Nataliia Maika1 , Natalia Kalyniuk
, Natalia Kalyniuk 2, Valentyna Sloma1
2, Valentyna Sloma1 , Liudmyla Sheremeta3*
, Liudmyla Sheremeta3* , Leonid Kravchuk2
, Leonid Kravchuk2 , Kateryna Stefanyshyn3
, Kateryna Stefanyshyn3 and Larysa Kravchuk4
and Larysa Kravchuk4
1Department of Civil Law and Process, Ternopil National Economic University, Ternopil, Ukraine.
2Department of Pedagogy of the Higher School and Social Science, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
3Department of the Ukrainian Language, I. Horbachevsky Ternopil National Medical University, Ternopil, Ukraine.
4Department of General Chemistry, I. HorbachevskyTernopil National Medical University, Ternopil, Ukraine.
Corresponding Author E-mail: liudmilasher64@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2180
Abstract
The feasibility of training future medical professionals on the basis of interdisciplinary integration is explored in the article. Analyzed through the lens of medical knowledge in legal knowledge, drug reimbursement as a process by which the health care system affects the availability of medicines and medical services to the public. The peculiarities of drug reimbursement in Ukraine have been investigated using the comparative legal method.
Keywords
Healthcare; Higher education; Human rights; Interdisciplinary integration; Reimbursement
Download this article as:| Copy the following to cite this article: Maika N, Kalyniuk N, Sloma V, Sheremeta L, Kravchuk L, Stefanyshyn K, Kravchuk L. Basic of Medicinal Products Reimbursement: A Comparative-legal Analysis to Ukraine: An update. Biomed Pharmacol J 2021;14(2). |
| Copy the following to cite this URL: Maika N, Kalyniuk N, Sloma V, Sheremeta L, Kravchuk L, Stefanyshyn K, Kravchuk L. Basic of Medicinal Products Reimbursement: A Comparative-legal Analysis to Ukraine: An update. Biomed Pharmacol J 2021;14(2). Available from: https://bit.ly/3i2c0kg |
Introduction
Historically, in the Soviet period of existence of Ukraine, topical issues of medical science and practice were studied by future specialists in the medical field in higher educational establishments within such disciplines as “Forensic medicine”, “Social hygiene”, “Organization of health care”. However, if this was justified earlier, today, as national healthcare legislation is rapidly evolving, implementing international human rights standards, knowledge of the legal bases of professional activity by future professionals in the medical and pharmaceutical industries is a need of the new age. Discipline “Medical Law” taught at I. Horbachevsky Ternopil National Medical University provides medical students with assimilation of the necessary amount of knowledge not only about the current legislation on regulation of medical activity in Ukraine, but also systematic material on the right to health and health care in the context of international and regional standards on human rights. In this study, we consider it appropriate to prove the need for professional training of future medical professionals on the basis of interdisciplinary integration through the case study of drug reimbursement. A successful combination of medical and legal knowledge will give rise to the formation of professional identity of future professionals in the medical field. The problem of drug reimbursement was chosen as an example because it is a common international practice in health care, the name of the process by which the health care system affects the availability of medicines and medical services to the public1. Basic components of health care and quality medical aid are the availability of socially important goods for the population, among which the medicinal products take one of the most important places.
Development Theoretical bases of the study
The state of health care and the quality of medical services delivery in Ukraine have long required major changes. The situation before 2017 in the field of pricing for medicines made it impossible to guarantee the constitutional rights of citizens to medical and pharmaceutical assistance 2.
Insufficient access to public funding of medicine in Ukraine complicates access to the medicinal products. Per capita health care spending in absolute terms is extremely small in Ukraine, compared to developed countries. As of 2007, the consumption of medicines in Ukraine was $ 22.6 per capita, being the lowest in Europe. Although, as a percentage of GDP, health care expenditures are quite decent, accounting for 7.8%. But the state’s role in these expenditures is no more than 3%3.
The 2018 World Health Organization (WHO) publication states that the expenditures of the state budget on health care as a share of gross domestic product have remained steadily low in recent years in Ukraine. Thus, they were 2.9% in 2015, which is well below the average for the WHO European Region countries (5%) and the European Union (6%). As a result, the proportion of patients’ expenses they pay out of their own funds, reached 48% in total health care expenditure in 2015, being one of the highest figures for the European region4.
