Yousra Nomier1, Gihan F. Asaad1,5*, Saeed Alshahrani1 , Seham Safhi2, Layla Medrba2, Najd Alharthi2, Zia Rehman3, Hassan Alhazmi3 and Syeda Sanobar4
, Seham Safhi2, Layla Medrba2, Najd Alharthi2, Zia Rehman3, Hassan Alhazmi3 and Syeda Sanobar4
1Department of Pharmacology and Toxicology, Pharmacy College, Jazan University-KSA.
2Pharmacy College, Jazan University, KSA.
3Departmentof Pharmaceutical Chemistry, College of Pharmacy, Jazan University, Jazan, KSA.
4Department of Pharmacognosy, Pharmacy College, Jazan University-KSA
5Department of Pharmacology, National Research Centre, Dokki-Giza-Egypt
Corresponding Author E-mail: dr_g.asaad@yahoo.com
DOI : https://dx.doi.org/10.13005/bpj/2112
Abstract
The chronic mild stress (CMS) model in rats is a classic example to further understanding of human psychopathology. This study aimed to evaluate the antidepressant and anxiolytic activity of Cymbopogon Flexuosusethanolic extractagainst CMS model induced in rats. The ethanolic extract of dried leaves of C. flexuosus was subjected to GCMS analysis using Thermo Scientific GC/MS equipped with AS 3000 auto samplers, trace ultra-GC and ISQ detector for determination of phytoconstituents. Rats were divided randomly into 7 groups (n=6). Group 1 was kept as normal unstressed rats given saline, Group 2 was unstressed rats given fluoxetine (1mg/kg), Groups from 3 to 7 were subjected to CMS model. Rats of group 3 served as control +ve. Thirty minutes before behavioral assessment, groups (4-7) were given fluoxetine (1mg/kg), extract at the dose of 50, 100, 200 mg/kg (p.o) respectively. Antidepressant and anxiolytic activities were assessed by activity box, open field activity, light and dark box and elevated plus maze. The neurotransmitter serotonin was determined in serum. The ethanolic extract of C. flexuosus at the doses of 50, 100, 200 mg/kg significantly ameliorated all the behavioral deficits that originated as a result of CMS. The group given C.flexuosus at a dose of 100 mg/kg showed the best effect among all other treated groups. Similar results were obtained on determining the serotonin levels in serum. It was concluded that the plant extract possesses a potential antidepressant and anxiolytic effect against chronic mild stress induced in rats.
Keywords
Anxiety; Behavior; Chronic Mild Stress; C. flexuosus; Fluoxetine; Serotonin; SSRIs
Download this article as:| Copy the following to cite this article: Nomier Y, Asaad G. F, Alshahrani S, Safhi S, Medrba L, Alharthi N, Rehman Z, Alhazmi H, Sanobar S. Antidepressant and Anxiolytic profiles of Cymbopogon Flexuosus Ethanolic extract in Chronic Unpredictable Mild Stress induced in rats. Biomed Pharmacol J 2021;14(1). |
| Copy the following to cite this URL: Nomier Y, Asaad G. F, Alshahrani S, Safhi S, Medrba L, Alharthi N, Rehman Z, Alhazmi H, Sanobar S. Antidepressant and Anxiolytic profiles of Cymbopogon Flexuosus Ethanolic extract in Chronic Unpredictable Mild Stress induced in rats. Biomed Pharmacol J 2021;14(1). Available from: https://bit.ly/2PlNb6s |
Introduction
chronic mild stress model is an approved method for inducingstressin experimental animals(1). It has been reported that exposure to scheduled unpredictable stressors induces substantialimpairment in behavioral status as locomotive and explorative behavior.chronic mild stress model has also been usedeffectively used as an animal model of depression associated with anxiety(2).
Many studiesusedrats to determinethe effect of many therapeutic agents such as fluoxetine (FLX), on behavioral impairment viz depression associated with anxietyusing elevated plus maze, forced swim test.Administration of fluoxetine in an acute or chronic manner was recorded to increase motor activity in familiar environment but enhancement of exploratory activity in the open field was exerted only with repeated administration(3).A major disadvantage of SSRI treatment is the delayed antidepressant response which is believed to be due to the time needed for improvement of serotonergic neurotransmission. This improvement is thought to be due to the desensitization of somatodendritic 5-HT1A autoreceptors(4).
Cymbopogon flexusousis placed in the genus Cymbopogon. The essential oil of C.flexusoushas shown an anti-inflammatory effect by inhibiting various inflammatory and immunomodulatory biomarkers(5). C.flexusousalso showed an inhibitory effect on the early phase hepatocarcinogenesis in rats after initiation with diethylnitrosamine(6). The Methanolic extract of C.citratus leaves; one of the Cymbopogonfamily increased the percentage of time-spent and the percentage of arm entries in the open arms and decreased the percentage of time-spent in the closed arms of the elevated plus-maze, and this effect was attributed to the high content of flavonoids in the methanolic extract(7).
