Sree Sudha TY1*, Yakaiah Vangoori2 and Anjaly Mary Varghese2
1Department of Pharmacology, AIIMS-Raipur, Chhattisgarh, India, 492099
2Department of Pharmacology, Santhiram Medical College and General Hospital, Nandyal, A.P, India, 518502
Corresponding Author E-mail: sudhambbs2010@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2135
Abstract
Objective: The present study was conducted to identify, analyze the causality, and severity of adverse drug reactions and to find out the factors associated with ADR related factors. Adverse effects of drugs are identified as one of the main reason for increasing in-patient number in the hospital. This has become financial burden and also rise in mortality rate in society. The main purpose of the pharmacovigilance program is to identify the risks linked with the use of drugs. This study may be useful to identify and prevent adverse effects caused by drugs to increase the quality of life and ability of doctors to treat consequences of ADRs more effectively. The main aim of the present study was to explore and identify adverse effects caused by drugs and to improve patient safety with pharmacovigilance activities.
Method: An observational study was conducted as part of Pharmacovigilance program for 14 months (January 2018 - Feb 2019). ADRs reported from hospital were filled into Suspected ADR forms - CDSCO forms. Causality assessment was done based on WHO- UMC causality scale and severity was assessed using Hartwig-Siegel scale. By taking history of the patient, and by regular monitoring of the inpatients, the causative factors for ADR related hospital admissions were evaluated.
Results: Total 145 ADR reports were analyzed. Most of the ADRs were observed in females (60%). Majority of ADRs were caused by NSAIDs (32.4%), followed by antimicrobials (20%). Most common organ systems involved was skin (38%). Causality assessment showed 85.5% ADRs as probable. 18.6% of ADRs were of severe type and 51% moderate. ADR related hospital admissions were found in 53 cases (36.5%) and 92 cases of ADRs occurred during hospitalization (63.4%).
Conclusion: The study generated a data of ADRs that is useful to the clinicians for optimum and safe use of drugs in day to day practice and help in creating ‘P’ drug list. The major reasons for ADR related hospital admissions are OTC and non-compliance. Hence a constructive Pharmacovigilance to minimize ADR related hospitalization, treatment cost, morbidity and mortalities are the need of the hour.
Keywords
Adverse Effects; NSAIDs; OTC; Pharmacovigilance
Download this article as:| Copy the following to cite this article: Sudha T. Y. S, Vangoori Y, Varghese A. V. A Profile of Adverse Drug Reactions in a Teritiary Care Teaching Hospital and Associated Factors. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Sudha T. Y. S, Vangoori Y, Varghese A. V. A Profile of Adverse Drug Reactions in a Teritiary Care Teaching Hospital and Associated Factors. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/3e9g9Rx |
Introduction
According to world health organization (WHO), the definition of adverse drug reaction is “any noxious, unintended, or undesired effect of a drug that occurs at doses used in human for prophylaxis, diagnosis, or therapy (1). An adverse effects of drugs are main causes for patient illness and has increased health care costs (2). Adverse effects related death rate is in between fourth and sixth leading causes of death in the USA (3). In India also, many scientific studies have revealed that adverse effects of drugs leads to 1.8% of death rate out of 0.7% of total admissions (4). According to WHO, Pharmacovigilance program was defines as “the science and activities relating to the detection, assessment, understanding, and prevention of adverse drugs reactions or any other drug-related problems” (5). Many research studies were proved that patients admitted with adverse effects of drugs ranges from 2.0 to 21.4% and 1.7 and 25.1% of inpatients were reported ADR during their stay in hospital. (6-9). various factors can influence the susceptibility of adverse effects of drugs are multiple drug therapy, disease severity, age, and the type and number of drugs prescribed [10-15]. In developing and developed nations, the frequency and type of adverse drug reactions are due to disease prevalence, access to medicines, drug use patterns, and drug management systems (16). Reporting of adverse reactions of drugs have become mandatory in hospitals to evaluate causes for adverse effects (17). This may be useful to detect reasons and to set preventable measures for better patient safety and it gives more knowledge to doctors to minimize the problems (18, 19).
The present study was undertaken to:
Identify, analyze the causality, and severity pattern of ADRs reported from various departments.
Identify the factors/reasons associated with ADR related hospital admissions
Evaluate ADRs that occurred during inpatient stay at the Hospital
Facilitate the development of a pharmacovigilance service in a tertiary care hospital.
Material and Methods
This was a prospective observational study conducted at ADR monitoring center of SANTHIRAM MEDICAL COLLEGE and Hospital, Nandyal.
The study was conducted as part of pharmacovigilance program over 14 months (Jan 2018 – Feb 2019).
A total of 145 cases reported over a period of 14 months were included in the study.
