Manuscript accepted on :30-Sep-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: M Zak Khalil
Second Review by: David Crompton
Savariraj Sahayam1, Davitraj Chellappan2, Sridharan Sriram1, Perumal Rajalakshmi1* and Pemaiah Brindha1
1Centre for Advanced Research in Indian System of Medicine(CARISM), School of Chemical and Biotechnology, SASTRA Deemed University, Thanjavur, Tamil Nadu, India.
2Central Animal Facility (CAF), School of Chemical and Biotechnology, SASTRA Deemed University, Thanjavur, Tamil Nadu, India.
Corresponding Author E-mail: rajalakshmi@carism.sastra.edu
DOI : https://dx.doi.org/10.13005/bpj/1981
Abstract
Siddha medicated oil Sivanarvembu kuzhi thylam is used in the management of various skin diseases such as leucoderma, leprosy, eczema, infected wounds, insect bites and boils. Ingredients of this formulation are roots of Indigofera aspalathoides, tubers of Corallocarpus epigaeus and seeds of Celastrus paniculatus. The present study aims to prepare the oil and to evaluate its safety and toxicity profiles by performing heavy metal analysis using Atomic Absorption Spectroscopy (AAS) through in vitro assays and carrying out in vivo acute toxicity studies in female Wistar rats. AAS results revealed that the heavy metals were present only in the below detectable level. In-vitro assay results clearly showed that the aqueous extract of selected drugs showed no toxicity in the healthy PBMCs at various concentrations. It was also observed that PBMCs showed an increase in cell proliferation at higher concentrations of each extract which also suggests that the selected drug may have a positive effect on cell survivability. No mortality was observed in the treated group of rats throughout the observation period of 14 days in acute toxicity studies. Based on the results of the research study, it is concluded that SVKT will not produce any toxic effects. . The acute oral LD50 of test substance was observed to be greater than 2000 mg/kg b.w. Based on the results of the research study, it is concluded that SVKT does not produce any toxic effects.
Keywords
Atomic Absorption Spectroscopy; Acute toxicity; in vitro; in-vivo; Sivanarvembu kuzhi thaylam; Wistar rats.
Download this article as:| Copy the following to cite this article: Sahayam S, Chellappan D, Sriram S, Rajalakshmi P, Pemaiah Brindha P. Acute Toxicity Profiles of Siddha Medicine Sivanarvembu kuzhi thailam on Rats. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Sahayam S, Chellappan D, Sriram S, Rajalakshmi P, Pemaiah Brindha P. Acute Toxicity Profiles of Siddha Medicine Sivanarvembu kuzhi thailam on Rats. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/34YwW3u |
Introduction
The traditional Siddha system is one of the most ancient systems of Indian medicine, and unique to the southern part of India. Siddhars are the founders of this system, and they classified Siddha medicines according to the mode of administration into two categories 32 types of internal medicines and 32 types of external medicines1. The common preparations of internal medicines include kudineer (decoctions), churnam (medicated powders), legium (medicated jam),nei (medicated ghee), mathirai (pills), vadagam (sweet pills), rasayanam (medicated Granules),parpam (calcined metals and minerals) and chenduram (red oxide)2.
Thaylam is one among them and is used both internally and externally. Thaylam is a medicated oil preparation and is prepared by boiling the decoction, juice, milk and pastes of herbal drugs with oils as mentioned for a specific period and filtered. It retains its medicinal value for one year1.
Some of the thaylam are prepared by a process termed “kuzhi thylam” in siddha terminology. In this process the constituent drugs are crushed coarsely and charged into an earthen pot, the bottom of which is perforated with some holes through which iron wires are passed and drawn out and tied to converge. The mouth of the vessel is covered with a lid and sealed. A pit is dug into which the pot will just fit in. At the bottom of the pit, a collecting vessel is placed and centered. The pot is placed in it so that the convergent wires are pointing to the mouth of the collection vessel. Cow dung cakes are arranged around the pot and they are set to fire. The set up is dismantled after it is completely cooled down and the oil is collected in the vessel at the bottom and is taken for further use2&3.