Nowadays, developed countries are investing in traditional herbal medicine research to overcome the detrimental adverse effects and drawbacks of synthetic agents. As examples for these drawbacks; the delayed response and the need of repeated administration of SSRI which may lead to incidence of toxicity.The current study was conducted to assess the antidepressant and anxiolytic activity of C.flexusousethanolic extract against chronic mild stress model in rats in refer tofluoxetine.
Materials and Methods
Preparation of leave extract of C.flexuosus
C.flexuosus leaves were collected from Jazan botanical garden. Identified by the botanist from Jazan University, Pharmacy College and the specimen was kept in the campus. Crude drug (leaves) was left for drying under shade, grinded coarsely by using a mortar and pestle. About 25 gm of powder was weighed, processed for hot percolation method with various solvents like ethyl alcohol, Ethyl acetate, Chloroform depending on their polarity level. Before starting, the process packing was carefully done by placing cotton bed on the upper and lower level of the powder, to avoid any blockage. Temperature of the solvents was adjusted to its boiling point in a heating mantle. Cool water supply was provided for condensation process. Extraction process was stopped after16 hours. Extracts were condensed to remove the solvents and stored for further studies. Solvents and Fluoxetine hydrochloride procured from Sigma Aldrich, Germany.
Preliminary phytochemical screening
Preliminary studies were done to identify the different chemical constituents like alkaloids, flavonoids, steroids, tannins, glycosides, triterpenoids and saponins(8).
Physiochemical properties used for identification of C.flexuosus
GC/MS analysis
The ethanolic extract of dried leaves of C.flexuosus was subjected to GCMS analysis using Thermo Scientific GC/MS equipped with AS 3000 auto samplers, trace ultra-GC and ISQ detector. The separation was achieved using capillary TR-5MS column (30 m × 0.25 mm ID × 0.25 μm) and helium carrier gas at a flow rate of 1.2 mL/min using our earlier reported method of sesame oil(9). Ethanolic extract (1mL) was diluted to 10 mL v/v with absolute ethanol, sonicated for 2 minutes, filtered through 0.45μm filter and injected in split less mode (2 µL). The injection port, ion source and MS line transfer temperatures were set at 320oC, 320oC and 340oC respectively. The oven temperature was initially set at 70oC with holding time for 5 minutes and subsequently ramped to 205oC, 280oC, 290oC and 300oC at rate of 5oC/min with 5 min holding time at ramping stage. For GC-MS detection, an electron ionization system with ionization energy of 70 eV with 0.6 s scan time was used. The spectra were recorded using positive ion mode within the mass range of 20-800 amu. A delay time of 4 min was selected to avoid initial solvent peaks. The mass spectra were interpreted using Xcalibur software and the fragmentation patterns of mass spectra were compared with database using the built-in NIST library. The relative percentage amount of each component was calculated by comparing its average peak area to the total area.
Compound identification
The components ethanolic extract of dried leaves of C.flexuosus were identified by using match factor (SI) and reverse match factor (RSI) thresholds of 900 and above between measured spectrum and standard library spectrum. Percentage areas of each component were obtained by using Xcalibur software without use of any internal standard and are uncorrected.
Experimental protocol
Animals
Forty-two adult female wistar rats (150-180g) were obtained from the animal breeding unit at the Medical College-Jazan University. Animals were kept in a cleansed, clear poly propylene cages in groups of four in each cage maintained at 25 ± 2ºC with 12 hours light and dark cycle with free access to cubes of food and water ad libitum. The examination was done between 8.00 am to 3.00 pm. All experimental procedures were conducted according to the recommendations of the proper care and use of laboratory animals and following the recommendations of the National Institutes of Health guide for Care and Use of Laboratory Animals.
Acute toxicity studies
Toxicity studies were carried out as per the OECD guidelines no 423(8). No deaths or toxicity signs were reported after administering the extract orally in doses up to 2000mg/kg. Accordingly, different doses of 50, 100 & 200 mg/kg body weight were chosen. Forty-two animals were erratically branched into two main groups (Unstressed and stressed groups)
-Group I (Unstressed)
Unstressed rats + saline (0.9% Nacl)
Unstressed rats + Fluoxetine (1 mg/kg)
-Group II (Chronic mild stress) (CMS)
CMS rats + saline (0.9% Nacl)
CMS rats + Fluoxetine (1 mg/kg)
CMS rats +ethanolic extract of C.flexuosus (50 mg/kg)
CMS rats + ethanolic extract of C.flexuosus (100 mg/kg)
CMS rats + ethanolic extract of C.flexuosus (200 mg/kg)
All drugs were given per os (p.o.) daily 30 minutes
before exposing to daily schedule of CMS for 14 days (Table No. 1).
