Wayan Niryana*, Putu Eka Mardhika, Nyoman Golden, Tjokorda GB Mahadewa and Sri Maliawan
Department of Neurosurgery, Faculty of Medicine, Universitas Udayana, Sanglah Hospital, Bali, Indonesia
Corresponding Author Email: niryanawayan@gmail.com
DOI : https://dx.doi.org/10.13005/bpj/1995
Abstract
Introduction: Hemorrhagic stroke is currently the most common cause of disability and results in high mortality compared to ischemic stroke. Surgical technique for treating supratentorial spontaneous intracerebral hemorrhage are varied. There are two procedure that still controversial which are decompressive craniectomy and craniotomy osteoplasty. In this study, we performed meta analysis to compare functional outcome between two procedures in managing spontaneous supratentorial intracerebral hemorrhage. Method: We performed systematic electronic searching in PubMed database. We included all full-text study in Bahasa or English. Glasgow Outome Scale was used to compared functional outcome between these two procedures. Meta analysis was performed using software Review Manager 5.3. Result: We found five eligible articles that met our inclusion and exclusion criteria. We performed meta analysis with random effect model because of high heterogeneity between studies (I2 = 85%; X2 = 26.47). We found that pooled risk ratio between decompressive craniectomy and craniotomy osteoplasty on poor outcome was 1.12 (p = 0.30; 95% CI: 0.90 – 1.41). Conclusion: There is no superiority between both procedures regarding management of acute spontaneous supratentorial intracerebral hemorrhage.
Keywords
Craniotomy Osteoplasty; Decompressive Craniectomy; Glasgow Outcome Scale; Spontaneous Intracerebral Hematoma; Supratentorial
Download this article as:| Copy the following to cite this article: Niryana W, Mardhika P. E, Golden N, Mahadewa T. G. B, Maliawan S. Comparison of Functional Outcome between Decompressive Craniectomy and Craniotomy Osteoplasty in Acute Spontaneous Supratentorial Intracerebral Hemorrhage: A Systematic Review and Meta Analysis. Biomed Pharmacol J 2020;13(3). |
| Copy the following to cite this URL: Niryana W, Mardhika P. E, Golden N, Mahadewa T. G. B, Maliawan S. Comparison of Functional Outcome between Decompressive Craniectomy and Craniotomy Osteoplasty in Acute Spontaneous Supratentorial Intracerebral Hemorrhage: A Systematic Review and Meta Analysis. Biomed Pharmacol J 2020;13(3). Available from: https://bit.ly/32R8bVS |
Introduction
Hemorrhagic stroke is a major cause of long-term disability and is associated with higher mortality compared to ischemic stroke.1 Arteriosclerotic hypertension is the most common cause of primary hemorrhage followed by other causes such as vascular malformations and amyloid angiopathy.2 Clot evacuation is the treatment of choice in indicated patients. Evacuation of clot is favourable because the rationale to prevent the toxic effects of clot degradation and the mass effect of hematoma.3
Operative treatment provide favourable outcome compared to conservative treatment in patients with indication. The indication are patients with presenting GCS 4 – 8, volume of 31 – 60 ml, presence of midline shift more than 5 mm, an intraventricular extension, and without pupillary asymmetry.4 Surgical treatment was also reported capable to reduce short term and long term mortality compared to conservative treatment.5
Craniotomy with hematoma evacuation is preferred for the surgical management of ICH because it provides favourable clinical outcome in patients with supratentorial spontaneous intracerebral hemorrhage (sICH).6 However, evacuation of hematoma alone may not be enough to relieve intracranial hypertension. The ICP can increase again and reach pathological value after hematoma evacuation because of loss of cerebral autoregulation.7
Decompressive craniectomy also seems to provide promising result regarding management of supratentorial sICH.[8-10] DC was able to reduce rate of in-hospital mortality and provide better functional outcome compared to non operative patients.8,9 However, the benefits of DC must be weighed against the risks. Various complications can occur after DC. They are epidural and subdural hematomas; subdural effusions or hygromas; brain herniation, wound infection and prolong healing; hydrocephalus; trephined syndrome; bone flap resorption and others. Survivors have to undergo a second surgical intervention to repair the cranial defect (cranioplasty) after decompressive craniectomy.10
However, the superiority between these procedures is still controversial in treating supratentorial sICH. This study aimed to compare the functional outcome between two procedures in managing supratentorial sICH through systematic review and meta-analysis.