In the present study a polyherbal medicated oil “Sivanarvembu kuzhi thylam” (SVKT) is selected which is used for skin/dermal problems, infected wounds, insect bites and boils4. The present study aims to prepare the oil and evaluate the heavy metal limits and also carry out in-vitro and in-vivo acute toxicity profiles in female wistar rats. It has three ingredients namely roots of Indigofera aspalathoides, tubers of Corallocarpus epigaeus and seeds of Celastrus paniculatus2.
In the present scenario mercury, lead, cadmium and arsenic are common heavy metals in all edible products. These are toxic to human beings if we are exposed to longer periods5. To prove the safety of SVKT – heavy metal analysis was performed using Atomic Absorption Spectroscopy (AAS) for the oil and its main ingredient Indigofera aspalathoides (Sivanarvembu choornam –SVC). In this work, in vitro and in vivo acute oral toxicity profiles were also determined with a view to assess the non-toxic nature of the selected formulation.
Materials and Methods
Plant drugs used as ingredients
Roots of Indigofera aspalathoides, tubers of Corallocarpus epigaeus and seeds of Celastrus paniculatus2 (Figure 1) were procured from the local herbal market Thanjavur, Tamil Nadu, India, identified and authenticated in the NABL accredited Pharmacognosy lab of CARISM, SASTRA University.
 |
Figure 1: Herbal ingredients of SVKT |
In the Siddha system, Indigofera aspalathoides is called as sivanarvembu or ghanthaari. It acts as a stimulant and demulcent. It is used for treating all types of skin diseases including leprosy, fissure of palms and soles itching, fungal infection of the skin, insect bites and chronic wounds and also makes the skin shining. It is also used in treating asthma and flatulence. This root relieves the tooth pain, mouth ulcer and swelling6. It has anti-inflammatory activity and cures psoriasis disease which was proven by Baskar et.al, (2015) in their in vivo research7. This plant has anti diabetic activity8. Besides, its anticancer potential was also proved through the in-silico studies by Krishnasamy et.al, 20159. It is also the main ingredient of a Siddha formulation namely Makavallati ilakam2. Earlier studies reported its antibacterial activity10 and very high anti-inflammatory activity11.
The second drug is Celastrus paniculatus seeds. In Siddha, it called as vazhuluvai, kanguni, mallkanguni and athiparicham. It acts as an aphrodisiac, stimulant, alterative, diaphoretic and as nervine tonic. It is used for treating diarrhea, dysentery, amebic colitis, irritable bowel syndrome, enteritis, cough, hemiplegia, joint pain, pricking pain of the limb and also used to heal chronic wounds. A review article explains that it can act as an antiviral, antibacterial, insecticidal, anti-inflammatory, anti-spermatogenic, sedative, anti-fatigue, analgesic, hypolipidemic, arthritogenic, antirheumatic, aphrodisiac, emetic, laxative and also as a nervine tonic12. Herbal oil extracted from this seed is called Jyotishmati oil in Ayurveda. It has a medicinal effect on the Central Nervous System. It is employed to treat acute and chronic immobilization stress. The oil obtained from the seeds possesses sedative and anticonvulsant properties13. It is the main ingredient in many Siddha formulations like Irasakanti meluku, Idi vallathi melugu, Karudankilangu enney and Makavallati ilakam2.
The third ingredient is the tuber of Corallocarpus epigaeus. In Tamil, it is called agasakarudan kizhangu, kollancovai and pei–seenthil. It has alterative and tonic properties. It is useful in anemia, body pain, internal body heat, itching, eczema and herpes zoster. It acts as an antidote for heavy poison. A research paper explained that this tuber has antifungal activity13. Its anti diabetic activity was proved with ethanol extract by Gnananath et al., (2013)14. An In vivo screening proved its analgesic, anti-inflammatory and anti-pyretic activities15. It is one of the ingredients in a Siddha herbo – metallic formulation of rasaganthi melugu16.