Induction of chronic mild stress (CMS)
Animals of the CMS group were exhibited to a routine of deep-rooted mild restlessness displayed beneath over a portion of time for 14 days period, in the period of time animals stayed in their hutch under usual circumstances. Behavioral activities were tested at the end of the experiment(10).
Table 1: Chronic Mild Stress (CMS) schedule.
| Time | CMS | Day | S.No |
| 11:00 am | Exposed to 4°C for 50 minutes | Day1 | 1. |
| 11:00 am | 60 mins cage agitation (60 rpm) | Day2 | 2. |
| 11:00 am | 60 mins restrained stress (wire grid) | Day3 | 3. |
| 11:00 am to 11:00 pm | 12 hrs. water deprivation | Day4 | 4. |
| 11:00 am to 02:00 pm | 3 hrs. light off day time | Day5 | 5. |
| 11:00 am | 60 mins Noise Stress | Day6 | 6. |
| 11:00 am | 60 mins restraint stress(tube) | Day7 | 7. |
Animals of the CMS group were exposed to a schedule of chronic mild stress shown above over a period of 14 days, while animals of unstressed group were kept in their cages.
Behavioral assessment of anxiolytic activity
Activity box
To judge the locomotor actions experiment was performed in a regular environment inside an activity box. Apparatus chosen for this work were created by clear Perspex (26x26x26 cm) embedded in powdered wood(11). Trail was carried out in a noiseless area which was in the influence of white light as represented by 15 minutes earlier than monitoring the activity of animals were placed in the hutch to get adapted and reading were noted for 10 minutes.
Open Field Activity
The estimation of experimental actions in a new surrounding was carriedin an open field apparatus. Open field apparatus used in current experiment had a square area (76x76cm) with walls 42cm high. Ground was separated by bands into 25 equal squares(12).
To evaluate the actions, rats were left in the center squarer of the open field. Numbers of square crossed with all four paws were noted for 5 minutes.
Light Dark Box Activity
Actions in a light-dark box were used as animal model of anxiety. The trial was performed in a locally made compartment box. The compartment of equal size (26 x 26 x 26 cm), with an access (12 x 12 cm) between the compartments, differed in their sensory properties. The coverings and walls of one compartment were light (transparent) and other dark (black)(13). To examine the activity, rat was set in the center of the light compartment of the box. Entries and time consumed in the light compartment were observed for a close off time of 5 min. Entry into a compartment of the box is explained like the positioning of all four paws in the compartment of the activity box.
Elevated Plus Maze Test
Elevated plus maze was widely used to examine the tranquilizing actions.To carry out the plus maze model a specially designed apparatus was used.it was constructed in the lab. Totally, it consists of four arms in which two ere open and two were closed. Length (50cm) and breadth (10 cm) of the arms was kept identical and a connection was given from the center of5 cm2.Rat was held about 60 cm of peak from the ground in the center of the plus maze and time consumed and the entries in the open arm were computed for difference of 5 minutes(14).
Serotonin ELISA test
At the end of the experiment, serum samples were collected for measuring serotonin levels using commercialized ELISA kits (cat. no. ab133053; Abcam) according to the manufacturer’s protocol. The operational procedures were performed according to the manufacturer’s instructions.
Statistical analysis
Complete data was represented as mean ± SD and the figures were examined by using one-way analysis of variance (ANOVA) followed by Dunnett’s “t” test for confirmation. P< 0.05 is considered as significance.
Results
Phytochemical constituents in c. flexuous
Preliminary tests showed the presence of alkaloids, flavonoids, tannins, carbohydrates, glycosides and triterpenoids in C. flexuous as shown in Table No.2.
Table 2: The following constituents were found in C. flexuous after preliminary tests.
| Name of the constituents | Presence (+)/Absence (-) |
| Alkaloids | + |
| Flavonoids | + |
| Steroids | – |
| Tannins | + |
| Carbohydrates | + |
| Glycosides | + |
| Triterpenoids | + |
GC/MS analysis
The ethanolic extract of dried leaves of C.flexuosus appeared as a dark green color liquid with very mild fragrant odor. The GC-MS analysis revealed the presence of esters (29.9%), acetals (15.05%), terpenes (10.49%), phytosterols (7.75%), aldehydes (7.25%) fatty acid hydrocarbons (5.97%), monosaccharides (4.68%), fatty acids (2.52%), fatty acid alcohols (2.54%) and phenolic compounds (1.36%). A total of forty-one compounds were identified in preliminary GCMS screening accounting for 90.58 % based on mass balance with respect to total peak area. Peaks below 900 points of match factor (SI) and reverse match factor (RSI) thresholds were not considered to compensate the internal standard corrections in Fig.1. Isoamyl acetate (14.42%), isovaleraldehyde diethyl acetal (10.2%), geraniol (7.24%), 1-o-methylfructose (4.68%), ethylmorpholinyl (phenyl) acetate (4.52%), dibutyl phthalate (3.61%), ethyl butanoate (3.46%), p-hydroxybenzaldehyde (2.98%), butraldehyde diethyl acetal (2.89%), 5-hydroxymethylfurfural (2.65%), phytol (2.31%), ethyl linoleate (1.65%), heptacosane (2.10%) were the principal constituents found in extract. The details of all identified compounds with their retention time, molecular weight, molecular formula and % peak area etc. are represented in Table No. 3.
