, Ge WJ, Li RF, Qiu XJ, et al. Mechanism of apoptosis induction in human hepatocellular carcinoma cells following treatment with a gecko peptides mixture. Biomedical reports; 2016;5:73-78.
CrossRef - Vethakanraj HS, Babu TA, Sudarsanan GB, Duraisamy PK, Kumar SA. Targeting ceramide metabolic pathway induces apoptosis in human breast cancer cell lines. Biochem Biophys Res Commun; 2015;464:833-839.
CrossRef - Elumalai P, Gunadharini DN, Senthilkumar K., Banudevi S, Arunkumar R, Benson CS, et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicology letters. 2012; 215:131-142.
CrossRef - Umayaparvathi S, Meenakshi S, Vimalraj V, Arumugam M, Sivagami G, Balasubramanian T. Antioxidant activity and anticancer effect of bioactive peptide from enzymatic hydrolysate of oyster (Saccostrea cucullata). Biomedicine & Preventive Nutrition.2014;4:343-353.
CrossRef - Ebrahim K, Shirazi FH, Vatanpour H, Kobarfard F, Rabiei H. Anticancer activity of cobra venom polypeptide, cytotoxin-II, against human breast adenocarcinoma cell line (MCF-7) via the induction of apoptosis. J Breast Cancer.2014; 17:314-322.
CrossRef - Hsin YH, Chen CF, Huang S, Shih TS, Lai PS, Chueh PJ. The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicology letters. 2008;179:130-139.
CrossRef - Xie CM, Chan WY, Yu S, Zhao J, Cheng CH. Bufalin induces autophagy-mediated cell death in human colon cancer cells through reactive oxygen species generation and JNK activation. Free Radical Biol Med. 2011;51:1365-1375.
CrossRef - Ortiz-Martinez M, de Mejia EG, García-Lara S, Aguilar O, Lopez-Castillo LM, Otero-Pappatheodorou JT. Antiproliferative effect of peptide fractions isolated from a quality protein maize, a white hybrid maize, and their derived peptides on hepatocarcinoma human HepG2 cells. J Funct Foods. 2017; 34:36-48.
CrossRef - Pritchard, S.R., Phillips, M., Kailasapathy, K. Identification of bioactive peptides in commercial Cheddar cheese. Food research international.; 2010; 43(5): 1545-1548.
CrossRef - Neves, A.C., Harnedy, P.A., O’Keeffe, M.B., FitzGerald, R.J. Bioactive peptides from Atlantic salmon (Salmo salar) with angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory, and antioxidant activities. Food Chemistry.; 2017; 218: 396-405.
CrossRef - Ahmed, A.S., El-Bassiony, T., Elmalt, L.M., Ibrahim, H.R. Identification of potent antioxidant bioactive peptides from goat milk proteins. Food Research International.; 2015; 74: 80-88.
CrossRef - Kumar, L.V., Shakila, R.J., Jeyasekaran, G. In vitro Anti-Cancer, Anti-Diabetic, Anti-Inflammation and Wound Healing Properties of Collagen Peptides Derived from Unicorn Leatherjacket (Aluterus Monoceros) at Different Hydrolysis. Turkish Journal of Fisheries and Aquatic Sciences;2019; 19(7): 551-560.
CrossRef - Li, M., Zhang, Y., Xia, S., Ding, X. Finding and isolation of novel peptides with anti-proliferation ability of hepatocellular carcinoma cells from mung bean protein hydrolysates. Journal of Functional Foods;2019; 62.
CrossRef - Liu, K., Liu, P.C., Liu, R., Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Medical science monitor basic research.;2015; 21: 15–20.
CrossRef - Taniya, M.S., Reshma, M.V., Shanimol, P.S., Krishnan, G., Priya, S. Bioactive peptides from amaranth seed protein hydrolysates induced apoptosis and antimigratory effects in breast cancer cells. Food Bioscience., 2020.
CrossRef - Eruslanov, E., Kusmartsev, S. Identification of ROS using oxidized DCFDA and flow-cytometry. In Advanced protocols in oxidative stress II. Humana Press, Totowa, NJ.;2010; 594: 57–72.
CrossRef - Brodská, B., Holoubek, A. Generation of reactive oxygen species during apoptosis induced by DNA-damaging agents and/or histone deacetylase inhibitors. Oxidative medicine and cellular longevity; 2011.
CrossRef