Methods
Eligibility Criteria
The eligibility criteria for the included studies are based on the PICO framework. The PICO criteria can be seen in Table 1.
Table 1: PICO criteria of the study
| Patient | Supratentorial sICH |
| Intervention | Decompressive Craniectomy |
| Comparator | Craniotomy Osteoplasty |
| Outcome | Glasgow Outcome Scale |
We included all studies comparing decompressive craniectomy and osteoplasty craniotomy in supratentorial sICH patients. Exclusion criteria were review, cadaveric, anatomy, animal, qualitative and economic studies. Articles made by similar authors at similar institutions are subject to critical evaluation to prevent sample duplication. Articles in Bahasa and English were included in this review. Other languages were first translated and decided by the author whether included or not. We do not limit articles based on year of publication.
We included articles with participants aged 18 years or older of both genders with supratentorial sICH. Supratentorial SICH was defined as an acute hematoma in the brain parenchyma that is confirmed by hyperdense lesions on a CT scan of the head without a history of trauma. We include participants from all countries and setting.
The intervention of interest were decompressive craniectomy and craniotomy osteoplasty. Craniotomy osteoplasty was defined as a procedure to open the skull and evacuating blood clots followed by direct bone replacement on the defect. Decompressive craniectomy is defined as the procedure of removing a portion of the skull temporarily after blood clot evacuation. Articles that did not explain the surgical procedure and combined the techniques of interest with other techniques were excluded.
The outcome in this review was the Glasgow Outcome Scale (GOS) after the surgical procedure. GOS is dichotomized into favorable and poor outcome. Favorable were defined as GOS 4-5, while poor were defined as GOS 1-3.
Search Strategy
We extract the eligibility criteria (PICO) into keywords using the Boolean operator. We performed a systematic search using keywords ((craniotomy) AND (craniectomy) AND (spontaneous intracerebral hemorrhage)) in the PubMed database to find eligible studies.
Study selection was carried out by WN and EM to reduce the possibility of excluding relevant studies. When disagreement occured between the two authors, the decision of the other authors were used to reach a conclusion. Duplicate article notes were removed. Titles and abstracts are then filtered and irrelevant studies were removed. Further evaluation was carried out in the article for compliance with inclusion and exclusion criteria. Finally, studies were evaluated for quality before being included in this review.
Data Collection Process
Data collection was done by using an electronic data collection form. The collected data were combined and managed with software Review Manager 5.3.
Data Items
We collected data items such as the author’s name, year of publication, research method, sample size, participant diagnosis, age, surgical technique, and GOS. GOS is dichotomized into favorable and poor outcome. The risk ratio (RR) of each study was calculated and a meta-analysis was conducted.
Assessment of Quality of Study
Studies that pass the inclusion and exclusion criteria were assessed for quality to ensure the validity and reliability of research. We conducted quality assessments using standardized critical assessment tools to minimize the possibility of bias in the selection of studies by two independent authors. We used the Joanna Briggs Institute (JBI) critical assessment tool based on research designs to conduct quality assessments. The third author’s decision was used when disagreement occured.
We used cut off point to determine the quality of study. The cut point in was half of the total score in each JBI critical assessment checklist. Low-quality study was defined as scores below the cut-off point while conversely was referred as high-quality studies.
Synthesis of Result
The RR of outcome of each study were pooled and analysed. We used software Review Manager 5.3 to perform meta analysis. We performed random effect model because of high heterogeneity among studies.
Results
We found five articles that met our inclusion and exclusion criteria after systematic searching in Pubmed database (Figure 1). Four of the articles were observational study, while one article was randomized controlled trial. All of 5 articles were considered as good quality based on JBI critical appraisal checklist. Summary of finding and complete characteristic of study can be seen in Table 2, Table 3, and Table 4.