The main problem for the availability of medicinal products to the population was that the state did not have sufficient leverage to influence their pricing. As a consequence, there was an unreasonable price increase for major drugs on the pharmaceutical market, which puts a burden on the end consumer, i.e. on a patient. The grounds and consequences of monopolization in the pharmaceutical market required specific state interventions through regulatory mechanisms.
The aforementioned clearly indicates that the relevance of the right regulation of the reimbursement mechanism for medicinal products in Ukraine requires substantial improvement, which will stimulate the commercial activity of pharmaceutical manufacturers and will significantly increase the availability of medical services in Ukraine.
The emergence of new models and new forms of activity in the analyzed sector requires increased attention from the authorities and effective levers of legal regulation. In order to intensify the economic activity of the subjects of such a market, various forms of state participation, which are predominantly incentive, are considered among other things. But the involvement of the state is explained not only by the desire to stimulate and develop the pharmaceutical industry, but also by social responsibility to citizens for the implementation of state guarantees of access to medical care. The latter is achieved through a reimbursement mechanism.
Studies of the content, order, conclusion, execution and termination of the drug reimbursement agreement, its importance in pharmaceutical practice were carried out by lots of scientists 5-13.
Methodology
In order to achieve this goal and to solve the main tasks, as well as to substantiate the results of the study, common law and special methods used in legal science were applied.
In the course of the study, the following methods of scientific cognition were used: comparative-legal, formal-logical, dialectical, system-structural analysis and synthesis. Research and substantiation of the basic concepts used in the work were performed, first of all, using the dialectical method as a general scientific method of knowledge of social and legal phenomena in the context of their development, changes and contradictions. Due to the formal-logical construction and principles of dialectics, a holistic definition was given for each of the concepts in the development and in the manifestation of all the separate (contradictory) issues of reimbursement, which were defined in the analysis. System-structural analysis has made it possible to determine the place of reimbursement as an integral part of the principle implementation, entrenched in Art.49 of the Constitution of Ukraine – accessibility of medical care. Consistent use of the comparative legal method has led to the study of relevant experience in other countries and allowed us to make constructive formulations of the basic provisions of the work that can be applied in different legal systems of the present.
Results
Reimbursement is the basis for the successful implementation of the availability of medicines to patients. This system is considered as a socio-economic system, which aims at ensuring the availability of medicines and pharmaceutical assistance as a whole, the subject of which is the authorized bodies making compensation payments from certain sources of funding. The object of the system is certain categories of diseases and patients.
Socio-economic strategies for the functioning of the reimbursement system are conditionally divided into two systems: state system of medical and pharmaceutical assistance (having a purely social character); non-state system (the private nature of pharmaceutical assistance prevails). Under the conditions of functioning of the state reimbursement system of medicines, they are generally issued as a share of primary care delivery and inpatient treatment; they are also financed from the sources of state budget, social insurance funds and compulsory health insurance 14.
Reimbursement works in many countries around the world and is an effective system for providing patients with treatment. The state or insurance company reimburses the cost of certain medicines for certain categories of diseases. In each country, the state decides on what rules the drug reimbursement system will have. It also determines the sources of funding for the program, the conditions for granting compensation for medicines, the methods of price regulation by the state, the principles of selection of diseases and medicines to be reimbursed, etc.
Taking into account the European experience and drug prices, Ukraine has taken on the experience of developed countries and prepared its Affordable Medicines Program (hereinafter AMP). Prior to the introduction of the AMP, there was no system of state reimbursement for prescription drugs in the outpatient sector. This program became effective on April 1, 2017, which provides for a new price regulation of drugs for the treatment of 3 categories of diseases (cardiovascular disease (hereinafter – CVD), type II diabetes mellitus (hereinafter – DM) and bronchial asthma (hereinafter: (BA), as well as the procedure for granting subventions from the state budget for reimbursement of medicines 15.