The suspected adverse drug reactions reported to the pharmacovigilance center were filled into CDSCO reporting forms.
Temporal relationship between drug and event was assessed using WHO – UMC causality assessment scale.
The Severity of reactions was assessed using Hartwig and Siegel scale.
By taking thorough history of the patient, identified the causative factors for ADR related hospital admissions.
By regular monitoring of the inpatient and by using several parameters like data evaluation, drug and reaction characteristics, and treatment of reaction and its outcomes, evaluated ADRs that occur in hospitalized patients.
The data were analyzed and shown as number of cases and percentages in each table.
Results
A total of 145 ADRs were reported over 14 months. Of these, 40% were in males and 60% were in females (Figure: 1).
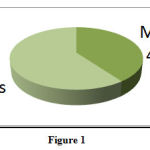 |
Figure 1 |
Gender – Wise distribution of ADRs
AGE wise Distribution of ADRs
Highest number of ADRs reported in the age group of 21- 40 years of age followed by >60 years of age group (Figure: 2).
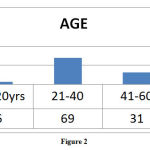 |
Figure 2 |
Department wise distribution of ADRs
Maximum number of cases were reported from the department of medicine (41.8%) followed by Dermatology (22%) and department of surgery (12.4%). (Figure: 3).
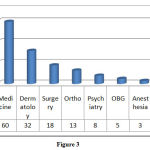 |
Figure 3 |
ADRs in various drug classes
Among the suspected drugs causing ADRs, highest percentage 32.4% accounted for NSAIDs, antimicrobial agents (AMAs) accounted for 20% of the total cases followed by other drugs accounted for 30.3% (Figure 4).
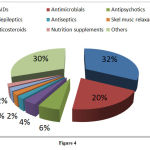 |
Figure 4 |
Organ systems involved in ADRs
The most common adverse drug reactions involved skin and appendages (37.9%) and CNS (23.5%) (Figure 5).
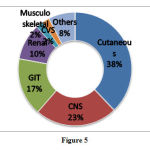 |
Figure 5 |
Causality Assessment
Assessment of ADRs using WHO-UMC causality assessment scale showed that 85.5% of ADRs were probable, 12.4% were classified as possible and 2.1% were certain to have occurred due to drug administration (Figure 6).
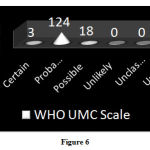 |
Figure 6 |
Severity Assessment- Hartwig-Siegel Scale.
Reported ADRs were mainly mild to moderate in severity (Figure: 7).
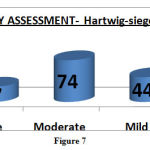 |
Figure 7 |
Frequency Distribution of Hospital Admissions due to ADR (Table : 1)
The patients got admitted due to adverse drug reactions is 53 out of 145 patients and high percentage was noted under severity i.e; 45.2%. All these 53 cases got admitted due to factors like self medication , over the counter use of drugs (OTC), non –compliance.
| Age | No : of Cases | Gender | Severity | Outcome | |||
| Male | Female | Mild | Moderate | Severe |
Recovered–
53 (100% ) |
||
| 1 m-20 yrs | 3 | 24
(45.2%) |
29
(54.7%) |
11
(20.7%) |
18
(33.9%) |
24
(45.2%) |
|
| 21-40 | 20 | ||||||
| 41-60 | 10 | ||||||
| >60 | 20 | ||||||
| Total:53
(36.5%) |
|||||||
ADRs occurred in Hospitalized Patients (Table :2)
The adverse drug reactions occurred in Hospitalized Patients is 92 out of 145 and higher percentage seen in females . 59.7% were moderate reactions and all cases were recovered.
| Age | No : of Cases | Gender | Severity | Outcome | |||
| Male | Female | Mild | Moderate | Severe | Recovered
92 (100% ) |
||
| 1m-20 yrs | 3 | 37
(40.2% ) |
55
(59.8% ) |
34
(37% ) |
55
(59.7% ) |
3
(3.2% ) |
|
| 21-40 | 53 | ||||||
| 41-60 | 18 | ||||||
| >60 | 18 | ||||||
| Total – 92
(63.5%) |
|||||||
Discussion
The fundamental role of Pharmacovigilance centers is to collect and process data regarding ADRs and to support hospitals in the identification of these reactions (20). The centers’ actions serve to reduce risks related to medication usage, improve patients’ quality of life, prevent iatrogenic diseases, and minimize health expenses. Pharmacovigilance program of India (PVPI) is monitoring peripheral ADR reporting centers to decrease drug induced complications, better patient compliance, quality of life and to reduce cost of health care. It was reported that many physiological systems were affected by drugs but highest affected system is skin that is about 38%. Its clinical symptoms appeared as multiple fluid filled lesions, skin rashes, flushing, and dried skin. In the present study, NSAIDs have shown highest prevalence of adverse effects among many drugs used in various conditions. Rechallenge was not done for ADRs because of ethical reasons which may affect the causality assessment. The treatment expenditure of ADRs was not assessed.
