Fariha Fatima, Fardan Qadeer, Afroz Abidi, and Dilshad Ali Rizvi
Department of Pharmacology, Era’s Lucknow Medical College and Hospital, Lucknow, Uttar Pradesh, India.
Corresponding Author E-mail : drfarihafshikoh@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/2091
Abstract
Alzheimer’s disease is associated with progressive neurological and cognitive decline and is the commonest cause of dementia in 70% population globally. Several natural and synthetic agents are incessantly being explored as potential therapeutic targets for disease modification and treatment to reduce the suffering of the patients as well as to alleviate the huge financial burden on the healthcare system. Punica granatum contains polyphenols and flavonoids which are reported to offer neuroprotection. Statins on the other hand, serve as potent cholesterol lowering agents which target the pathophysiology of the disease. Twenty-four male wistar rats were divided into 4 groups of six rats each. They were fed high fat diet for two months.The rats in the respective groups were given Punica granatum juice, Rosuvastatin, standard treatment comprising of Donepezil and distilled water. The analysis was done at baseline and at the end of the study. Behavioral tests and histopathological analysis depicted marked improvement in cognitive and memory functions in Punica granatum group. Rosuvastatin group however showed improvement which was not as pronounced as achieved by the Punica granatum group. The present study was done to discern the effects of test agents such as Punica granatum and Rosuvastatin in memory deficits associated with Alzheimer’s disease. Marked improvement in dementia was observed in Punica granatum juice group in the High Fat Diet induced model of Alzheimer’s disease. Hence, Punica granatum offers significant neuroprotection as compared to the Rosuvastatin group and its potential can be explored in further studies to consolidate its role in amelioration of the disease progress and its treatment.
Keywords
Beta Amyloid; Cholinesterase Inhibitors; Cook’s Pole Climbing Apparatus; Morris Water Maze; Neuro Degeneration; Passive Avoidance Response; Punica Granatum; Statins
Download this article as:| Copy the following to cite this article: Fatima F, Qadeer F, Abidi A, Rizvi D. A, Evaluating the Role of Punica Granatum and Rosuvastatin in an Experimental Model of Alzheimer’s Disease. Biomed Pharmacol J 2020;13(4). |
| Copy the following to cite this URL: Fatima F, Qadeer F, Abidi A, Rizvi D. A, Evaluating the Role of Punica Granatum and Rosuvastatin in an Experimental Model of Alzheimer’s Disease. Biomed Pharmacol J 2020;13(4). Available from: https://bit.ly/3s0T3kC |
Introduction
Alzheimer disease (AD) is the most common neurodegenerative disease leading to a progressive decline in cognitive function and is the most common cause of dementia. It is characterized by a decline in memory, language, problem solving and other cognitive skills that affects a persons’ ability to perform everyday activities1
This decline occurs because neurons involved in cognitive functions get damaged. It often starts with mild symptoms progressing towards moderate disease and finally leads to bed bound state culminating in irreparable brain damage. The prevalence of AD increases exponentially from approximately 2% at the age of 60– 65 years to more than 30%–35% in people aged greater than 80 years. It affects about 17-25 million elderly people around the globe and nearly 4 million people in India alone accounting for approximately 70% of dementia worldwide making it a global health crisis that needs to be addressed.2
The pathophysiology of Alzheimer’s disease is directly related to the cholinergic deficit of neurons beginning in the hippocampal region which is involved in memory and learning. It later progresses towards dilatation of ventricles and shrinkage of cortex.3 Current treatment is aimed at alleviating its symptoms only failing to target its cure.
Augmentation of cholinergic transmission by cholinesterase inhibitors such as Donepezil, Rivastigmine and Galantamine is currently the mainstay of therapy in mild to moderate disease.4
The cholinesterase inhibitors cannot reverse the disease pathology leading to their eventual loss of their effectiveness. Hence, there is an urgent need to discover and introduce new agents in the therapy of the disease.5
It has been proposed in various studies that statins play a beneficial role in Alzheimer’s pathology due to its potent cholesterol lowering mechanisms which alter beta amyloid (AB) levels. These findings raise the possibility that treating human subjects with cholesterol lowering agents can possibly decrease the risk of developing the disease.6,7
Several studies support the evidence that certain fruits have shown to possess powerful neuroprotective properties. One such fruit is Punica granatum(commonly known as Pomegranate)which alleviates conditions like hypertension, hypercholesterolemia, oxidative stress, inflammation also suppresses PGE2 production and COX2 expression in IL-1 stimulated SK-N-SH neuronal cells as stated in previous studies hence, it can serve as a potential candidate for preventing the development and progression of AD.8,9,10
AD exerts a huge financial burden on the health care system with no definitive treatment, therefore, the present research has been undertaken to find a solution by exploring herbal preparations as well as medications which could either help prevent the disease or cure the existing pathological state.