For the successful implementation of the AMP, a number of legal acts were adopted. On the one hand, they were aimed at ensuring the availability of medicines and on the other hand, at the effective use of budget funds aimed at providing subsidies for the reimbursement of medicines for the outpatient treatment of certain diseases. In particular, the Resolution No.152 of the Cabinet of Ministers of Ukraine as of March 17, 2017 “On Ensuring the Availability of Medicines” (hereinafter – Resolution No. 152)16 approved the “Procedure for Reimbursement of the Cost of Medicines”17 and “Procedure for Determining the Amount of Reimbursement for the Cost of Medicines to be Compensated”18, which relieved the tension that has arisen in the pharmaceutical market in the field of price regulation of some drugs for the treatment of cardiovascular diseases, type II diabetes and bronchial asthma, as well as regulated the mechanism of compensation of their value.
The marginal price for admission to the Affordable Medicines Program is determined by comparing the prices of drugs with one active substance in 5 neighbouring countries: Poland, Slovakia, Hungary, Czech Republic and Latvia.
Less than 1.5% of medicines are marketed by the state at the expense of public funds. Eastern Europe has one third of the medicines on the market being reimbursed, and the developed European countries have even more.
As a rule, it is expected in almost all EU countries that the patient will participate in the payment of the medicines he needs in the form of minimum additional payment or as a percentage of the prescription cost.
Ukraine has chosen for itself the model of full reimbursement of the drug at the lowest price, for others – patient pays the difference in a pharmacy institution. This, in turn, improves access to treatment for all segments of the population.
In order to determine the amount of reimbursement of the cost of drugs, the Ministry of Health of Ukraine (hereinafter – MoH) is obliged to establish a Register of Medicinal Products, the value of which is to be reimbursed. According to the Order, the trade names of medicinal products are entered in the Register, the wholesale-selling price for packing of which does not exceed the marginal wholesale-selling price in terms of the daily dose of the medicinal product.
Updating the Register of marginal wholesale prices for medicinal products is planned to be carried out twice a year: as of January 1 and July 1 of the current year. Recalculation of the Register of marginal wholesale prices for medicinal products should also be made in case of change of the official hryvnia exchange rate to the US Dollar, established by the National Bank of Ukraine for more than 5 percent per month or 10 percent for the quarter 19.
In order to obtain a full or partial reimbursement of medicines, pharmacy institutions must apply to the budget institutions for concluding an Agreement on Reimbursement of Medicines. The institutions, in turn, should, on the basis of concluded agreements, form a list of pharmacy institutions providing medication under the Program and create adequate information support for consumers. The Program is intended to compensate only for the cost of the cheapest medicines that have applied for the Affordable Medicines Program. It means that patients will be able to get these drugs for free. More expensive drugs, the price of which does not exceed the benchmark level, will be available for patients by paying the difference between the minimum price and the retail price of the selected drug.
Medicines, the cost of which exceeds the reference price in 5 neighbouring countries, are not covered by the reimbursement program and therefore the subventions for reimbursement of such medicines are not provided by the Program 17.
Back in 2000, after the usual centralized health care system for the post-Soviet countries, Bulgaria was one of the first countries to apply reimbursement. In case of outpatient treatment, a patient receives a prescription drug from a pharmacy, and the National Health Insurance Fund of Bulgaria reimburses the pharmacy for a portion or all of its value. Drugs, according to the degree of disease risk, the availability of alternatives and other factors, fall into groups with different levels of compensation – 100%, 75% and 50%. In other words, the patient is either completely free of charge for the medication, or he/she pays some of the cost – 25% or 50%. The patient is entitled to 100% reimbursement of the cost of the medication only under the condition of passing a physical evaluation board. The budget of the National Health Insurance Fund consists of insurance premiums paid by employed citizens. For certain categories of citizens who do not work (students, disabled, pensioners, unemployed, children, etc.), the premiums are paid by the state 20.
Bulgaria’s experience in implementing the drug reimbursement system (cost recovery) has been taken over by Ukraine in the outpatient segment.
We can note the positive experience of Bulgaria, which could be adopted in the future:
1) creating a register of patients participating in the AMP will allow to plan budget expenditures effectively, as well as will create an effective administration system;
2) not only the price but also the indicators of quality and efficiency should be a criterion for including the drug in the list for reimbursement when forming the Register of Medicinal Products, the cost of which is to be reimbursed;
3) the system of reimbursement should be built on the insurance principle of accumulation of funds, not based on budgetary expenditures.