Preparation of the oil
All the ingredients were powdered coarsely using a lab mill and then mixed as per the procedure given in the Siddha formulary of India and Formulary of Siddha medicine. Indigofera whole plant and Celastrus seeds were ground with Corallocarbus tuber juice and cakes were made and kept aside for semi drying. An earthen pot with few holes in the bottom was taken and thin wires were bent such that they converge and these converged wires were placed in the holes of the bottom of the earthen pot a few inches away from the base. This set pot was filled with the semi-dried cakes prepared using herbal ingredients. The mouth part was sealed with a suitable earthen plate using mud clay dipped gada cloth. The oil was prepared through the destructive distillation of the plant ingredients and collected by placing a collecting vessel in the center of the pit (Figure 2). Heat was applied only to the pot by igniting cow dung cakes arranged around and above the pot. After cooling removed the upper pot and collected the oil in the bottom vessel. Oil had blackish brown color and fire smoke smell.
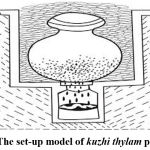 |
Figure 2: The set-up model of kuzhi thylam preparation |
Atomic Absorption Spectroscopy (AAS)
It is a heavy metal analyzing instrument according to Beer-Lambert’s law, the model is Analyst 400 (for flame), HGA 900 (for graphite furnace), Perkin Elmer. If a solution is aspirated into a flame, a vapor that contains atoms of the metal may be formed. Some of the gaseous metal atoms may be raised to an energy level which is sufficiently high to permit the emission of radiation characteristic of the metal. However, a much larger number of atoms will normally remain unexcited or ground state. These ground state atoms are capable of absorbing radiant energy of their specific resonance wavelength. The extent of absorption will be proportional to the number of ground-state atoms present in the flame. Based on this concept presence of heavy metal is calculated.
In vitro toxicity study
MTT assay procedure
In vitro cytotoxicity was evaluated for the aqueous extract of SVKT and the main ingredient SVC (dry powder of Indigofera aspalathoides) employing MTT assay as per standard textual procedures of Mossman T, (1983)19. This assay was performed against freshly collected healthy Peripheral Blood Mononuclear Cells (PBMCs). PBMCs were treated with various concentrations of selected drugs and incubated for 24 h at 37 °C in humidified 5% CO2. After 24 hours, 10 μL aliquots of serial dilutions of plant powder and oil aqueous extract (1000-1.95 μg/ml) were added to cells and incubated for 48 h. Cell viability was assessed through the MTT assay. Briefly, 25 μL of MTT (5 mg/mL) was added and the cells were incubated for an additional 3 h. Thereafter, cells were lysed and the dark blue crystals solubilized with 100 μL of a solution containing 50% N, N-dimethylformamide and 20% Sodium dodecyl sulfate. The optical density of each well was measured using the Epoch microplate spectrophotometer (BioTek, USA) set at 590 nm filter.
Acute Toxicology Study Design
The acute oral toxicity of SVKT was performed as per OECD-425 guidelines20. The study was conducted after approval by the Institutional Animal Ethical Committee, SASTRA Deemed University. (IAEC Approval Number: 310 /SASTRA/IAEC/RPP). Five healthy female Wistar rats of age 8 to 12 weeks, which were nulliparous and non-pregnant were chosen for the conduct of the present study. All the experimental animals were maintained under standard conditions (Temperature: 22±3°C and relative humidity: 50 to 70%) during the experimental period. The animals were provided with standard rodent pellet feed (Altromin, Germany) and RO water ad libitum and were acclimatized for seven days before the conduct of the study.
SVKT was suspended in distilled water, administered to all the animals at a dose of 2000 mg/kg body weight and were observed for 14 days. All the animals fasted overnight before drug treatment. The test substance was administered by oral gavages using a syringe and stainless steel ball-tipped oral gavage needle. During the observation period, all the animals were observed twice every day for mortality. Bodyweight of each animal was recorded just prior to the test substance treatment (Day 0), Day 7 and 14 using an electronic animal weighing balance (Sartorius AG, Germany). The feed intake for individual animals was recorded daily for the entire study period.
All the animals were observed individually after the treatment of the test substance during the entire observation period for the presence of any signs of toxicity including alopecia, catalepsy, chromodacryorrhea, clonic, coma, convulsion, diarrhoea, dullness, excessive grooming, change in gait, hyperactivity, lacrimation, nasal discharge, nasal irritation, piloerection, polyuria, prostration, repetitive circling, respiratory distress, salivation, scaling, tonic, tremor and urogenital staining. After 14 days of the observation period, all the animals were euthanized using carbon-di-oxide and were subjected to gross pathology.