Hence, the purpose pf the study was to investigate the neuroprotective role of Punica granatum and Rosuvastatin in high fat diet induced memory deficits in AD.
Material and Methods
Study Design
Experimental
Study Period
60days
Animals
Male Wistar rats (Rattus norvegicus) of 150-200gm weight. Animals were obtained from CPCSEA-certified animal house (CDRI, Lucknow).
The animals were maintained in cages, under a temperature of 25 ± 2°C and 45-55% relative humidity, with a 12-hour light/dark cycle. They were fed with standard pellet diet and water ad libitum.
Ethical Consideration
All experiments were performed after approval from Institutional Animal Ethics Committee of Era’s Lucknow Medical College as per the guidelines of Animal Care by CPCSEA.
Approval No: ELMC/PHAR/IAEC-11
Drugs and Chemicals
Test Drugs
Punica granatum(Pomegranate)
The fruit was purchased from the market and was authenticated at NBRI, Lucknow.
Preparation of Juice
Juice from fresh Punica granatum fruit was extracted by squeezing the pulp and seeds in a fine muslin cloth and were collected in a separate container.11
The dose of juice was taken to be 500mg/kg body according to the previous studies.12 This juice was administered via oral gavage tube.
Rosuvastatin
The oral tablet was pulverized and dissolved in distilled water and administered to experimental animals via oral gavage tube.
Dose of Rosuvastatin was taken 10mg/kg taken from the previous studies.13
Standard Treatment
Donepezil
The oral tablet was pulverized and dissolved in distilled water and administered to experimental animals via oral gavage tube.
The dose of Donepezil was taken from previous studies as 0.5 mg/kg body weight orally for 6 weeks followed by 1mg/kg till 8 weeks.14
High Fat Diet: A model of Alzheimer’s Disease
Cell culture and in vivo animal studies have shown that reducing cholesterol can inhibit Aß synthesis. Degeneration that occurs in AD is mediated through the modulation of cholesterol signalling in the brain.15 Intake of diets high in saturated fat and simple carbohydrates is associated with cognitive impairments in both humans and rodents.16 It has been reported that feeding rats this type of high-energy (HE) diet has different effects on different types of learning and memory processes.17
High fat diet was purchased from Hindustan Lever Limited, Mumbai, India with the following composition:
Feed Contents
| CONTENT | PERCENTAGE |
| Fat | 35% |
| Crude protein | 20% |
| Carbohydrates | 40% |
| Vitamins & minerals | 5% |
| Metabolic energy of diet
|
5130 kcal/kg
61% of this energy was from fat |
Methodology
The rats were trained for 1 week prior to the start of the experiment. They were divided into 4 groups of 6 rats each. Total of 24 rats were taken. The baseline behavioural assessment was done in rats at the start of the experiment i.e. at day 0.
The rats were administered the HFD, test(Rosuvastatin & Punica granatum juice) and standard drug(Donepezil) for 60 days, in all the groups and behavioural assessment on the Cook’s Pole Climbing Response apparatus, Morris Water Maze Response and Passive Avoidance Response Apparatus was done on the 60th day and the rats from all the groups were sacrificed following anesthesia by i.p. ketamine and histopathological analysis was carried out.
| GROUP | NAME | INTERVENTION |
| 1. | AMNESIC CONTROL | Distilled Water + HFD |
| 2. | STANDARD TREATMENT | DONEPEZIL(0.5mg/kg orally for 6 weeks followed by 1mg/kg till 60h day + HFD till 60th day |
| 3. | ROSUVASTATIN | ROSUVASTATIN(10mg/kg orally) till 60th day + HFD till 60th day |
| 4. | PUNICA GRANATUM JUICE | PUNICA GRANATUM JUICE(500mg/kg orally) till 60th day+ HFD 60th day |
Behavioural Analysis
Behavioural tests were performed to functionally validate Alzheimer’s disease (AD) models and assess treatments.18 Following methods to assess hippocampus-dependent memory functions were used:
The Morris water maze: measures spatial reference memory.