The Government of France applies in its model a strict control over the prices of medicines to be compensated. Therefore, the mechanism for setting prices and determining the list of medicines to be compensated is quite complex. After obtaining a marketing authorization, the options of market access are determined by a special committee, which, after reviewing the comprehensive dossier for the product, determines the level of compensation based on the pharmacoeconomic efficacy indices depending on the severity of the disease.
In our view, the positive side is that the French government has not limited itself to a list of particular categories of diseases.
The experience of Belgium during the formation of reimbursement system shows us that since 2001, the Ministry of Economy has applied a system of marginal prices for all registered medicines. The Ministry of Health and Social Welfare evaluates the therapeutic and economic value of medicines by comparing them to analogues. Representatives of insurance companies, manufacturers, wholesalers, pharmacies and patients are involved in agreeing of prices. The reimbursement commission includes representatives of insurance companies, medical and pharmaceutical associations, as well as independent experts. The final decision on reimbursement is made by the Ministry: if the decision is not made within 180 days, the price offered by the manufacturer is accepted. Prices for all reimbursed medicines are reviewed in 1.5-3 years.
In Spain, in order to obtain the status of the medicinal product to be reimbursed in medical practice, an application is submitted to the authorized Directorate for Provision of Medicinal Products to the Population. If the drug has not received the status of reimbursed, pricing is flexible. There are four levels of compensation: 100% for inpatient medications only, 70% for some chronic conditions, 60% for most medicines, 0% for over-the-counter medicines.21
Having analyzed the experience of European countries, we can point out that the compensation of medicines does not only depend on the cost of medicines, but on the needs of the consumer, which makes it possible to exercise the right to medical care in full.
In addition, we see that collective decision-making to include drugs in the list of available medicines is more vital. We believe that representatives of insurance companies, medical and pharmaceutical associations, as well as independent professionals must be involved in making such decisions.
Separately, we want to focus on the issuing of medicines participating in the AMP. For the purpose of controlling the targeted use of subventions, the issue of medicines under the Program is expected to be made only on prescription. From April 1 of 2019, patients using the AMP started receiving medication on electronic prescription. It can only be obtained from a family doctor, therapist or pediatrician with whom the patient has a signed declaration (medical service agreement).
Thus, as of today, the adoption of the laws of Ukraine “On Electronic Documents and Electronic Document Management”22, “On State Financial Guarantees of Health Care of the Population”23 was a prerequisite for adoption of the Order of the Ministry of Health of Ukraine No. 35 dated 18.04.2018 “On Amendments to the Order No.360 of the Ministry of Health of Ukraine as of July 19, 2005”24.
The main novelty of this legal act is the legal introduction of such concept as “electronic prescription”, which is written according to the same rules as the usual prescription, authorized by the person in the information (information and telecommunication) system of the entity and signed by electronic digital signature using a strengthened public key certificate.
The electronic prescription was made possible by the transfer of the AMP to the National Health Service of Ukraine (hereinafter NHSU). This will allow patients to receive prescription drugs at any pharmacy in Ukraine that has a contract with the NHS, without reference to the area where the prescription was issued. This will make the program even more accessible to patients.Schematically, the AMPprocess in actioncan be represented as follows (Figure1).
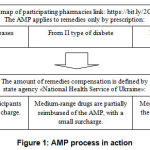 |
Figure 1: AMP process in action |
Approval of dispensing in a pharmacy of a medicinal product is possible by prescription only if it belongs to one of three groups of diseases: cardiovascular type, bronchial asthma, type II diabetes mellitus. Reimbursement is carried out by the National Health Service of Ukraine. In turn, patients buy medicines from pharmacies from the list located at https://bit.ly/2CRGorZon, with a small surcharge or take them for free.Practically it looks like this: to receive the selected medication free of charge or with a small surcharge, you need to see a doctor, get a properly prescribed prescription with the active ingredient, come to the pharmacy participating in the program, and get the selected remedies.