 |
Figure 1: Total ion chromatogram showing chemical compounds of ethanolic leaf extract of C. flexuosus using gas chromatography mass spectrometry (GCMS). |
Table 3: Chemical compounds of Ethanolic extract of of C.flexuosus leaves using gas chromatography mass spectrometry (GCMS).
| Peak No | RT | Name of Compound | Molecular Formula | Molecular Weight | % Peak Area | Chemical Class |
| 1 | 5.21 | 4-Chloromethylene-2-phenyl-4,5-Dihydrooxazole-5-one | C10H6ClNO2 | 207.61 | 0.48 | Heterocyclic (oxazolone) |
| 2 | 5.41 | Neohexene | C6H12 | 84.16 | 0.36 | Fatty acid hydrocarbon
(Aliphatic alkene) |
| 3 | 5.50 | Ethyl butanoate | C6H12O2 | 116.16 | 3.46 | Fatty acid ester |
| 4 | 5.93 | Acetaldehyde ethyl propyl acetal | C7H16O2 | 132.20 | 1.96 | Acetal |
| 5 | 6.50 | Butyraldehyde diethyl acetal | C8H18O2 | 146.23 | 2.89 | Acetal |
| 6 | 6.94 | 1-O-Methyl fructose | C7H14O6 | 194.18 | 4.68 | Monosaccharide |
| 7 | 7.33 | Isoamyl acetate | C7H14O2 | 130.18 | 14.42 | Carboxylic acid ester |
| 8 | 8.56 | Isovaleraldehyde diethyl acetal | C9H20O2 | 160.25 | 10.20 | Acetal |
| 9 | 9.14 | Benzyl chloride | C7H7Cl | 126.58 | 1.08 | Substituted toluene |
| 10 | 9.49 | Benzyl Alcohol | C7H8O | 108.14 | 0.68 | Aromatic alcohol |
| 11 | 14.85 | Trimethylsilyl p-(trimethylsilyloxy) benzoate | C13H22O3Si2 | 282.48 | 0.81 | Silyl ester |
| 12 | 15.73 | 4-Ethoxy-2-butanone | C6H12O2 | 116.12 | 0.29 | Ether |
| 13 | 16.93 | O-tolualdehyde | C8H8O | 120.15 | 0.94 | Aromatic aldehyde |
| 14 | 19.94 | 5-hydroxymethylfurfural | C6H6O3 | 126.11 | 2.65 | Aromatic aldehyde |
| 15 | 20.89 | p-Hydroxybenzaldehyde | C7H6O2 | 122.12 | 2.98 | Aromatic aldehyde |
| 16 | 21.30 | 9-Octadecen-12-ynoic acid methyl ester | C19H32O2 | 292.50 | 0.37 | Fatty acid hydrocarbon
(Aliphatic alkyne ester) |
| 17 | 25.42 | 3,5-Dichloro-2,4-dimethylphenol | C8H8Cl2O | 191.05 | 1.36 | Phenol |
| 18 | 26.02 | Dihydroactinolide | C11H16O2 | 180.24 | 0.26 | Benzofuran |
| 19 | 34.92 | Nonadecene | C19H38 | 266.50 | 1.26 | Fatty acid hydrocarbon |
| 20 | 36.76 | Nonadecane | C19H40 | 268.50 | 0.46 | Fatty acid hydrocarbon |
| 21 | 38.59 | N-Methylsaccharin | C8H7NO3S | 197.21 | 2.20 | Benzisothiazole |
| 22 | 39.34 | 2-Hexylcinnamaldehyde | C15H20O | 216.32 | 0.70 | Aromatic aldehyde |
| 23 | 40.01 | 2-Myristynoyl pantetheine | C25H44N2O5S | 484.21 | 0.54 | Thioester |
| 24 | 40.39 | Eicosyne | C20H38
|
278.50 | 1.09 | Fatty acid hydrocarbon
Aliphatic alkyne |
| 25 | 40.85 | Phytol | C20H40O | 296.50 | 2.31 | Acyclic diterpene alcohol |
| 26 | 41.41 | Cetyl alcohol | C16H34O | 242.44 | 1.28 | Long-chain fatty alcohol |
| 27 | 42.95 | Geraniol | C10H18O | 154.25 | 7.24 | Acyclic monoterpene |
| 28 | 43.40 | Dibutyl phthalate | C16H22O4 | 278.34 | 3.61 | Benzoic acid ester |
| 29 | 44.02 | 6-Pentadecen-1-ol | C15H30O | 226.10 | 0.58 | Long-chain fatty alcohol |
| 30 | 48.75 | Dihomo gamma linolenic acid | C20H34O2 | 306.50 | 0.87 | Essential fatty acid |
| 31 | 52.88 | Ethyl linolenate | C20H34O2 | 306.50 | 1.65 | Essential fatty acid ester |
| 32 | 56.10 | Heptacosane | C27H56 | 380.70 | 2.10 | Aliphatic alkane |
| 33 | 57.28 | Cholesterol benzoate | C34H50O2 | 490.80 | 0.22 | Steroid & Benzoic acid ester |
| 34 | 57.94 | Ethyl 4-morpholinyl(phenyl)acetate | C14H19NO3 | 249.30 | 4.52 | Morpholine ester |
| 35 | 74.94 | Isooctyl phthalate | C24H38O4 | 390.60 | 1.15 | Phthalate ester |
| 36 | 75.97 | Ethyl cholate | C26H44O5 | 436.60 | 0.68 | Prenol lipids ester |
| 37 | 76.32 | Dioctyl isophthalate | C24H38O4 | 390.60 | 0.50 | Phthalate ester |