Conclusion
Study generated a data of ADRs, which will give information to the clinicians for optimum and safe use of drugs in day to day practices and helps in creating their own ‘P’ drug list. The major reasons for frequency distribution of HOSPITAL ADMISSIONS due to ADR are OTC and non-compliance. In our study out of 145 cases, 53 cases were accounted for hospital admissions due to ADRs. out of 53 cases , 24 (45.2%) cases were severe .To reduce prolong hospitalization , treatment cost, morbidity and mortalities must need a constructive pharmacovigilance like Awareness and sensitization programmes among medical and non-health professionals and also must for people who are illiterate and no medical knowledge, whom using drugs over many years without knowing of their noxious effects. Pharmacovigilance is a continuous ongoing process which ensures the safety of the patients.
On the basis of our results we feel that NSAID use could be moderated by increased knowledge of their noxious effects on continuous use over many years because NSAIDs are available over the counter.
Conflict of interest
None declared
Funding Sources
No funding sources
References
- World Health Organization: Internal drug monitoring: the role of the hospital. In technical report series no.425.Geneva, Switzerland: World Health Organization; 1966:1-24.
- Classen DC, Pestotnik SL, Evans RS, Lloyd JF, Burke JP. Adverse drug events in hospitalized patients, Excesslength of stay, extra costs and attributable mortality, JAMA. 1997; 227 (4):301-6.
CrossRef - Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279(15):1200-5.
CrossRef - Ramesh M, Pandit J, Parthasarathi G. Adverse drug reactions in a south Indian hospital – Their severity and cost involved. Pharmacoepidemiol Drug Saf. 2003;12(8):687-92.
CrossRef - Arulmani R, Rajendran SD, Suresh B. Adverse drug reaction monitoring in a secondary care hospital in South India. Br J Clin Pharmacol. 2008; 65(2):210-6.
CrossRef - Marcia Germana Alves de Araujo Lobo et al., Adverse drug reaction monitoring: support for pharmacovigilance at a tertiary care hospital in northern Brazil. BMC Pharmacology and Toxicology. 2013:14:5.
CrossRef - Brandão A, Vasconcelos F: A tênue fronteira entre a cura e o malefício. Pharmacia Brasileira 2000, 22:36–39.
- Einarson TR: Drug-related hospital admissions. Ann Pharmacother 1993, 27:832–840.
CrossRef - Lazarou J, Pomeranz BH, Corey PN: Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA 1998, 279:1200–1205.
CrossRef - Magalhães SMS, Carvalho WS: Reações Adversas a Medicamentos. In Ciências Farmacêuticas. Uma Abordagem em Farmácia Hospitalar. São Paulo: Atheneu; 2001:125–146.
- Passarelli MCG: Reações adversas a medicamentos em uma população idosa hospitalizada. PhD thesis. Universidade de São Paulo, Faculdade de Medicina; 2005.
- Lobstein R, Lakin A, Koren G: Pharmacokinetic Changes during pregnancy and Their clinical Relevance. Clinical Pharmacokinet 1997, 33:328–343.
CrossRef - Wong A: Os usos inadequados e os efeitos adversos de medicamentos na prática clínica. J Pediatr (Rio J) 2003, 79:379–380.
CrossRef - Tatro DS: Textbook of therapeutics, drug and disease management. Baltimore: William and Wilkins; 1996.
CrossRef - May RJ: Adverse drug reactions and interactions, Pharmacoterapy: a pathophysiologic approach. Norwalk: Appleton and Lange; 1997:101–116.
CrossRef - Sobravime: Declaração de Berlim sobre Farmacovigilância. São Paulo; 2005.
CrossRef - Van Grootheest K, Olsson S, Couper M, de Jong-van den Berg L: Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf 2004, 3:457–464.
CrossRef - Gallelli L, Ferreri G, Colosimo M, Pirritano D, Flocco MA, Pelaia G, et al: Retrospective analysis of adverse drug reactions to bronchodilatorsobserved in two pulmonary divisions of Catanzaro, Italy. Pharmacol Res 2003, 47(Suppl 6):493–499.
CrossRef - Wu WK: Evaluation of outpatient adverse drug reactions leading to hospitalization. Am J Health Syst Pharm 2003, 60(Suppl 3):253–259.
CrossRef - Gomes MJV, Reis AAMM: Ciências Farmacêuticas: Uma Abordagem em Farmácia Hospitalar. São Paulo: Atheneu; 2001.