Similar programs operate in European countries. The difference there is that there pharmacies are health care facilities that focus on profits from trading under a similar program. At the same time, the state pays European pharmacies for patient care – 15-19 euros for each patient 25.
However, not everything is as great as it seems at first glance. In this context, it is impossible not to raise the issue of implementation of E-prescriptions. Of course, we support this progressive step in the healthcare system, which has been successfully operating in many countries around the world, but today we also have to face a number of problems.
The first problem was the lack of a transitional period of enforcing or testing, and now the system is technically malfunctioning. Therefore, doctors are now again duplicating prescriptions on paper or printing them if possible.
The second problem is that there is a category of patients that was not considered – older population that does not always find common ground with modern gadgets. Therefore, we believe that it is advisable to have paper prescriptions that are digitalized along with electronic prescriptions.
The third problem is the registration of pharmacies in the system, their reporting and the need to pay for each processed prescription. Pharmacies have to incur additional expenses.
Again, let us turn to the statistics data. If by April 1, 2019, every third pharmacy had a signed contract for reimbursement of medicines, then according to today’s data, such number of pharmacies is constantly decreasing.
Legislators are also ambiguous about this issue. As of 09.07.2019, non-affiliated people’s deputy Andriy Derkach filed a lawsuit against the Cabinet of Ministers of Ukraine on the implementation of the reimbursement of medicinal products exclusively by electronic prescriptions as of April 1. The plaintiff requests the court to declare a certain paragraph of the government decree “Some Issues of Drug Reimbursement” as illegal and invalid, namely – since April 1, 2019, drug reimbursement shall be carried out solely by electronic prescriptions issued through the electronic health care system in accordance with the Order of the approved resolution of the CMU “On Availability of Medicines”26.
Of course, in the first stage of the introduction of E-prescription, one can hear thoughts about not accepting such a mechanism. It is clear that objective arguments can be made for this: more time is needed to write a prescription, because the doctor is not yet accustomed to the new system, or the pharmacy will have a queue when dispensing medications by electronic prescriptions. However, we consider this to be a temporary inconvenience in the transition period, as with any other reform. Thanks to the transparency of the system, the state will be able to manage the processes of payments, which should help to save budget funds for their redistribution to the most priority measures in the field of health care.
Electronic health systems in the world offer the prospect of easy data sharing between health care facilities at various levels, which have already been implemented in many countries. Moreover, cross-border exchange of patient information and electronic prescriptions becomes possible. The most famous in this aspect is the European project – European Patient Smart Open Services (epSOS), in which 23 countries participated. Its purpose is to provide healthcare professionals with basic information on patients’ case histories and the nature of care provided for them 27.
An urgent challenge for Ukraine is to ensure the protection of personal data contained in electronic systems.
Thus, the updated rules on personal data processing established by the General Data Protection Regulation (EU Regulation 2016/679 as of 27 April 2016, or GDPR – General Data Protection Regulation) have come into force in the EU. This Regulation has direct effect in all 28 EU countries and replaces the Framework Directive on Personal Data Protection 95/46/EU as of October 24, 1995. An important nuance of the GDPR is the extraterritorial principle of the new European rules on personal data processing. The new regulation provides EU residents with the tools to fully control their personal data. Since May 2018, responsibility for violating personal data processing rules has increased: according to GDPR, fines amount to € 20 million, or 4% of an entity’s annual income.
Pursuant to this Regulation, personal data is any information relating to an identified natural person (data subject), by which he or she can be directly or indirectly identified. Such information shall include, but not limited to, name, location, online identifier, or one or more factors specific to that person’s physical, physiological, genetic, mental, economic, cultural, or social identity (p.1 of Art.4). The definition is broad and clear enough to realize that even IPs can also be personal data. It is important to note that there are certain types of personal data that fall into the category of special or sensitive personal data. This is information that reveals racial or ethnic origin, political views, religious or philosophical beliefs, as well as union membership. In addition, this group includes genetic, biometric data used to identify a person, health status, information relating to sexual life or sexual orientation (Art.9).GDPR has an extraterritorial effect and applies to all companies processing personal data of EU residents and citizens, regardless of the location of such company 28.