| 38 | 79.92 | Campesterol | C28H48O | 400.70 | 1.46 | Steroid (Phytosterol) |
| 39 | 80.92 | Stigmasterol | C29H48O | 412.70 | 2.70 | Steroid (Phytosterol) |
| 40 | 82.42 | Beta Sitosterol | C29H50O | 414.70 | 2.65 | Steroid (Phytosterol) |
| 41 | 83.89 | Beta-Amyrin | C30H50O | 426.70 | 0.94 | Pentacyclic triterpenoid (oleanane) |
Acute oral toxicity study
The ethanolic extract of C.flexuosus was orally given to various groups of rat’s at doses of 5, 50, 300 and 2000 mg/kg body weight, respectively. Readings were noted after 48 hours of keen observation to check the normal behavior and examine if there were any issue with nervous problems. Rats given the larger doses of C.flexuosus(2000 mg/kg body weight, p.o.) had zero death, dose levels at 1/10th (200 mg/kg body weight, p.o.), 1/20th (100 mg/kg body weight, p.o.) and 1/40th (50 mg/kg body weight, p.o.) among this excessive doses were chosen for the anxiolytic activity
Behavioral assessment of ethanolic extract of C. flexuosus
Activity cage test
Group subjected to CMS model showed a significant decrease in locomotor and exploratory activity of the rats as compared to both unstressed animals given saline(192 crossing/10min) (*p<0.05) or fluoxetine(169.8 crossing/10min) (ap<0.05) (1mg/kg). Stressed group given fluoxetine (1 mg/kg) showed (65.6) significant (bp<0.05) increase in activity as compared to stressed groups given saline (9.6 crossing/10min). Stressed groups given C. flexuosus extract 50 mg/kg (88.4crossing/10min),100 mg/kg(122.8crossing/10min),200 mg/kg (95.8crossing/10min) showed a significant (bp<0.05) increase in the locomotor activity when compared to stressed group (9.6crossing/10min). Group given C. flexuosus extract 100mg/kg (122.8crossing/10min) showed better effect than the two other doses and it also showed better effect than stressed group given fluoxetine. The results were depicted in Fig.2.
 |
Figure 2: Activity cage test: Effect of C.flexuosus at a dose of 50, 100 and 200 mg/kg in stressed rats compared to fluoxetine 1mg/kg. |
All data represented as mean ± SD. * p < 0.05 compared to unstressed+saline group, a p < 0.05 compared to unstressed + fluoxetine group, bp < 0.05 stressed group. (n = 6).
Open field activity
In theopen field test (OFT), Group subjected to CMS model showed a significant decrease in the number of square crossings frequencies(22 crossings/5min) as compared to both unstressed animals given saline (69crossings/5min) (*p<0.05) or unstressed animals given fluoxetine (34crossings/5min) (ap<0.05). Stressed group given fluoxetine(1 mg/kg) showed significant (bp<0.05) increase in number of square crossings(51 crossings/5min) as compared to stressed groups (21.8 crossings/5min). Stressed groups given C. flexuosus extract(50,100,200 mg/kg)showed a significant (bp<0.05) increase in the number of square crossings (51, 59, 55 crossings/5min), respectively, ascompared to stressed group given saline (21.83crossings/5min). Amongst the three doses of the test extract, 100 mg/kg was found to show better effect than the other two doses (50 mg/kg and 200 mg/kg) and the (CMS+Fluoxetine) group.The outcome of the anxiolytic effect was shown in Fig.3.
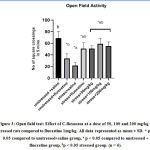 |
Figure 3: Open field test: Effect of C.flexuosus at a dose of 50, 100 and 200 mg/kg in stressed rats compared to fluoxetine 1mg/kg. |
All data represented as mean ± SD. * p < 0.05 compared to unstressed+saline group, a p < 0.05 compared to unstressed + fluoxetine group, bp < 0.05 stressed group. (n = 6).