The Morris Water Maze (MWM) is a test of spatial learning for rodents that relies on distal cues to navigate from start locations around the perimeter of an open swimming arena to locate a submerged escape platform.
Procedure
Rats were trained prior to the start of the experiment for 1 week. The water in the maze was made opaque by adding sufficient quantities of milk powder to it. Animals were placed in the maze and allowed to explore the maze to find the hidden platform. Time taken by the rat to find the hidden platform was noted in seconds known as the “escape latency.” Analysis was done at baseline day 0 & day 60. Escape latency in seconds was then recorded and taken as an end point
Passive Avoidance Response
It is based on negative reinforcement. The response was recorded to examine the long-term memory.
Procedure
Rats were trained prior to the start of the experiment for 1 week. A wooden platform was placed on the grid floor carrying foot shock (50 Hz, 1.5 mA) Each rat is placed on the wooden platform. The time taken by the rat to step down and place all four paws on the grid floor carrying shock known as “step down latency” in seconds is recorded. Step down Latency (SDL) in seconds was recorded as end point.
Cook’s Pole Climbing Apparatus
The rats were trained for conditioned avoidance response by using Cook’s Pole Climbing Apparatus.19
Procedure
Rats were trained 1 week prior to the start of the experiment. Each rat was allowed to acclimatize and explore the apparatus for 1 minute. The buzzer was then sounded. 5 seconds after switching on the buzzer, mild electric shocks were administered through the stainless steel grid floor. The time taken by the rat to climb the wooden pole in the center known as “escape latency” is recorded. As soon as the rat climbed the pole, both the buzzer and foot-shock were switched off. Rats with escape latency within 5 seconds were included in the experiment. Escape latency in seconds was recorded as end point measure.
Histopathological Analysis of Brain
After the behavioural analysis for assessment of behaviour, learning and memory, the rats were then sacrificed following anaesthesia by an i.p. injection of ketamine (100 mg/kg) by decapitation and the whole brain of each animal was rapidly dissected, thoroughly washed with isotonic saline and then divided mid-sagittally into two halves. Each half was then fixed in formalin buffer (10%) for histopathological investigation. The brain was then cut into sections for preparation of slides and fixed. The slides were then viewed under 10X & 40X magnifications under microscope.
Statistical Analysis
The data obtained was tabulated and subjected to descriptive analysis. The different groups were compared using ANOVA followed by Post Hoc Dunnet T3 Test. Values before and after treatment in each group were compared using Paired ‘t’ test. All statistical analysis were done using Graph pad Prism software (version 6.02) p value < 0.05 was considered as significant.
Observation and Results
Baseline characteristics at day 0 were assessed in all the rats and did not differ significantly. The behavioural assessment was done on the Cook’s Pole Apparatus, Passive Avoidance Response Apparatus and Morris Water Maze Response Apparatus and there was no significant change in the latency periods in all the three tests.
High Fat Diet model was administered to induce dementia in all the groups pretreated with vehicle, test and the standard treatment. The behavioural assessment was done on day 60 showed a statistically significant change in all the groups on the Cook’s Pole Apparatus, Passive Avoidance Response Apparatus and Morris Water Maze Response Apparatus and also there was a significant deterioration from the baseline behaviour, hence, indicating the establishment of a model for Alzheimer’s disease.
Effect of Test Drugs on Escape Latency by Cook’s Pole Apparatus
When the comparison was done with the control group; a significant improvement was observed in all the groups, the Standard treatment group, Rosuvastatin and PJ groups at day 60 (p<0.0001). The Control group elicited significantly poorer memory function, learning skills and behaviour in terms of high escape latency as compared to the standard treatment group (p<0.0001).The remaining groups (Rosuvastatin and PJ groups) showed comparable results with the Standard treatment group. [Table 1]
Table 1: Effect of Test Drugs on Escape Latency by Cook’s Pole Apparatus
| DAY 0 | DAY 60 | Paired t test:
|
|||
| GROUPS | MEAN (sec) | 95% CI | MEAN (sec) | 95% CI | p value |
| CONTROL | 4.8 | 4.245-5.355 | 16.17** + | 14.13-18.12 | <0.0001 |
| STD TREATMENT | 5.6 | 2.741-8.459 | 4.833# | 1.909-7.758 | 0.6836 |
| ROSUVASTATIN | 6.6
|
3.132-10.07 | 4.833# | 1.78-8.887 | 0.2746 |
| PJ | 7.2 | 6.161-8.239 | 5# | 2.607-7.393 | 0.1138 |
| ANOVA F value
p value |
1.425
0.262 |
29.29
<0.0001 |
|||
** When compared with standard; p<0.05 # When compared with control; p<0.05+ When compared to day 0; p<0.05 (STD: standard; PJ: Punica granatum juice)
Effect of Test Drugs on Step Down Latency by Passive Avoidance Response
Mean baseline values of step down latencies at day 0 did not vary significantly amongst different groups (p=0.6704). Hence, all the groups at baseline showed comparable results to each other. [Table 2]
A significant improvement in the step down latency in the Standard treatment group and PJ group was noted at day 60 from the baseline at day 0 (p=0.0185, p=0.0180 respectively).