Results
AAS results
The AAS results (Table 1) revealed that the levels of heavy metal in the herbal oil SVKT was below the detectable limit (BDL). This type of AAS method was previously used by Bhattacharjee et al., 2017 for determining heavy metal contents in plants 21.One more Siddha research paper declared that heavy metal limits in BDL .
Table 1: Heavy metal analysis by atomic absorption spectrometry(AAS)
|
Sample name |
Lead (ppm) |
Cadmium (ppm) |
Mercury (ppm) |
Arsenic (ppm) |
|
SVKT |
BDL |
BDL |
BDL |
BDL |
In vitro toxicity study
The results clearly showed that the aqueous extract of selected drugs SVKT and SVC showed no toxicity to healthy PBMCs at different concentrations used. It was also observed that PBMCs showed an increase in cell proliferation at higher concentrations. Figure.3 explains the toxicity of SVKT and SVC on normal PBMC.
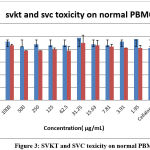 |
Figure 3: SVKT and SVC toxicity on normal PBMC |
In vivo Acute oral toxicity study (AOT)
Acute oral toxicity study for SVKT was done in female rats at a dose of 2000 mg/kg body weight as a single administration following OECD guideline 42520. In the fourteen days of study period we observed the animal death, weekly body (Table 2), Daily feed intake was measured and remained unaffected (Table 3), noted any signs of toxicity such as changes in respiration, circulation, autonomic and central nervous system, behavioural pattern were observed during the entire observation period.
Table 2: Weekly Mean Body Weight Changes In Rats
|
Animal ID |
Sex |
Body Weight(g) | ||
| Day 0 | Day 7 | Day 14 | ||
| 4319 | Female | 202.66 | 204.85 | 217.34 |
| 4320 | Female | 193.21 | 195.45 | 197.15 |
| 4321 | Female | 196.41 | 196.21 | 204.81 |
| 4322 | Female | 195.30 | 200.89 | 202.47 |
| 4323 | Female | 193.18 | 187.36 | 190.35 |
| Mean | 196.15 | 196.95 | 202.42 | |
| Standard deviation | 3.89 | 6.57 | 10.02 | |
Table 3: Daily feed intake
| Animal
ID |
Sex | Day | |||||||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | ||||
| 4319 | F |
5.69 |
12.86 |
14.25 |
9.90 |
12.12 |
11.28 |
11.95 |
12.68 |
11.44 |
16.21 |
11.54 |
9.91 |
13.90 |
12.15 |
||
| 4320 | F |
12.35 |
17.32 |
12.51 |
14.52 |
7.95 |
10.07 |
11.45 |
12.96 |
11.06 |
9.04 |
13.24 |
8.64 |
9.43 |
12.91 |
||
| 4321 | F |
11.18 |
11.78 |
11.73 |
12.96 |
12.06 |
9.11 |
16.48 |
16.22 |
12.40 |
11.22 |
12.37 |
11.47 |
12.49 |
16.00 |
||
| 4322 | F |
11.58 |
13.06 |
11.77 |
12.22 |
11.03 |
11.05 |
15.33 |
11.16 |
13.48 |
12.10 |
13.26 |
10.41 |
9.57 |
16.48 |
||
| 4323 | F |
10.38 |
12.97 |
13.75 |
9.65 |
10.47 |
6.83 |
13.73 |
9.41 |
13.85 |
8.34 |
11.50 |
10.87 |
10.10 |
13.69 |
||
| Mean |
10.24 |
13.60 |
12.80 |
11.85 |
10.73 |
9.67 |
13.79 |
12.49 |
12.45 |
11.38 |
12.38 |
10.26 |
11.10 |
14.25 |
|||
| SD |
2.64 |
2.14 |
1.15 |
2.07 |
1.70 |
1.81 |
2.15 |
2.52 |
1.22 |
3.11 |
0.87 |
1.07 |
1.99 |
1.91 |
|||
Discussion
AAS results
From this results we concluded that SVKT has no any heavy or toxic metal. Table.1 clearly shown this. One more Siddha research paper declared that heavy metal limits in BDL22 .