We performed random effect model analysis with high heterogeneity (I2 = 85%; X2 = 26.47) and we found pooled risk ratio between decompressive craniectomy and craniotomy osteoplasty on poor outcome was 1.12 (p = 0.30; 95% CI: 0.90 – 1.41) (Table 5). The forest plot of the meta analysis can be seen in Figure 2.
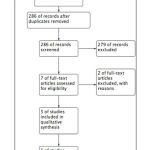 |
Figure 1: PRISMA study flow diagram |
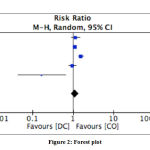 |
Figure 2: Forest plot |
Table 2: Summary of findings for the main comparison
| Study Author | Type of Study | Level of Evidence | Subject Condition | Intervention | n | Control | n | Outcome |
| Ghani et al. 2008 [11] | Observational study, prospective cohort | 1b | Spontaneous supratentorial ICH, GCS 5 – 12 with disturbed consciousness, hematoma volume > 30 ml and/or evidence of mass effect based on midline shift at the level of third ventricle. | DC | 15 | CO | 21 | DC: 14 poor outcome, CO: 17 poor outcome |
| Kim 2018 et al. 2011 [12] | Observational study, retrospective cohort | 1b | Spontaneous ICH confirmed by CT; ICH volume > 50 ml; surgical evacuation within 24 hours of the ictus; age 30 – 85 years old | DC | 125 | CO | 139 | DC: 121 poor outcome, CO: 121 poor outcome |
| Li et al. 2013 [13] | Observational study, retrospective cohort | 1b | Basal ganglia haemorrhage | DC | 68 | CO | 185 | DC: 60 poor outcome, CO: 105 poor outcome |
| Ma et al. 2010 [14] | Observational study, retrospective cohort | 1b | Spontaneous basal ganglia hemorrhage and rapid neurological deterioration | DC | 38 | CO | 46 | DC: 30 poor outcome, CO: 38 poor outcome |
| Moussa et al. 2016 [15] | Prospective randomized controlled clinical trial | Aged from 18 – 80 years old, large hemispheric hypertensive ICH with volume > 60 ml | DC | 20 | CO | 20 | DC: 2 poor outcome, CO: 12 poor outcome |
Table 3: Characteristics of study of Ghani et al. and Kim et al.
| Functional outcome at 6 months in surgical treatment of spontaneous supratentorial intracerebral haemorrhage. (Ghani et al. 2008)[11] | |
| Methods | Prospective cohort |
| Participants | Inclusion criteria:
· Patients with evidence of spontaneous supratentorial ICH on intial CT brain · GCS 5 – 12 with obvious hemiplegia or disturbed consciousness · CT brain with hematoma volume > 30 ml and/or evidence of mass effect based on midline shift at the level of third ventricle. Exclusion criteria · Hemorrhage was due to an aneurysm and/or angiographically proven arteriovenous malformation · Intracerebral hemorrhage secondary to trauma or tumour · Cerebellar or brainstem haemorrhage · Severe preexisting physical or mental disability · Severe comorbidity |
| Intervention | Intervention group
· Decompressive craniectomy: 15 patients Comparison group · Craniotomy clot evacuation: 21 patients |
| Outcomes | Primary outcomes is Glasgow Outcome Scale (GOS) at six months |
| Comparison of craniotomy and decompressive craniectomy in large supratentorial intracerebral hemorrhage. (Kim et al. 2018)[12] | |
| Methods | Retrospective cohort |
| Participants | Inclusion criteria:
· Patients with spontaneous ICH confirmed by CT · ICH volume > 50 ml · Surgical evacuation within 24 hours of the ictus; · Age 30 – 85 years old Exclusion criteria · Coagulopathy including INR >1.2 and anticoagulation therapy · Traumatic ICH · Intracranial or systemic infection · Severe cardiac, hepatic, renal, or pulmonary dysfunction · Neoplasm leading to ICH · History of stroke with neurological deficits · Intracranial aneurysm or other vascular malformation Endoscopic or stereotactic guided hematoma aspiration |
| Intervention | Intervention group
Decompressive craniectomy: 125 patients Comparison group Craniotomy clot evacuation: 139 patients |
| Outcomes | Primary outcomes is GOS at 12 months after surgery. |
Table 4: Characteristics of study of Li et al., Ma et al., and Moussa et al
| Surgical treatment for large spontaneous basal ganglia hemorrhage: retrospective analysis of 253 cases. (Li et al. 2013)[13] | |
| Methods | Retrospective cohort |
| Participants | Inclusion criteria:
· Patients with basal ganglia haemorrhage Exclusion criteria · Traumatic brain injury · Typical aneurysm · Vascular malformation hemorrhage |