The rest of the groups (Control, Rosuvastatin) also reported a rise in the mean step down latency values at day 60 as compared to day 0 but this increase was not statistically significant. [Table 2]
The mean step down latencies reported a significant surge in the Standard treatment, Rosuvastatin and PJ groups at day 60 as compared to the Control group(p=0.0069). The Control group noted a High Fat Diet induced deterioration in the mean step down latency in comparison to the Standard treatment group (p=0.0069) while Rosuvastatin & PJ groups showed values comparable to the standard treatment. [Table 2]
Table 2: Effect Of Test Drugs On Step Down Latency By Passive Avoidance Response
| GROUPS | DAY 0
|
DAY 60
|
Paired t test:
|
||||
| MEAN (sec) | 95% CI | MEAN (sec) | 95% CI | p value | |||
| CONTROL | 1.8 | 0.7611-2.839 | 4** | 2.673-5.327 | 0.0743 | ||
| STD TREATMENT | 4.2 | 1.236-7.164 | 16.67# + | 7.478-25.87 | 0.0185 | ||
| ROSUVASTATIN | 5.6 | 3.18-8.02 | 12.5# | 6.074-18.93 | 0.0759 | ||
| PJ | 3.6 | 2.184-5.016 | 11# + | 6.802-15.2 | 0.0180 | ||
| ANOVA F value
p value |
0.5949
0.6704 |
4.528
0.0069 |
|||||
** When compared with standard; p<0.05
# When compared with control; p<0.05
+ When compared to day 0; p<0.05
(STD: standard; PJ: Punica granatum juice)
Effect of Test Drugs on Escape Latency by Morris Water Maze Response
High fat diet induced a rise in the mean escape latency at day 60 from the baseline at day 0 (p=0.0079). Although the rest of the groups at day 60 also differed from the baseline but this variation was not statistically significant. [Table 3]
Administration of Standard treatment, Rosuvastatin & PJ prevented the high fat diet induced rise in escape latency at day 60 as compared to the Control group(p=0.0092). A significant increase in the Control group was noted as compared to the Standard treatment group (p=0.0092), whereas, the rest of the groups showed improvement similar to the Standard treatment group. [Table 3]
Table 3: Effect of Test Drugs on Escape Latency by Morris Water Maze Response
| GROUPS | DAY 0
|
DAY 60
|
Paired t test:
|
||||
| MEAN (sec) | 95% CI | MEAN (sec) | 95% CI | p value | |||
| CONTROL | 3 | 2.122-3.878 | 9.5** + | 6.004-13 | 0.0079 | ||
| STD TREATMENT | 3.6 | 2.92-4.28 | 5.5# | 2.626-8.374 | 0.0978 | ||
| ROSUVASTATIN | 4 | 2.758-5.242 | 5.5# | 2.951-8.049 | 0.2804 | ||
| PJ | 4.6 | 3.184-6.016 | 4.833# | 4.043-5.623 | 0.8276 | ||
| ANOVA F value
p value |
2.417
0.0826 |
4.251
0.0092 |
|||||
** When compared with standard; p<0.05
# When compared with control; p<0.05
+ When compared to day 0;
p<0.05 (STD: standard; PJ: Punica granatum juice)
Histopathological Analysis
The specimen of brain was subjected to histopathological examination under light microscope after staining with Hematoxylin and Eosin (H & E) stain.