In the legislation of Ukraine, the protection of personal data is based on the legislation on protection of information in the information and telecommunication systems, and the processing of personal data in the electronic health care system is carried out in compliance with the requirements of the Law of Ukraine “On Personal Data Protection”20, 29, the Law of Ukraine “On State Financial Guarantees for Medical Care of the Population”23 stipulates that access to a patient’s data is possible only with the consent of the latter (except in cases of signs of a direct threat to the patient’s life, the inability to obtain the consent of such patient or his legal representatives, and by court decision). There are no other specific regulations to ensure the safety of patients’ personal data, if any.
Thus, we believe that the introduction of E-prescriptions without special legal support, including without the solution of problems of technical equipment, and, as a consequence, addressing the issue of protection of patients’ personal data in accordance with GDPR, is a priority part of the AMP implementation.
Conclusions
The peculiarity of the national AMP program is that medicines can be obtained free of charge or with a partial surcharge in pharmacies included in a special register, provided that the disease belongs to only one of the three groups of diseases. We consider it necessary to expand the list of diseases that will be included in the AMP and the preferential interest of pharmacies, which have not yet been included in the electronic map of pharmacies.
In the event of a malfunction of the electronic system, electronic prescriptions must be duplicated on paper, which is convenient for elderly patients who do not know how to handle digital devices and inconvenient for doctors who use their material resources. At the same time, additional costs incurred by pharmacies in connection with writing paper prescriptions are not compensated by the state.
The protection of personal data is the main legal concern when dealing with the electronic AMP system.
References
- Melnychuk I., Fedchyshyn N., Pylypyshyn O., Vykhrushch A. Philosophical and Cultural Aspects of Medical Profession: Philosophical and Conceptual Peculiarities. Cultura: International Journal of Philosophy of Culture and Axiology,2019; 16(1): 165-174.
CrossRef - Ministry of Health of Ukraine. The first month of robots with the “Accessible Liki” programs. Ministry of Health of Ukraine; 2017.Available from: https://moz.gov.ua/article/news/pershij-misjac-roboti-programi-dostupni-liki
- Ignatov V. Ukrainian pharmaceutical market: industry regulation 2013-2014. Аua, 2014. Available from: https://www.apteka.ua/ article/279870
- WorldHealthOrganisation. Evaluation of the Affordable Medicines Programme in Ukraine. Available from: https://www.euro. who.int/ data/assets/pdf_file/0019/400429/52308-WHO-Affordable-Medicines-Programme-Ukraine-UKR_low_V7.pdf
- Brozek G., Lawson J., Shpakou A., Fedortsiv O., Grishchuk L. A., Rennie D., Zejda J. Childhood asthma prevalence and risk factors in three Eastern European countries – the Belarus, Ukraine, Poland Asthma Study (BUPAS): An international prevalence study. BMC Pulmonary Medicine, 2016; 16(11).
CrossRef - GammieT, Lu Y., Babar Z.U.Access to orphan drugs: a comprehensive review of legislations, regulations and policies in 35 countries. PLoS One,2015; 10(10).
CrossRef - Gonçalves Advanced therapy medicinal products: value judgement and ethical evaluation in health technology assessment. Eur J HealthEcon, 2020; 21: 311-320.
CrossRef - Jönsson B., Hampson G., Michaels J., Towse A., Schulenburg J-M., Wong O. Advanced therapy medicinal products and health technology assessment principles and practices for value-based and sustainable healthcare. Eur J HealthEcon, 2019; 20(3): 427-438.
CrossRef - Jørgensen J., Servos S., Kefalas P.The potential price and access implications of the cost-utility and budget impact methodologies applied by NICE in England and ICER in the US for a novel gene therapy in Parkinson’s disease. J MarkAccessHealthPolicy, 2018; 6(1).
CrossRef - Logoyda L., Korobko D., Ivanusa I., Serhii K. Development of the methodology of the chromatographic determination of nifedipine in medicines. Asian J Pharm Clin Res, 2017; 10(3): 149-152.
CrossRef - Logoyda L., Korobko D., Samohalska O., Berdey I., Kuchmerovska T. Development of methodology for identification of captopril in medicines.Asian J Pharm, 2016; 10(3): 168-171.