Light and dark box
In the light and dark box, group subjected to CMS model without any treatment showed a significant decrease in time spent in light box of light dark transition box (9.6 sec.) as compared to both unstressed animals given saline (192 sec) (*p<0.05) or fluoxetine (169.8 sec) (ap<0.05). Stressed group given fluoxetine (1 mg/kg) showed significant (bp<0.05) increase in time spent in light box of light dark transition box(65.6 sec) as compared to stressed groups. Stressed groups given C. flexuosus extract 50,100,200 mg/kg showed a significant (bp<0.05) increase in the time spent in light box of light dark transition box (88.4, 122.8, 95.8 sec) respectively, when compared to depressed group. Amongst the three doses of the test extract, 100 mg/kg was found to exert better effect as compared than the other two doses (50 mg/kg and 200 mg/kg body weight) and the (CMS+fluoxetine) group.The consequences of the anti-anxiety property was reflected in the Fig .4.
 |
Figure 4: Light and dark box test: Effect of C.flexuosus at a dose of 50, 100 and 200 mg/kg in stressed rats compared to fluoxetine 1mg/kg. |
All data represented as mean ± SD. * p < 0.05 compared to unstressed+saline group, a p < 0.05 compared to unstressed + fluoxetine group, bp < 0.05 stressed group. (n = 6).
Elevated plus maze
In the Elevated Plus Maze, group subjected to CMS model without any treatment showed a significant decrease in time spent in open arm of the elevated plus maze (11.6 sec) as compared to both unstressed animals given saline (192 sec) (*p<0.05) or fluoxetine (141.8 sec) (ap<0.05). Stressed group given fluoxetine (1 mg/kg) showed significant increase (bp<0.05) in time spent in open arm of the elevated plus maze(85.6 sec) as compared to stressed group. Stressed groups given C. flexuosus extract 50,100,200 mg/kg showed a significant increase (bp<0.05) in the time spent in open arm of the elevated plus maze(88.4, 121.17, 95.8 sec) respectively,when compared to stressed group. Amongst the three doses of the test extract, 100 mg/kg was found to show better effect than the other two doses (50 mg/kg and 200 mg/kg body weight) andthe (CMS+fluoxetine) group. The aggregate effect of the anxiolytic property was depicted in the Fig.5.
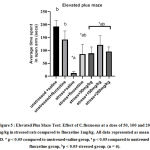 |
Figure 5 : Elevated Plus Maze Test: Effect of C.flexuosus at a dose of 50, 100 and 200 mg/kg in stressed rats compared to fluoxetine 1mg/kg. |
All data represented as mean ± SD. * p < 0.05 compared to unstressed+saline group, a p < 0.05 compared to unstressed + fluoxetine group, bp < 0.05 stressed group. (n = 6).
Serotonin ELISA test
Group subjected to CMS model without any treatment showed a significant decrease in serotonin level (1002.85±0.003 mmol/L) as compared to both unstressed animals given saline (1107.14±0.001 mmol/L) (*p<0.05) or fluoxetine (1116.07±0.002 mmol/L) (ap<0.05). Stressed group given fluoxetine (1 mg/kg) showed significant increase (bp<0.05) in serotonin level(1036.78±0.002 mmol/L) as compared to stressed group. Stressed groups given C. flexuosus extract 50,100,200 mg/kg showed a significant increase (bp<0.05) in serotonin level(1050.57±0.002, 1062.5±0.003, 1053.57±0.004 mmol/L) respectively, when compared to stressed group. The aggregate effect of the anxiolytic property was depicted in the table (4).
Table 4: Effect of C. flexuosus extract on the concentrations of serotonin in serum samples.
| Groups | Concentration of Serotonin (mmol/L) |
| Unstressed+saline | 1107.14±0.001 |
| Unstressed+Fluoxetine | 1116.07±0.002 |
| Stressed+saline | 1002.85±0.003* a |
| Stressed+Fluoxetine | 1036.78±0.002b |
| Stressed+ C.flexuosus (50mg/kg) | 1050.57±0.002 b |
| Stressed+ C.flexuosus (100mg/kg) | 1062.5±0.003 b |
| Stressed+ C.flexuosus (200mg/kg) | 1053.57±0.004 b |
All data represented as mean ± SD. * p < 0.05 compared to unstressed+saline group, a p < 0.05 compared to unstressed + fluoxetine group, bp < 0.05 stressed group. (n = 6).