In vitro toxicity study
It was also observed that PBMCs showed an increase in cell proliferation at higher concentrations of each extract which also suggests that selected drugs may have a positive effect on cell survivability. In these results compared to SVC and SVKT has high cell proliferation activity. This type of in vitro study was carried out previously by Christian G J et.al., 2016 in the Siddha medicine Kariuppu mezhugu23.
In vivo Acute oral toxicity study (AOT)
No mortality was observed in any treated rats during the experimental period and all animals survived up to 14 days after the administration of SVKT24. It was observed from the present study that a slight increase in weekly body weight was found in SVKT treated animals on Day 14 (Table.2). Daily feed intake was measured and remained unaffected throughout the experimental period (Table.3). No visible signs of toxicity such as changes in respiration, circulation, autonomic and central nervous system, behavioral pattern were observed during the entire observation period.
No test compound-related findings were observed at necropsy. All gross observations were agonal (abnormal pattern of breathing) in nature and bore no relation to treatment with the test substance. All animals survived until the end of the study. With this data, we concluded that SVKT did not produce any toxicity to humans. A similar in vivo toxicological study was done on a Siddha medicine Vajjiravalli chooranam and concluded that herbal medicines have a non-toxic effect even if used long time for therapeutic purpose 25.
Conclusion
To confirm the therapeutic safety of SVKT, the acute toxicity test was carried out. AAS results revealed that the toxic metals are present only below the detectable level. SVKT was orally given at a higher dose of 2 gm/kg to the wistar rats. Single-dose oral administration of the test substance did not produce any adverse toxic effect on the body weight changes, feed and the gross anatomy of treated female wistar rats. The acute oral LD50 of the test substance was observed to be greater than 2000 mg/kg b.w. Clinically SVKT has used by the Siddha medicine practionares and its therapeutic effects is already proven one. This research work is a small step to prove this in scientifically.
Acknowledgment
Authors place their deep sense of gratitude to our Honorable Chairman, SASTRA Deemed University for suggesting the problem and motivating traditional medicinal research. We profoundly thank our Honorable Vice-Chancellor and Dean Planning and Development for their constant encouragement, support and for providing the necessary infrastructure for our research.
References
- Thyagarajan R. Gunapadam Thaathu Jeeva Vaguppu, 2nd and 3rd part, 4thedn, Department of Indian Medicine and Homeopathy, Chennai.(2004).
- , The Siddha Formulary of India .Part I. Ministry of Health and Family Welfare, Government of India, New Delhi.(1992).
- Sambasivam Pillai TV. Tamil Dictionary Vol.1-5. Fourth edition, Publisher; Tamilnadu Siddha Medical Council, Chennai. (1992).
- Kuppusamy Mudaliyar KN, UthamarayanKS. Siddhavaithiya thirattu. Tamilnadu Siddha Medical Council, Chennai.(1998).
- World Health Organization. WHO guidelines for assessing quality of herbal medicines with reference to contaminants and residues, World Health Organization. 2007.
- Murugesa Mudaliyar KS, Gunapadam Mooligai Vaguppu. Fourth edition, Tamilnadu Siddha Medical Council, Chennai (1988).
- Baskar Ananda Raj V, Senthil Kumar KL, S Suresh Kumar. Traditional Indian Medicinal Plants as a potential Anti inflammatory phytomedicine for Psoriasis control. Journal of Pharmacognosy and Phytochemistry; 4(3): 118-122 (2015).
- Vijayalakshmi K, Immanuel Selvaraj C, Sindhu S, Arumugam P. In vitro investigation of anti diabetic potential of selected traditional medicinal plants. International Journal of Pharmacognosy and Phytochemical Research. 2014;6(4): 856-861.
- Krishnasamy L, Masilamani Selvam M, Jayanthi K. Novel in silico approach of anti-cancer activity by inhibiting hemopexin proteins with indigoferaaspalathoides plant constituents at active site. Asian Journal of Pharmaceutical and Clinical Research; 8(3): 159-164(2015).
- Ariharan VN, Meena Devi VN, Parameswaran NK and Nagendra Prasad P. Anti bacterial activity of Sivanar Vembu (Indigofera aspalathoides) against some human pathogenic bacteria. Journal of Chemical and Pharmaceutical Research; 7(3): 937-941(2015).