| Intervention | Intervention group
· Decompressive craniectomy: 68 patients Comparison group · Craniotomy osteoplasty: 185 patients |
| Outcomes | Primary outcomes was GOS at 3 months post operatively |
| Decompressive craniectomy in addition to hematoma evacuation improves mortality of patients with spontaneous basal ganglia hemorrhage. (Ma et al. 2010)[14] | |
| Methods | Retrospective cohort |
| Participants | Inclusion criteria:
· Patient with spontaneous basal ganglia hemorrhage identified by non contrast CT · Rapid neurological deterioration Exclusion criteria · ICH from cerebral aneurysm, arteriovenous malformation, brain tumor or head trauma |
| Intervention | Intervention group
· Decompressive craniectomy: 38 patients Comparison group · Craniotomy clot evacuation: 46 patients |
| Outcomes | GOS at 6 months post operatively |
| Decompressive craniectomy and expansive duraplasty with evacuation of hypertensive intracerebral hematoma, a randomized controlled trial. (Moussa et al. 2016)[15] | |
| Methods | Prospective randomized controlled trial |
| Participants | Inclusion criteria:
· Patient aged from 18 – 80 years old · Confirmed large hemispheric hypertensive ICH that had a measured hematoma volume at least 60 ml on CT Exclusion criteria · Patients with associated major comorbidity such as cancer, long term uncontrolled diabetes mellitus, severe dementia, renal, hepatic or respiratory failure |
| Intervention | Intervention group
· Decompressive craniectomy: 20 patients Comparison group · Craniotomy clot evacuation: 20 patients |
| Outcomes | Primary outcomes is GOS at three and six months post operation |
Table 5: Pooled meta-analysis of included study
| Study | DC | CO | Weight | Risk Ratio
M-H, Random, 95% CI |
||
| Events | Total | Events | Total | |||
| Ghani 2008[11] | 14 | 15 | 17 | 21 | 21.3% | 1.15 [0.90, 1.48] |
| Kim 2018[12] | 121 | 125 | 121 | 139 | 28.0% | 1.11 [1.04, 1.19] |
| Li 2013[13] | 60 | 68 | 105 | 185 | 25.4% | 1.55 [1.33, 1.81] |
| Ma 2009[14] | 30 | 38 | 38 | 46 | 22.9% | 0.96 [0.77, 1.18] |
| Moussa 2016[15] | 2 | 20 | 12 | 20 | 2.4% | 0.17 [0.04, 0.65] |
| Total (95% CI) | 266 | 411 | 100.0% | 1.12 [0.90, 1.41] | ||
| Total events | 227 | 293 | ||||
| Heterogeneity: Tau2 = 0.04; Chi2 = 26.47, df = 4 (P < 0.0001); I2 = 85% | ||||||
| Test for overall effect: Z = 1.04 (P = 0.30) | ||||||
Discussion
Hemorrhagic stroke is mostly cause by hypertensive arteriosclerosis. 2 Hypertension could cause degenerative changes in small perforating arteries. It is thought that these changes could lead to increase likelihood of rupture. It was supported by the fact that hypertensive hemorrhage has a tendency to occur in the deep brain structures supplied by these vessels.16
Beside the hypertension, increased alcohol intake could cause brain hemorrhage also. Alcohol intake was postulated to produce platelet / coagulopathy dysfunction and endothelial damage which subsequently caused rupture of blood vessels. Increased high density lipoprotein cholesterol, low total cholesterol, and high non-density lipoprotein cholesterol are also associated with sICH.16
Cerebral amyloid angiopathy is also a cause of hemorrhagic stroke. Deposition of β-amyloid in cortical blood vessels is the main cause of this angiopathy. Blood vessels continue to weaken and the tendency to rupture increases. This pathogenesis makes CAA an independent risk factor for lobar sICH. The presence of E2 and E4 alleles of apolipoprotein E is associated with an increase in β-amyloid deposition in the vessel wall and, consequently, the risk of bleeding. In addition, secondary sICH can occur due to coagulopathy, vascular malformation rupture, cerebral venous thrombosis, mycotic aneurysm rupture, moyamoya, tumors, hemorrhagic conversion from ischemic stroke, or vasculitis.16
In this meta analysis, we found that both procedure provide similar clinical outcome to patients with supratentorial sICH. However, we provide several theoritical pathopyhsiology of intracerebral hematoma that can be used as consideration to choose the surgical technique.