Discussion
The current study aimed to investigate that whether administration of C.flexuosusethanolic extract at three dose levels (50, 100 and 200 mg/kg) could ameliorate the behavioral deficits induced by chronic mild stress (CMS) in adult female wistar rats. The behavioral impairment was induced by CMS as it is considered a valid method of depression associated with anxiety(15). The potential antidepressant and anxiolytic effect of C.flexuosuswas estimated in refer to the standard SSRI; fluoxetine.Animals of the CMS group exposed to a schedule of chronic mild stress shown above over a period of 14 daysshowed a significant change in behavioral parameters, such as significant decrease inlocomotor activity and explorative activity, significant decrease in the number of square crossings frequencies, significant decrease in time spent in light box of light dark transition box and a significant decrease in time spent in open arm of the elevated plus maze. All out results were similar to previous studies conducted by Gihan et al,(2020)(16). These behavioural deficit due to CMS model could be attributed to depression developed due to significant decrease in serotonin level as compared to control unstressed group(17).
The current study showed that fluoxetine (1mg/kg)significantly increased the number of square crossings frequencies, time spent in light box of light dark transition box and time spent in open arm of the elevated plus maze in CMS animals. Same results was obtained in previous study(3).It was also obvious that fluoxetine increased the locomotor activity in normal unstressed animal more than stressed animals and this result was obtained in a previous study and attributed to the limited beneficial effects or even adverse effects on anxiety and depression of acute fluoxetine administration(18). These results are due to the serotonergic effect of fluoxetine that acts as an indirect agonist, stimulating multiple 5-HT receptors.
Stressed groups administered C. flexuosus at doses of (50, 100 and 200 mg/kg) showed a prominent antidepressant and anxiolytic effect with no significant difference than fluoxetine except for the extract at a dose of (100 mg/kg) that exerted better effect than fluoxetine and extract (50 and 200 mg/kg). The ameliorative effects of the extract were represented as significant increase in locomotor activity, number of square crossings frequencies, time spent in light box of light dark transition box and time spent in open arm of the elevated plus maze in CMS animals. As per the physiochemical study we can correlate these effects to the flavonoid content of the extract and topossible agonistic effect on GABA/benzodiazepine receptor complex, or via antagonizing the 5-HT1B receptor or agonize the 5-HT1A receptor(19) that was confirmed by the significant increase of serotonin level. In addition, we attributed the anxiolytic activity exerted by C. flexuosus to its contents of Geraniol; an acyclic monoterpene and the main component in the lemon grass oil, which was present in comparatively lesser levels in the leaf extract. It has been reported previously that geraniol showed antidepressant and anxiolytic activities(20)(21). The major constituent in the leaves extract, isoamyl acetate, and isovaleraldehyde diethyl acetal, finds their use as a flavoring agent and in perfume industry. P-hydroxybenzaldehye is known to have dopamine beta-monooxygenase inhibitor activity, which was found to abolish cocaine induced anxiety in animal models(22). The nature and contents of chemical components in the leaf extract differed considerably form the literature(23)(24). Most of the GCMS analysis work available in the literature focuses on the study of extracted volatile oil from the leaves.It is imaginable that the method of anxiolytic action of C. flexuosuscould be interceded by synergistic action of these phytoconstituents.
Conclusion
It was concluded that ethanolic extract of C. flexuosus exerted a significant antidepressant and anxiolytic activities against stress induced in rats using CMS model. It was also reported repeatedly that administering the extract at a dose of 100 mg/kg orally gave the best effect amongst the three doses as well as better effect when compared to fluoxetine, which is an accredited standard for treatment of anxiety. Additional molecular studies can validate the precise underlying mechanism.We also recommend further clinical studies for the management of stress and muscle tension problems.
Acknowledgement
The authors gratefully acknowledge Dr. Sailaja Rao, Assistant Professor, College of Pharmacy, Jazan University for her support in reviewing this article. Complete preliminary and pharmacological work was carried out in the college of pharmacy, Jazan University. The authors are thankful to the authorities of Future scientist 6 – Jazan University – KSA for funding the project to carry out the successful trails.
Conflict of Interest
Authors declared that there is no conflict of interest
Contribution of Authors
I/we declare that this work was done by the author(s) named in the article and all the liabilities pertaining to claims relating to the content of this article will be borne by the authors.
Funding Source
Future scientist 6 program- Deanship of Scientific Research- Pharmacy College- Jazan University – KSA.
Grant Number: FR6-65
References
- D’Aquila PS, Peana AT, Carboni V, Serra G. Exploratory behaviour and grooming after repeated restraint and chronic mild stress: Effect of desipramine. Eur J Pharmacol. 2000;399(1):43–7.
CrossRef - Willner P. Reliability of the chronic mild stress model of depression: A user survey. Neurobiol Stress. 2017;6:68–77.
CrossRef - Farhan M, Haleem DJ. Anxiolytic profile of fluoxetine as monitored following repeated administration in animal rat model of chronic mild stress. Saudi Pharm J. 2016;24(5):571–8.