- Selvam C, Jachak Sanjay M, Oli R Gnana, Thilagavathi Ramasamy, Chakraborti Asit K, Bhutani KK. A new cyclooxygenase (COX) inhibitory pterocarpan from Indigofera aspalathoides: structure elucidation and determination of binding orientations in the active sites of the enzyme by molecular docking Tetrahedron Letters; 45 (22): 4311-4314 (2004).
CrossRef - Kamalinee A. Deodharand Nanda W. Shinde. Celastrus paniculatus; Medicinal and pharmacological properties: a review. International Journal of Development Research; 5 (9): 5526-5531(2015).
- Deodhar KA and Shinde.N.W. Celastruspaniculatus: Traditional uses and Ethnobotanical study. Indian Journal of Advances in Plant Research; 2(1):18-21(2015).
- Vasantha K, Priyadardhini S, Tresina Soris P and Mohan VR. Antifungal activity of corallocarpus epigaeus (hook. f.). Bioscience Discovery; 3(1):87-90 (2012).
- Kattamanchi Gnananath, Kontham Ramakanth Reddy, Gudur Pavan Kumar, Bheemanapally Krishna, Karka Srinivas Reddy, Avvari Sanjeeva Kumar. Evaluation of antidiabetic activity in Corallocarpus epigaeus rhizomes. International Current Pharmaceutical Journal;2(3): 53-56(2013).
CrossRef - Narendra Naik D, Suresh Babu VV, Sandeep Veda Narayana MS, Rangu Mahesh. In vivo screening of corallocarpusepigaeus tuber for its analgesic,anti-pyretic and antiinflammatory activities. International Journal of Phytopharmacology; 3(3): 241-244 (2012).
- Formulary of Siddha medicines. Indian Medical Practitioners’ Cooperative Pharmacy and Stores Ltd., Adyar, Madras. 1989.
- Sivakkumar and Antibacterial activity of topical siddha medicine sirattaithylam against selected human pathogens. World Journal of Pharmaceutical Research;4( 10): 2859-2866(2015).
- Mossman T. Rapid calorimetric assay for cellular growth and survival: application to proliferation and cytotoxic assays. Journal of Immunological Methods;16(65): 55-63 (1983).
CrossRef - OECD, Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure, OECD Guidelines for the Testing of Chemicals, Section 4, OECD Publishing, Paris, https://doi.org/10.1787/9789264071049-en ((2008).
CrossRef - Bhattacharjee A, Chakraborty A, Dutta D, Mukhopadhyay G. Standardization of Eclipta Alba by HPTLC, HPLC and AAS. Pharm Anal Acta; 8(4): 1-6 (2017).
- Nandhini E, Mohamed Musthafa M. and Nivetha G. Standardization of Siddha herbal drug – Maha manjishtathi kashayam. World journal of pharmaceutical and medical research. 2019;5(4):120-127.
- Christian G J , Muralidharan P, Ramaswamy R S.In vitro and in vivo toxicity study of Kariuppu mezhugu (NIS KM) a Siddha Herbo- mineral formulation. Sch. Acad. J. Biosci. 2016; 4(2):132-143
- Subramanion L. Jothy ,ZurainiZakaria, Yeng Chen, Yee Ling Lau, Lachimanan Yoga Latha and SreenivasanSasidharan. Acute Oral Toxicity of Methanolic Seed Extract of Cassia fistula in Mice.Molecules; 16 (6): 5268-5282 (2011).
- S,Kaniraja.S, Muthu Kumar. N.J, Banumathi.V. Toxicological Evaluation of Herbal Siddha Preparation Vajjiravalli Chooranam in Rats. International Journal of Pharmaceutical Science Invention; 07( 07): 01-05(2018).
Abbreviations
SVKT-sivanarvembu kuzhi thylam
WHO-World Health Organisation
AAS-Atomic Absorption Spectroscopy
SVC – sivanarvembuchoornam
PBMC-Peripheral blood mononuclear cells
IAEC-Institutional Animal Ethics committee
BDL-Below Detectable Limit
PPM-parts per million
OECD- Organization for Economic Co-operation and Development
SD-Standard Deviation
LD-Lethal Dose
(Visited 2,907 times, 1 visits today)