The development of PHE and cerebral dynamic autoregulation disturbance should be considered when choosing surgical technique of supratentorial sICH. All of these can cause detrimental effect even when the hematoma has been evacuated. Understanding of these pathophysiological event can provide better outcome of supratentorial sICH patients.
When bleeding occurs, initial tissue injury activates many secondary injury pathways, including the release of toxic hemolysis products, oxidative stress, and inflammatory response, which results in the development of perihematomal edema (PHE) in the initial acute stage.17-19 PHE is associated with poor outcome of sICH patients.18-20 Two aspects can contribute to the possible adverse effects of PHE. First, an increase in volume with tissue shifts and herniation has been recognized as a possible consequence of PHE and factors affecting mortality. Second, the pathophysiological background of PHE includes several pathways of destruction leading to damage and death of neurons, for example, excitotoxicity and release of glutamate (cytotoxic edema), induction of inflammatory and pro-apoptotic pathways, brain barrier damage (vasogenic edema), release of toxic blood decomposition products and thrombin, or early perihematomal hypoperfusion, so PHE can represent the process of neuron damage.21
Several studies have shown us that PHE can be predicted. Wagner et al., found that PHE is associated with higher sICH radioopacity on head CT scan.22 The absence of ipsilateral vein filling is also observed in about one third of patients with acute ICH, which may be associated with hypoperfusion after ICH and is strongly associated with the development of PHE. Identification of the status of cerebral venous filling may be a promising imaging marker for PHE and potential therapeutic targets on ICH.17 Feng et al reported that presence of Jugular Venous Reflux (JVR) is associated with PHE volume in sICH patients. It is plausible that JVR retrogradely transmits venous hypertension into the brain.23 Leasure et al., found that intensive blood pressure reduction could decrease PHE expansion in deep sICH.24
The cerebral dynamic autoregulation is also disturbed because of the hematoma. Larger hematoma volume is likely to independently predict poorer cerebral autoregulation status ipsilateral to hematoma.25
The PHE and disturbed cerebral dynamic autoregulation are the rationale of performing decompressive craniectomy in sICH patients because the ICP can increase to pathological value again even after hematoma evacuation and result in performing another operation. Decompressive craniectomy can provide additional room for the edema brain so the ICP is kept in normal value during acute phase. When the brain has relaxed, we can perform cranioplasty to replace the bone flap again as second procedure. However, a variety of complications can occur following DC. They include epidural and subdural hematomas; subdural effusions or hygromas; brain herniation; wound healing problems; hydrocephalus, trephined syndrome, bone flap resorption and others.10
Regarding the operating time, surgical management of sICH should be performed within 7 – 24 hours. If earlier, there is a higher rebleeding rate and if later, there is risk of being burdened by severe complications.26 In patients with poor GCS scores (3-4), the surgical approach loses evidence of efficacy. Similarly, surgery is not indicated in patients with higher GCS admission scores (> 13), for whom the initial approach should be medical. Hematoma evacuation should be considered, instead, in all patients with GCS admission scores ranging between 5 and 12.26
Conclusion
There is no superiority between two procedures regarding clinical outcome of acute spontaneous supratentorial intracerebral hemorrhage. Further studies are needed to provide better evidence regarding this controversy. We suggest that the first surgical planning for treating supratentorial sICH should be done with craniotomy osteoplasty technique, however surgeon should make preparation to perform decompressive craniectomy when severe brain edema occurred. The surgeon also need to assess preoperative risk factors of brain edema based on what have been described above.
Comflict of Interest
Authors declare no conflict of interest regarding this research.