Section of brain from Control group showed loss of fibrillary background with foci of eosinophilic cellular plaques under 10X. Under 40X magnification, eosinophilic tangles of tau protein were seen in the neuron cell bodies scattered in both the cortex and hippocampus. Focal sub pial aggregates and amyloid pools were also visible. [Figure 1B]
Histopathological examination of Rosuvastatin group showed traces of fibrillary background with lesser eosinophilic plaques under 10X. Under 40X magnification, tangles of tau protein were visible to a lesser extent in neuronal cell bodies scattered in both the cortex and hippocampus. Focal sub pial aggregates and amyloid pools were visible. [Figure 1C]
The histopathological examination of Punica granatum juice showed the presence of fibrillary background with reduced eosinophilic plaques under 10X. Under 40X magnification, there was a reduction in the number of tau protein tangles in neuronal cell bodies scattered in both the cortex and hippocampus. Focal sub pial aggregates and amyloid pools were visible. [Figure 1D] Figure 1e Shows The Standard Treatment Group With Almost Normal Cortical And Hippocampal Regions With Only A Few Tau Protein Tangles And Almost Negligible Amyloid Pools.
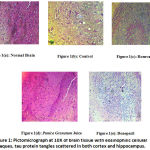 |
Figure 1: Pictomicrograph at 10X of brain tissue with eosinophilic cellular plaques, tau protein tangles scattered in both cortex and hippocampus. |
Discussion
Present study was done to discern the effects of the test agents Punica granatum juice and Rosuvastatin in memory deficits associated with dementia in an experimental model of Alzheimer’s disease in comparison to its standard treatment i.e. Donepezil.
Behavioural analysis was done to assess hippocampus-dependent memory functions on Cook’s Pole Response Apparatus, Passive Avoidance Response and Morris Water Maze apparatus. Histopathology was done to functionally and structurally validate the disease process.
High Fat Diet administration for 60days caused depreciation of memory and behaviour as recorded on Cook’s Pole Climbing response, Morris Water Maze and Passive Avoidance Response apparatus at day 66. It caused delayed reaction time when assessed at day 60 as compared to the baseline at day 0. Hence, it may be concluded that the disease was induced in rats after 60 days of high fat diet administration.15,20,21
This may also be used as a refined animal model of high fat diet induced Alzheimer’s disease causing no pain and suffering to experimental animals. The probable mechanism of action of High Fat Diet induced Alzheimer’s disease is that the High Fat Diet decreases the integrity of the blood brain barrier and results in cerebrovascular inflammation and neurotoxicity characteristic of the disease.22
It has been reported that Punica granatum polyphenols and extract inhibit nuclear factor of activated T-cell activity and microglial activation in Alzheimer’s brain.7,23,24
Marked improvement in dementia was also observed in Punica granatum juice group in High Fat Diet induced models of Alzheimer’s disease when the assessment was done at day 60 in comparison to the Control group.
Hence, to conclude Punica granatum juice has a remarkable protective role in memory function, learning, cognition & behaviour in High Fat Diet model of Alzheimer’s disease which was better than the Rosuvastatin treatment. Longer, more specific and dose responsive animal and human studies are required to further strength our present conclusion and to unveil the concerned mechanisms of actions of Punica granatum and Rosuvastatin in either halting the disease progress or preventing it.
Acknowledgments
Dr. Mohammad Tariq Salman, Professor, Department of Pharmacology, Hind Institute of Medical Sciences , Ataria
Conflict of Interest
None.
Funding Source
Self-Funded
References
- Gross A, Jones R, Habtemariam D, Fong T, Tommet D, Quach L et al. Delirium and Long-term Cognitive Trajectory Among Persons With Dementia. Arch Intern Med. 2012; 172(17):1324.
CrossRef - Raghavendra M, Maiti R, Kumar S, Acharya S. Role of Ocimum sanctum in the experimental model of Alzheimer′s disease in rats. Int J Green Pharm. 2009;3(1):6.
CrossRef - Shaffer J, Petrella J, Sheldon F, Choudhury K, Calhoun V, Coleman R et al. Predicting Cognitive Decline in Subjects at Risk for Alzheimer Disease by Using Combined Cerebrospinal Fluid, MR Imaging, and PET Biomarkers. Radiology. 2013; 266(2):583-591.
CrossRef - Birks J. Cochrane Database Syst Rev 2006 Jan 25; (1): CD005593 – Cholinesterase inhibitors for Alzheimer’s disease. psychoneuro. 2006;32(11):508-508.
CrossRef - Courtney C, Farrell D, Gray R, et al. Long-term donepezil treatment in 565 patients with Alzheimer’s disease (AD2000): randomised double-blind trial. The Lancet. 2004; 363(9427):2105-2115.