CrossRef - Roeder A.Internationaler Market Access am Beispiel Advanced Therapy Medicinal Product. In: Market Access in der Medizintechnik, T. Schubert, T. Vogelmann Eds. Wiesbaden, Springer Fachmedien Wiesbaden; 2019: 187-220.
CrossRef - Shukla V., Seoane-Vazquez E., Fawaz S., Brown L.M., Rodriguez-Monguio R. The landscape of cellular andgene therapy products: Cost, approvals, and discontinuations. Hum Gene Ther,2020; 30(3): 102-113.
CrossRef - Maidanyk R. Access tomedicinesinUkraine: theprincipleofcooperationandthelegalmodelofthepharmaceuticalmarket. Lawandcivilsociety, 2014; 1(6): 165-176.
- MinistryofHealthofUkraine.OnamendmentstotheRegisterofmedicinessubjecttoreimbursement. 2019, July 29. Available from: https://moz.gov.ua/article/ministry-mandates/nakaz-moz-ukraini-vid-20082019–1841-pro-vnesennja-zmin-do-reestru-likarskih-zasobiv-jaki-pidljagajut-reimbursacii-stanom-na-29-lipnja-2019-roku?preview=1
CrossRef - Ministry of Health. National Council of Prices and Reimbursement of Medicinal Products. Available from: http://www.mh.government.bg/en/ministry/commissions/national-council-prices-and-reimbursement-medicinal-products/
- Levchenko N. Mechanismofreimbursementofmedicines (reimbursement) underthegovernmentprogram “AffordableMedicines”. Publicadministrationandcustomsadministration, 2017; 2: 70-79.
- Verkhovna Rada of Ukraine. The procedure for determining the amount of reimbursement of medicines. 2017. Available from: https://zakon.rada.gov.ua/laws/show/152-2017-%D0%BF#n26
- VerkhovnaRadaofUkraine. About the statement of the Procedure for calculation of marginal wholesale and selling prices for medicines on the basis of reference prices. Available from: https://zakon.rada.gov.ua/laws/show/z0012-17#Text
- VerkhovnaRadaofUkraine. About personal data protection. Available from: https://zakon.rada.gov.ua/laws/show/2297-17#Text
- Kashkanova N. Theory and practice of intellectual property, 2015; 4: 80-88.
- VerkhovnaRadaofUkraine. About electronic documents and electronic document management. 2003. Available from: https://zakon.rada.gov.ua/laws/show/851-15#Text
- VerkhovnaRadaofUkraine.Aboutthestatefinancialguaranteesofmedicalserviceofthepopulation. 2018. Available from: https://zakon.rada.gov.ua/laws/show/2168-19#Text
- Verkhovna Rada of Ukraine. Amendments to the Rules for Prescribing Medicines and Medical Devices. 2018. Available from: https://zakon.rada.gov.ua/laws/show/z0502-18#Text
- “Affordable Medicines”: Problems and Prospects. Merezha komunalnykh aptek “Farmatsiia”; n.d.Available from:http://pharmacy.kiev.ua/novyny/Dostupni_liky_problemy_ta_perspektyvy.html
- Unifiedstateregisterofcourtdecisions.Ontheopeningofproceedingsinanadministrativecase № 640/11234/19 onappealingtheresolutionoftheCabinetofMinistersofUkraineofFebruary 27, 2019 № 135 “Someissuesofreimbursementofmedicines”. Availablefrom: http://reyestr.court.gov.ua/Review/85960705
- EuropeanCommission.epSOS, eHealth initiative to support medical assistance while travelling and living abroad. Commission and its politics. 2008. Available from: https://ec.europa.eu/digital-single-market/en/news/epsos-ehealth-initiative-support-medical-assistance-while-travelling-and-living-abroad
- VerkhovnaRadaofUkraine.Regulation (EC) No 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of individuals with regard to the processing of personal data and on the free movement of such data and repealing Directive 95/46 / EC (General Data Protection Regulation). 2016. Available from: https://zakon.rada.gov.ua/laws/show/984_008-16
- VerkhovnaRadaofUkraine.On ensuring the availability of m 2017. Available from: https://zakon.rada.gov.ua/laws/show/152-2017-%D0%BF#Text