Funding Source
There is no funding source.
Reference
- Roth C, Salehi M, Deinsberger W, Kaestner S, Engel H. Conservative versus operative treatment in supratentorial intracerebral hemorrhage – A survey among neurosurgeons and neurologist in Germany. Clin. Neurol. Neurosurg. 2019; 186: 105502. DOI: 10.1016/j.clineuro.2019.105502.
CrossRef - Rehman WA, Anwar MS. Surgical outcome of spontaneous supra tentorial intracerebral hemorrhage. Pak. J. Med. Sci. 2017; 33(4): 804 – 807.
CrossRef - Fung C, Murek M, Z’Graggen WJ, Krahenbuhl AK, Gautschi OP, Schucht P, et al. Decompressive hemicraniectomy in patients with supratentorial intracerebral hemorrhage. Stroke. 2012; 43(12): 3207-11. DOI: 10.1161/STROKEAHA.112.666537.
CrossRef - Urday S, Beslow LA, Dai F, Zhang F, Battey TW, Vashkevich A, et al. Rate of perihematomal edema expansion predicts outcome after intracerebral hemorrhage. Crit. Care. Med. 2016; 44(4): 790 – 7. DOI: 10.1097/CCM.0000000000001553.
CrossRef - Feng H, Zhang H, He W, Zhou J, Zhao X. Jugular venous reflux is associated with perihematomal edema after intracerebral hemorrhage. Biomed. Res. Int. 2017; 2017: 7514639. DOI: 10.1155/2017/7514639.
CrossRef - Gross BA, Jankowitz BT, Friedlander RM. Cerebral intraparenchymal hemorrhage: a review. JAMA. 2019; 321(13): 1295 – 1303. DOI: 10.1001/jama.2019.2413.
CrossRef - Takeuchi S, Wada K, Nagatani K, Otani N, Mori K. Decompressive hemicraniectomy for spontaneous intracerebral hemorrhage. Neurosurg. Focus. 2013; 4: 1 – 6.
CrossRef - Bhaskar MK, Kumar R, Ojha B, Singh SK, Verma N, Verma R, et al. A randomized controlled study of operative versus nonoperative treatment for large spontaneous supratentorial intracerebral hemorrhage. Neurol. India. 2017; 65(4): 752 – 758. DOI: 10.4103/neuroindia.NI_151_16.
CrossRef - Wagner I, Volbers B, Hilz MJ, Schwab S, Doerfler A, Staykov D. Radiopacity of intracerebral hemorrhage correlates with perihemorrhagic edema. Eur. J. Neurol. 2012; 19(3): 525 – 8. DOI: 10.1111/j.1468-1331.2011.03526.x.
CrossRef - Trifan G, Arshi B, Testai FD. Intraventricular hemorrhage severity as a predictor of outcome in intracerebral hemorrhage. Front. Neurol. 2019; 10: 2017. DOI: 10.3389/fneur.2019.00217.
CrossRef - Ghani AR, John JT, Idris Z, Ghazali MM, Murshid NL, Musa KI. Functional outcome at 6 months in surgical treatment of spontaneous supratentorial intracerebral haemorrhage. Malays. J. Med. Sci. 2008; 15(4): 48 – 55.
CrossRef - Kim DB, Park SK, Moon BH, Cho BR, Jang DK, Jang KS. Comparison of craniotomy and decompressive craniectomy in large supratentorial intracerebral hemorrhage. J. Clin. Neurosci. 2018; 50: 208 – 213. DOI: 10.1016/j.jocn.2018.01.066.
CrossRef - Li Q, Yang CH, Xu JG, Li H, You C. Surgical treatment for large spontaneous basal ganglia hemorrhage: retrospective analysis of 253 cases. Br. J. Neurosurg. 2013; 27(5): 617 – 21. DOI: 10.3109/02688697.2013.765938.
CrossRef - Ma L, Liu WG, Sheng HS, Fan J, Hu WW, Chen JS. Decompressive craniectomy in addition to hematoma evacuation improves mortality of patients with spontaneous basal ganglia hemorrhage. J. Strok.e Cerebrovasc. Dis. 2010; 19(4): 294 – 8. DOI: 10.1016/j.strokecerebrovasdis.2009.07.002.