CrossRef - Puglielli L, Tanzi R, Kovacs D. Alzheimer’s disease: the cholesterol connection. Nature Neuroscience. 2003;6(4):345-351.
CrossRef - Feng Y Wang X. Antioxidant Therapies for Alzheimer’s Disease. Oxidative Medicine and Cellular Longevity. 2012;2012:1-17.
CrossRef - Stover E, Mercure EW. The pomegranate: a new look at the fruit of paradise. HortScience. 2007; August 42(5):1088–92.
CrossRef - Asgary S, Javanmard S, Zarfeshany A. Potent health effects of pomegranate. Advanced Biomedical Research. 2014; 3(1):100.
CrossRef - Olajide OFiebich B. Pomegranate suppresses PGE2 production and COX-2 expression in IL-1β-stimulated SK-N-SH neuronal cells: implications for Alzheimer’s disease. Planta Med. 2013; 79(13).
CrossRef - Forouzanfar F, Afkhami Goli A, Asadpour E, Ghorbani A, Sadeghnia H. Protective Effect of Punica granatumL. against Serum/Glucose Deprivation-Induced PC12 Cells Injury. Evidence-Based Complementary and Alternative Medicine. 2013; 2013:1-9.
CrossRef - Jimenez del Rio M, Ramazanov A,Sikorski S,Ramazanov Z,Chkhikvishvili I.A new method of standardization of health promoting pomegranate fruit extract.Georgian Medical News 2006; 11(140).
- Maria T. Georgieva-Kotetarova*, Ivanka I. Kostadinova Effect of Atorvastatin and Rosuvastatin on Learning and Memory in Rats with Diazepam-Induced Amnesia Folia Medica 2013; 55(2): 58-65.
CrossRef - Ogura H, Kosasa T, Araki S, Yamanishi Y. Pharmacological properties of donepezil hydrochloride (Aricept), a drug for Alzheimer’s disease. Nihon Yakurigaku Zasshi.2000 Jan; 115(1):45-51.
CrossRef - Singh N, Jaggi A, Singh D, Ghulati P, Dalla Y. Potential of ezetimibe in memory deficits associated with dementia of alzheimer′s type in mice. Indian Journal of Pharmacology. 2009;41(6):262.
CrossRef - Ghribi O. Potential Mechanisms Linking Cholesterol to Alzheimer’s Disease-like Pathology in Rabbit Brain, Hippocampal Organotypic Slices, and Skeletal Muscle. J Alzheimers Dis. Dec2008;15(4):673-684).
CrossRef - Kanoski S Davidson T. Different patterns of memory impairments accompany short- and longer-term maintenance on a high-energy diet. Journal of Experimental Psychology: Animal Behavior Processes. 2010;36(2):313-319.
CrossRef - Eckert A, Hauptmann S, Scherping I, Meinhardt J, Rhein V, Dröse S, Brandt U, Fändrich M, Müller WE, Götz J (2008) Oligomeric and fibrillar species of beta-amyloid (Abeta42) both impair mitochondrial function in P301L tau transgenic mice. J Mol Med 86:1255-67.
CrossRef - Desai KM, Bhavsar VH, Dhumal VR, Kelkar VV. Disruptive effects of repeated electroconvulsive shock on retention of learned active avoidance is attenuated by carbamazepine. IRCS Med Sci. 1983; 11:516–7.
- Sharma S, Kumar A, Kaundal RK. Protective effects of 4 amino 1,8 naphthalimide in experimental diabetic nephropathy. Life sci. 2008;12(82): 570-6.
CrossRef - Dhingra D, Parle M, Kulkarni S. Memory enhancing activity of Glycyrrhiza glabra in mice. Journal of Ethnopharmacology. 2004;91(2-3):361-365.
CrossRef - Linnea R. Freeman, Vivian Haley-Zitlin, Dorothea S. Rosenberger, Ann-Charlotte Granholm. Damaging effects of a high-fat diet to the brain and cognition: A review of proposed mechanisms Nutr Neurosci. 2014 November ; 17(6): 241–251.
CrossRef - Jurenka JS. Therapeutic applications of pomegranate (Punica granatum L.): a review. Altern Med Rev. 2008 Jun; 13(2):128-44.
- Kiasalari Z, Khalili M, Shafiee S, Roghani M. The effect of Vitamin E on learning and memory deficits in intrahippocampal kainate-induced temporal lobe epilepsy in rats. Indian J Pharmacol 2016;48:11-4.
CrossRef