CrossRef - G. D, S.B. A, L.J. C. The role of early experience in shaping behavioral and brain development and its implications for social policy. Dev Psychopathol. 2000;
- Han X, Parker TL. Lemongrass (Cymbopogon flexuosus) essential oil demonstrated anti-inflammatory effect in pre-inflamed human dermal fibroblasts. Biochim Open. 2017;4:107–11.
CrossRef - Puatanachokchai R, Kishida H, Denda A, Murata N, Konishi Y, Vinitketkumnuen U, et al. Inhibitory effects of lemon grass (Cymbopogon citratus, Stapf) extract on the early phase of hepatocarcinogenesis after initiation with diethylnitrosamine in male Fischer 344 rats. Cancer Lett. 2002;183(1):9–15.
CrossRef - Shah G, Shiri R, Dhabiliya F, Nagpal N, Mann AS. Anti-anxiety activity of cymbopogon citratus (dc.) stapf leaves extracts on the elevated plus-maze model of anxiety in mice. Pharmacogn J. 2010;2(15):45–50.
CrossRef - OECD. OECD Guidline for the testing of chemicals 436 Acute inhalation toxicity- acute toxic class method. OECD Guidel Test Chem. 2009;1–27.
CrossRef - Ooi JP, Zarim NA, Lim V. Citrus aurontifolia and Cymbopogan flexuosus against Staphylococcus aureus and Escherichia coli. Malaysian J Med Heal Sci. 2019;15:37–42.
- Willner P. The chronic mild stress (CMS) model of depression: History, evaluation and usage. Neurobiology of Stress. 2017.
CrossRef - Bhattacharya SK, Satyan KS. Experimental methods for evaluation of psychotropic agents in rodents: I – Anti-anxiety agents. Vol. 35, Indian Journal of Experimental Biology. 1997. p. 565–75.
- COSTALL B, DOMENEY AM, GERRARD PA, KELLY ME, NAYLOR RJ. Zacopride: anxiolytic profile in rodent and primate models of anxiety. J Pharm Pharmacol. 1988;40(4):302–5.
CrossRef - Usha Rani P, Naidu MUR, Prasad VBN, Ramesh Kumar Rao T, Shobha JC. An evaluation of antidepressants in rheumatic pain conditions. Anesth Analg. 1996;83(2):371–5.
CrossRef - Pellow S, Chopin P, File SE, Briley M. Validation of open : closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14(3):149–67.
CrossRef - Matallana-Surget S, Meador JA, Joux F, Douki T. Effect of the GC content of DNA on the distribution of UVB-induced bipyrimidine photoproducts. Photochem Photobiol Sci. 2008;7(7):794–801.
CrossRef - Asaad GF, Ahmed RF, Nada SA, Eldenshary EEDS, Arafa NMS, Farid OAHA. Antidepressant activity of alpha-lactalbumin in chronic unpredictable stress model in swiss albino mice. Open Access Maced J Med Sci. 2020;8(A):93–9.
CrossRef - Joca SRL, Guimarães FS. Inhibition of neuronal nitric oxide synthase in the rat hippocampus induces antidepressant-like effects. Psychopharmacology (Berl). 2006;185(3):298–305.
CrossRef - Dulawa SC, Holick KA, Gundersen B, Hen R. Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology. 2004;29(7):1321–30.
CrossRef - Millan MJ, Hjorth S, Samanin R, Schreiber R, Jaffard R, De Ladonchamps B, et al. S 155355, A novel benzodioxopiperazine ligand of serotonin (5-HT)1(A) receptors: II. Modulation of hippocampal serotonin release in relation to potential anxiolytic properties. J Pharmacol Exp Ther. 1997;282(1):148–61.
- Mechan AO, Moran PM, Elliott MJ, Young AM, Joseph MH, Green RA. A comparison between Dark Agouti and Sprague-Dawley rats in their behaviour on the elevated plus-maze, open-field apparatus and activity meters, and their response to diazepam. Psychopharmacology (Berl). 2002;159(2):188–95.
CrossRef - Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Vol. 6, Pharmacognosy Reviews. 2012. p. 81–90.
CrossRef - Medeiros KAAL, dos Santos JR, Melo TC de S, de Souza MF, Santos L de G, de Gois AM, et al. Depressant effect of geraniol on the central nervous system of rats: Behavior and ECoG power spectra. Biomed J. 2018;41(5):298–305.
CrossRef - Pandey AK, Rai MK, Acharya D. Chemical Composition and Antimycotic Activity of the Essential Oils of Corn Mint (Mentha arvensis) and Lemon Grass (Cymbopogon flexuosus) Against Human Pathogenic Fungi. Pharm Biol. 2003;41(6):421–5.
CrossRef - Schank JR, Liles LC, Weinshenker D. Norepinephrine Signaling Through β-Adrenergic Receptors is Critical for Expression of Cocaine-Induced Anxiety. Biol Psychiatry. 2008;63(11):1007–12.
CrossRef