CrossRef - Moussa WM, Khedr W. Decompressive craniectomy and expansive duraplasty with evacuation of hypertensive intracerebral hematoma, a randomized controlled trial. Neurosurg. Rev. 2017; 40(1): 115 – 127. DOI: 10.1007/s10143-016-0743-6.
CrossRef - Gildersleeve KL, Hirzallah MI, Esquenazi Y, Moomaw CJ, Sekar P, Cai C, et al. Hemicraniectomy for supratentorial primary intracerebral hemorrhage: a retrospective, propensity score matched study. J. Stroke. Cerebrovasc. Dis. 2019; 28(11): 104361. DOI: 10.1016/j.strokecerebrovasdis.2019.104361.
CrossRef - Zheng J, Li H, Zhao HX, Guo R, Lin S, Dong W, et al. Surgery for patients with spontaneous deep supratentorial intracerebral hemorrhage: a retrospective case-control study using propensity score matching. Medicine (Baltimore). 2016; 95(11): e3024. DOI: 10.1097/MD.0000000000003024.
CrossRef - Chen L, Xu M, Yan S, Luo Z, Tong L, Lou M. Insufficient cerebral venous drainage predicts early edema in acute intracerebral hemorrhage. Neurology. 2019; 93(15): e1463-e1473. DOI: 10.1212/WNL.0000000000008242.
CrossRef - Volbers B, Giede-Jeppe A, Gerner ST, Sembill JA, Kuramatsu JB, Lang S, et al. Peak perihemorrhagic edema correlates with functional outcome in intracerebral hemorrhage. Neurology. 2018; 90(12): e1005 – e1012. DOI: 10.1212/WNL.0000000000005167.
CrossRef - Leasure AC, Qureshi AI, Murthy SB, Kamel H, Goldstein JN, Walsh KB, et al. Intensive blood pressure reduction and perihematomal edema expansion in deep intracerebral hemorrhage. Stroke. 2019; 50(8): 2016 – 2022. DOI: 10.1161/STROKEAHA.119.024838.
CrossRef - Ma H, Guo ZN, Sun X, Liu J, Lv S, Zhao L, et al. Hematoma volume is a predictive factor of disturbed autoregulation after spontaneous intracerebral hemorrhage. J. Neurol. Sci. 2017; 382: 96 – 100. DOI: 10.1016/j.jns.2017.09.035.
CrossRef - Luzzi S, Elia A, Del Maestro M, Morotti A, Elbabaa SK, et al. Indication, timing, and surgical treatment of spontaneous intracerebral hemorrhage: systematic review and proposal of a management algorithm. World. Neurosurg. 2019.
CrossRef - Grunwald Z, Beslow LA, Urday S, Vashkevich A, Ayres A, Greenberg SM, et al. Perihematomal edema expansion rates and patient outcomes in deep and lobar intracerebral hemorrhage. Neurocrit. care. 2017; 26(2): 205 – 212. DOI: 10.1007/s12028-016-0321-3.
CrossRef - Hessington A, Tsitsopoulos PP, Fahlstrom A, Marklund N. Favorable clinical outcome following surgical evacuation of deep-seated and lobar supratentorial intracerebral hemorrhage: a retrospective single-center analysis of 123 cases. Acta. Neurochir (Wien). 2018; 160(9): 1737 – 1747. DOI: 10.1007/s00701-018-3622-9.
CrossRef - Volbers B, Willfarth W, Kuramatsu JB, Struffert T, Dorfler A, Huttner HB. Impact of perihemorrhagic edema on short-term outcome after intracerebral hemorrhage. Neurocrit. Care. 2016; 24(3): 404 – 12. DOI: 10.1007/s12028-015-0185-y.
CrossRef - Esquenazi Y, Savitz SI, El Khoury R, McIntosh MA, Grotta JC, Tandon N. Decompressive hemicraniectomy with or without clot evacuation for large spontaneous supratentorial intracerebral hemorrhages. Clin. Neurol. Neurosurg. 2015; 128: 117 – 22. DOI: 10.1016/j.clineuro.2014.11.015.
CrossRef








